Abstract
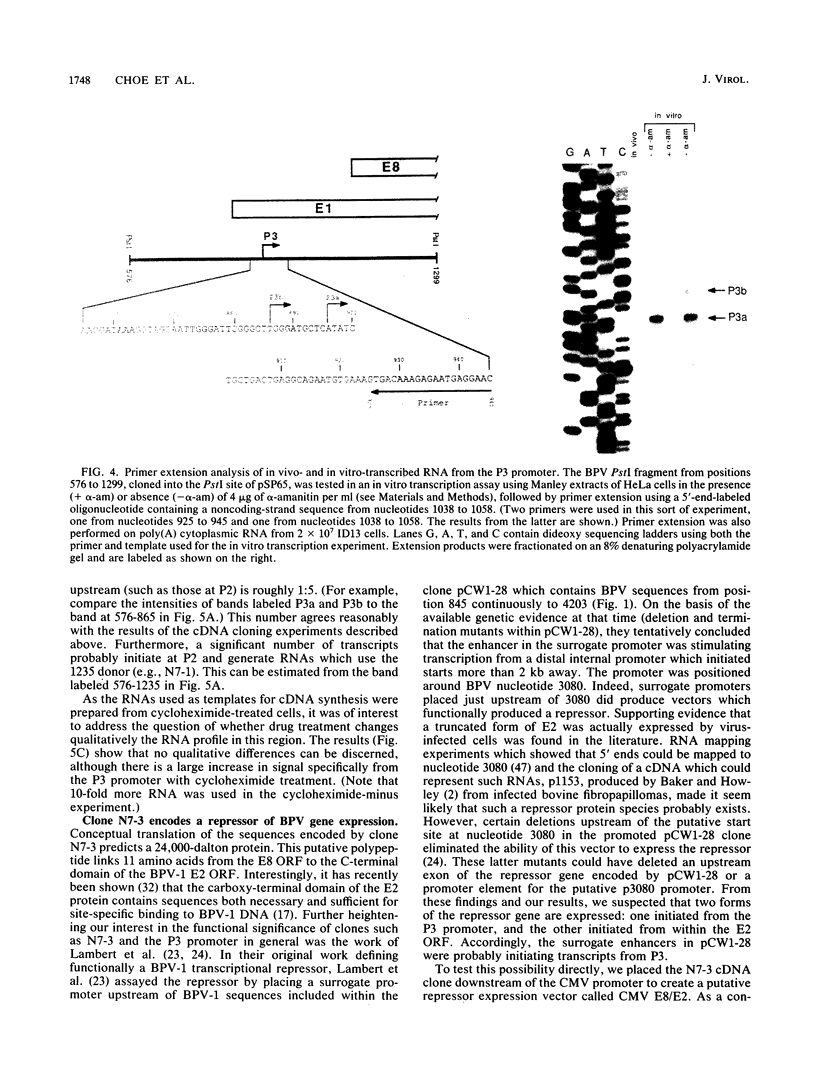
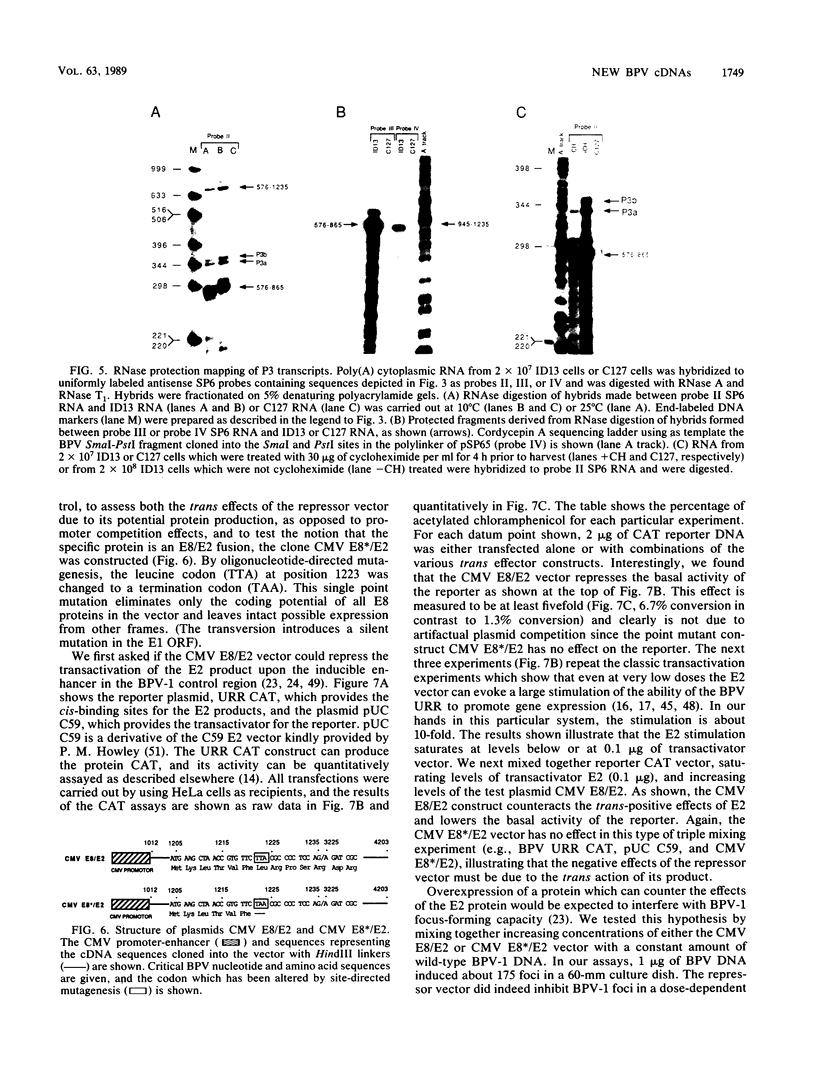
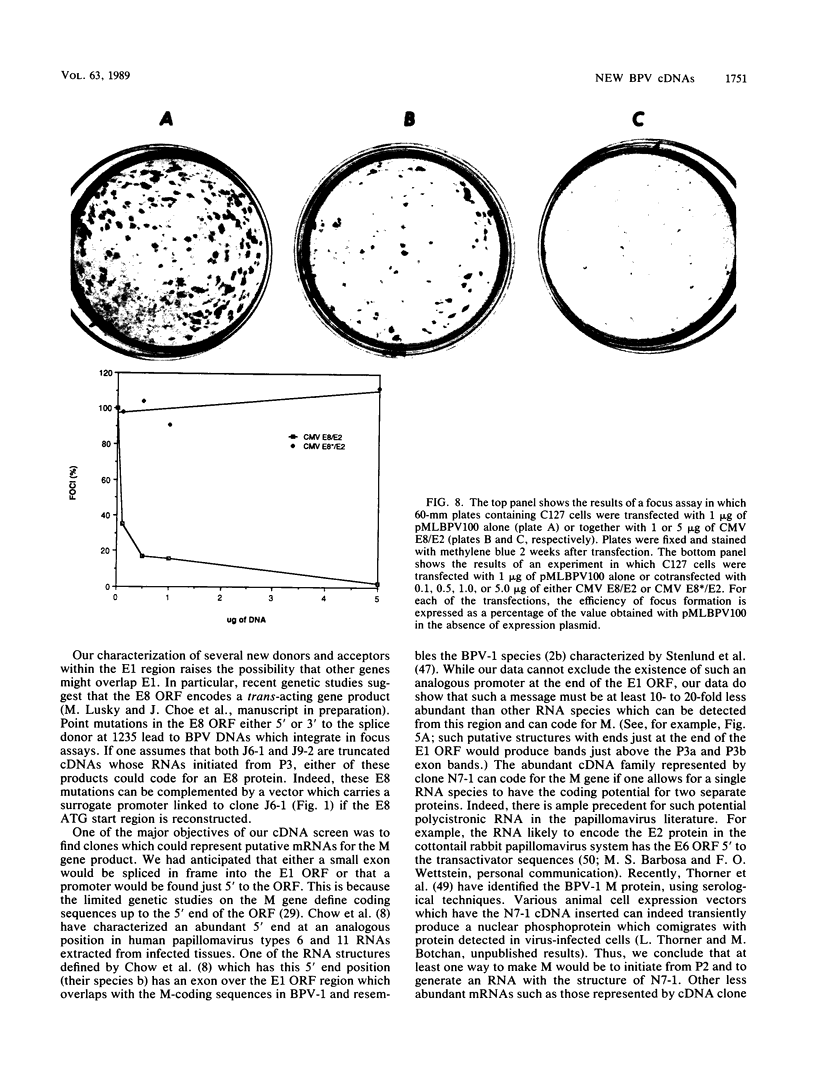
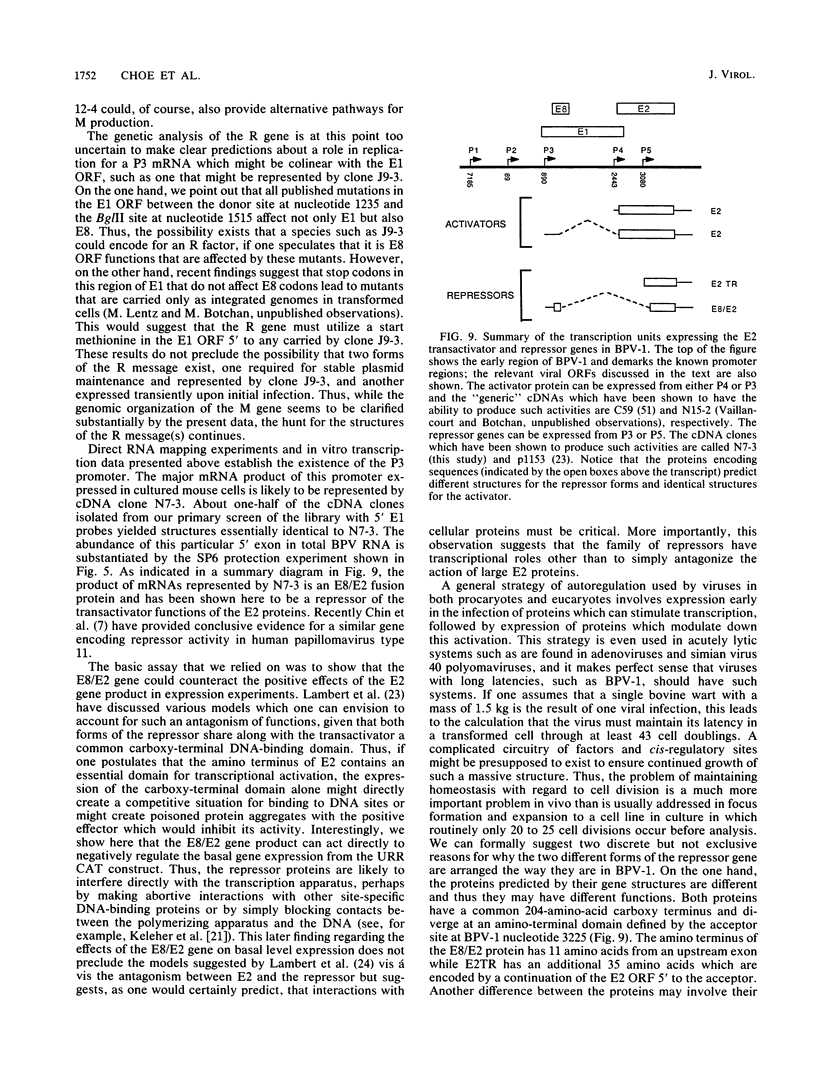
Genetic and biochemical evidence has established that the E2 open reading frame (ORF) of bovine papillomavirus type 1 encodes at least two different site-specific DNA-binding proteins, one which activates and the other which represses expression from a viral promoter (P. F. Lambert, B. A. Spalholz, and P. M. Howley, Cell 50:69-78, 1987). We have obtained data which show that a second form of the repressor gene is expressed in transformed cells harboring stable viral plasmids. The structural details of this gene have been discerned by cDNA cloning, by RNase protection, and by primer extension analysis of in vivo RNA. Moreover, data from in vitro transcription experiments support the notion that this form of the E2 repressor is expressed from a novel viral promoter and that a small exon from another ORF is linked to an active repressor domain in E2. Thus, two different forms of the repressor are expressed from different promoters and might be independently regulated either in the cell cycle or in different tissue types. We show by functional in vivo assays utilizing a cDNA vector encoding this gene that the trans-acting factor has in vivo activities similar to those of the known repressor. Our screen of a cDNA library for cDNA clones representing bovine papillomavirus transcripts has also revealed a number of other novel structures defining new donor and acceptor RNA-processing sites. Notably, clones which conceptually can be translated to yield an E7 protein, the viral M gene, and the entire E2 ORF have been characterized. Finally, truncated versions of putative E8 cDNAs were also obtained.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahola H., Stenlund A., Moreno-López J., Pettersson U. Promoters and processing sites within the transforming region of bovine papillomavirus type 1. J Virol. 1987 Jul;61(7):2240–2244. doi: 10.1128/jvi.61.7.2240-2244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Howley P. M. Differential promoter utilization by the bovine papillomavirus in transformed cells and productively infected wart tissues. EMBO J. 1987 Apr;6(4):1027–1035. doi: 10.1002/j.1460-2075.1987.tb04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berg L., Lusky M., Stenlund A., Botchan M. R. Repression of bovine papilloma virus replication is mediated by a virally encoded trans-acting factor. Cell. 1986 Aug 29;46(5):753–762. doi: 10.1016/0092-8674(86)90351-x. [DOI] [PubMed] [Google Scholar]
- Burnett S., Moreno-Lopez J., Pettersson U. Messenger RNAs from the E1 region of bovine papillomavirus type 1 detected in virus-infected bovine cells. Nucleic Acids Res. 1987 Nov 11;15(21):8607–8620. doi: 10.1093/nar/15.21.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M. T., Hirochika R., Hirochika H., Broker T. R., Chow L. T. Regulation of human papillomavirus type 11 enhancer and E6 promoter by activating and repressing proteins from the E2 open reading frame: functional and biochemical studies. J Virol. 1988 Aug;62(8):2994–3002. doi: 10.1128/jvi.62.8.2994-3002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Nasseri M., Wolinsky S. M., Broker T. R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987 Aug;61(8):2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Settleman J. Bovine papillomavirus mutant temperature sensitive for transformation, replication and transactivation. EMBO J. 1988 Apr;7(4):1197–1204. doi: 10.1002/j.1460-2075.1988.tb02931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haugen T. H., Cripe T. P., Ginder G. D., Karin M., Turek L. P. Trans-activation of an upstream early gene promoter of bovine papilloma virus-1 by a product of the viral E2 gene. EMBO J. 1987 Jan;6(1):145–152. doi: 10.1002/j.1460-2075.1987.tb04732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Androphy E. J., Lowy D. R., Schiller J. T. The specific DNA recognition sequence of the bovine papillomavirus E2 protein is an E2-dependent enhancer. EMBO J. 1988 Feb;7(2):525–531. doi: 10.1002/j.1460-2075.1988.tb02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P. L., Howley P. M. Mutational analysis of the 3' open reading frames and the splice junction at nucleotide 3225 of bovine papillomavirus type 1. J Virol. 1987 Dec;61(12):3889–3895. doi: 10.1128/jvi.61.12.3889-3895.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert N. L., Schiller J. T., Lowy D. R., Androphy E. J. Bovine papilloma virus-transformed cells contain multiple E2 proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5864–5868. doi: 10.1073/pnas.85.16.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher C. A., Goutte C., Johnson A. D. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell. 1988 Jun 17;53(6):927–936. doi: 10.1016/s0092-8674(88)90449-7. [DOI] [PubMed] [Google Scholar]
- Kleiner E., Dietrich W., Pfister H. Differential regulation of papilloma virus early gene expression in transformed fibroblasts and carcinoma cell lines. EMBO J. 1986 Aug;5(8):1945–1950. doi: 10.1002/j.1460-2075.1986.tb04448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Animal papillomaviruses. Microbiol Rev. 1982 Jun;46(2):191–207. doi: 10.1128/mr.46.2.191-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. A bovine papillomavirus type 1-encoded modulator function is dispensable for transient viral replication but is required for establishment of the stable plasmid state. J Virol. 1986 Nov;60(2):729–742. doi: 10.1128/jvi.60.2.729-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Transient replication of bovine papilloma virus type 1 plasmids: cis and trans requirements. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3609–3613. doi: 10.1073/pnas.83.11.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A. A., Schlegel R., Howley P. M. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988 Feb;7(2):533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Negative control of DNA replication in composite SV40-bovine papilloma virus plasmids. Cell. 1986 Aug 29;46(5):741–752. doi: 10.1016/0092-8674(86)90350-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Lambert P. F., Yee C. L., Howley P. M. Bovine papillomavirus transcriptional regulation: localization of the E2-responsive elements of the long control region. J Virol. 1987 Jul;61(7):2128–2137. doi: 10.1128/jvi.61.7.2128-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Stenlund A., Bream G. L., Botchan M. R. A promoter with an internal regulatory domain is part of the origin of replication in BPV-1. Science. 1987 Jun 26;236(4809):1666–1671. doi: 10.1126/science.3037693. [DOI] [PubMed] [Google Scholar]
- Stenlund A., Zabielski J., Ahola H., Moreno-Lopez J., Pettersson U. Messenger RNAs from the transforming region of bovine papilloma virus type I. J Mol Biol. 1985 Apr 20;182(4):541–554. doi: 10.1016/0022-2836(85)90240-2. [DOI] [PubMed] [Google Scholar]
- Thierry F., Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987 Nov;6(11):3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner L., Bucay N., Choe J., Botchan M. The product of the bovine papillomavirus type 1 modulator gene (M) is a phosphoprotein. J Virol. 1988 Jul;62(7):2474–2482. doi: 10.1128/jvi.62.7.2474-2482.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Barbosa M. S., Nasseri M. Identification of the major cottontail rabbit papillomavirus late RNA cap site and mapping and quantitation of an E2 and minor E6 coding mRNA in papillomas and carcinomas. Virology. 1987 Aug;159(2):321–328. doi: 10.1016/0042-6822(87)90470-3. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]