Abstract
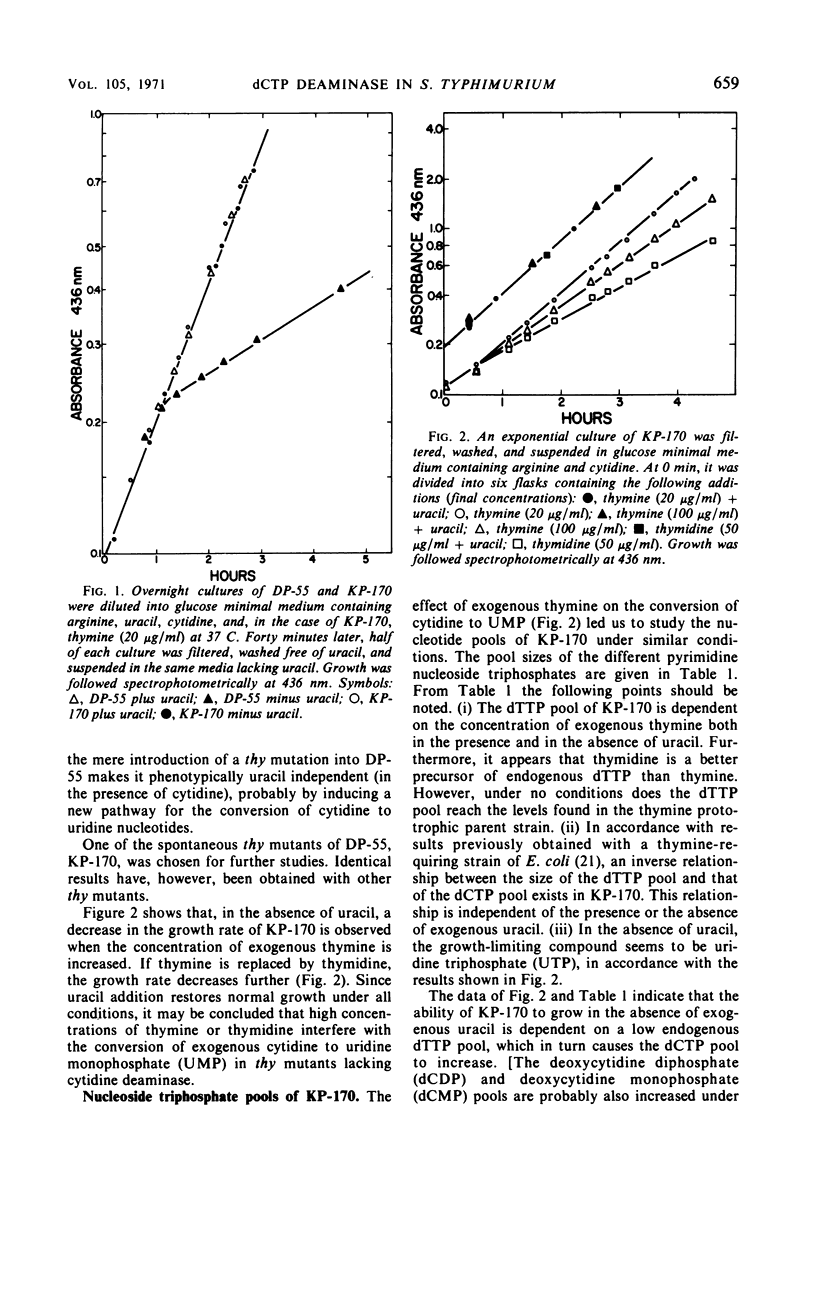
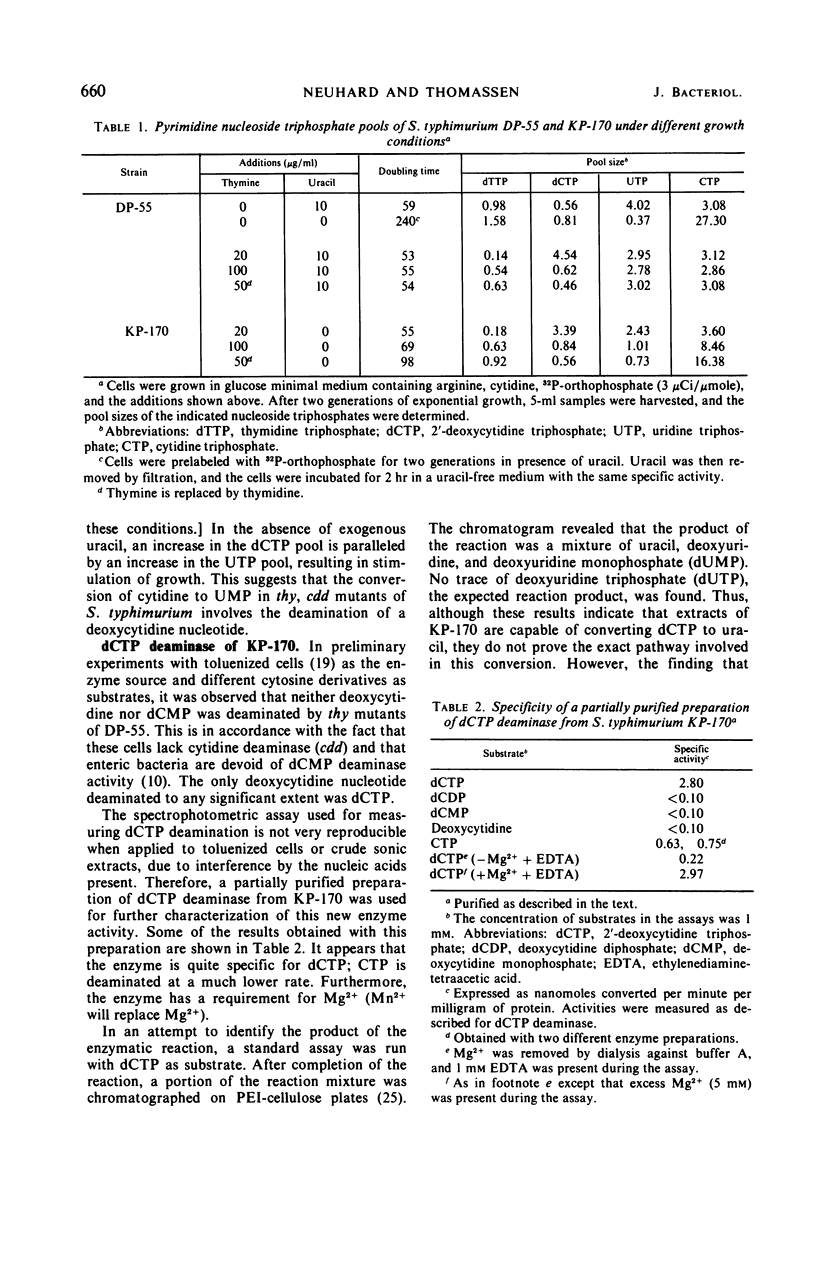
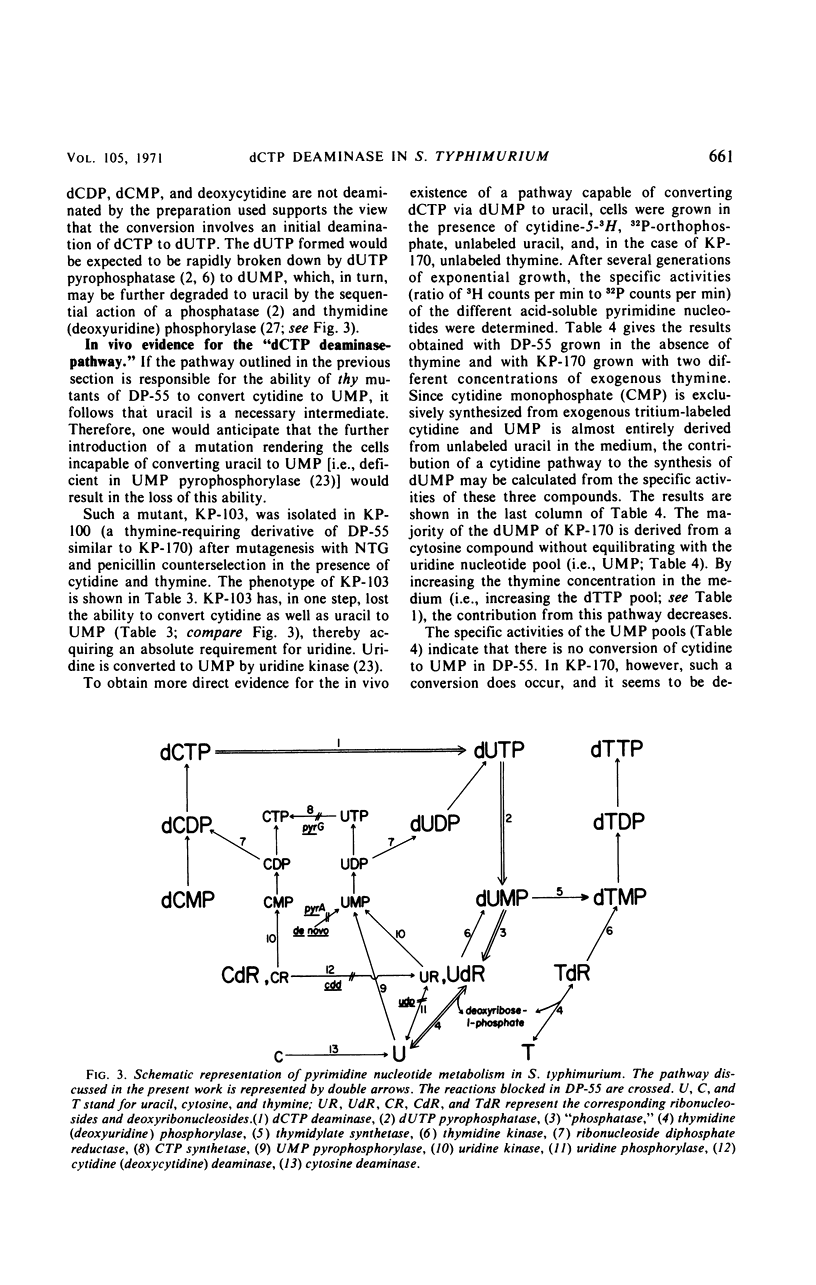
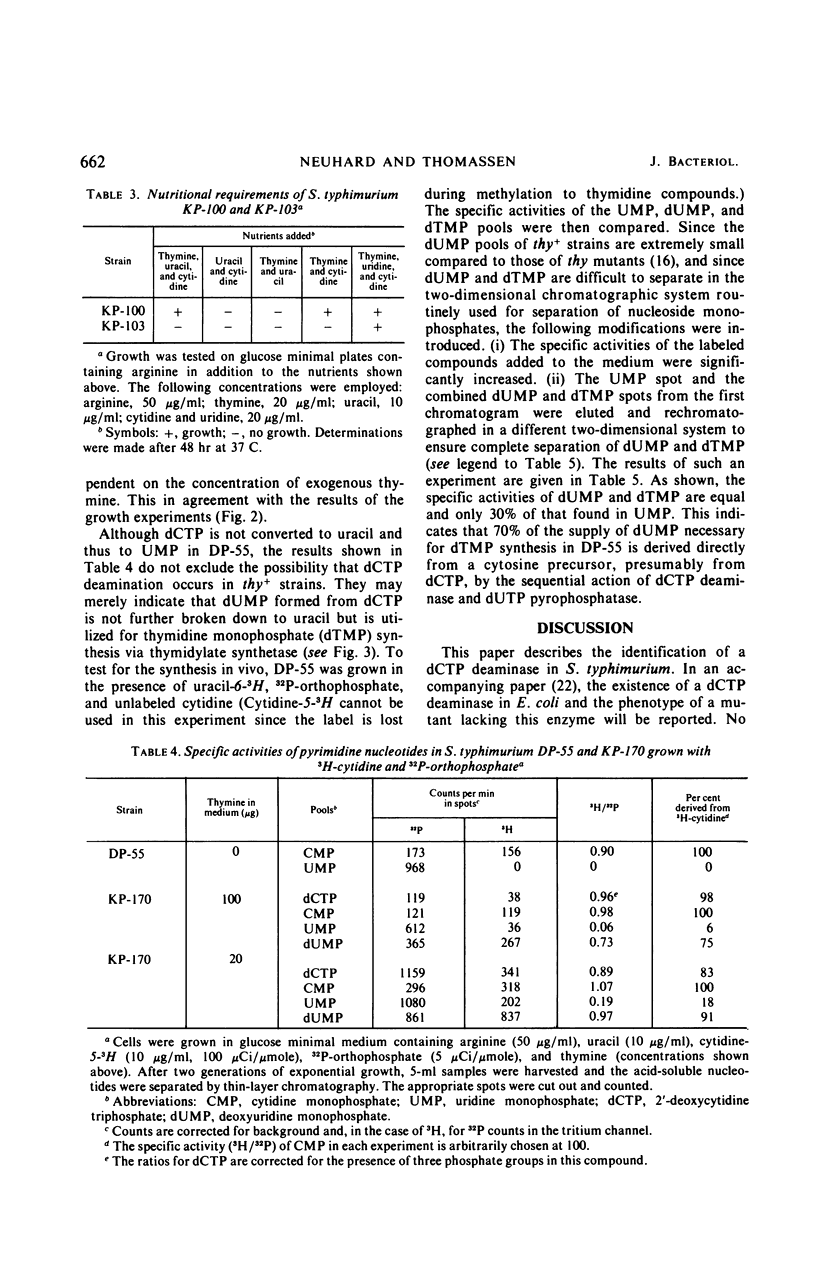
The biosynthesis of 2′-deoxyuridine monophosphate (dUMP) has been studied in a cytidine- and uracil-requiring mutant of Salmonella typhimurium (DP-55). The dUMP pool and the thymidine monophosphate (dTMP) pool of DP-55, grown in the presence of 3H-uracil and unlabeled cytidine, are found to have the same specific activities. However, only 30% of the dUMP and the dTMP is synthesized from a uridine nucleotide. Seventy per cent is derived directly from a cytosine compound. The identification and partial purification of a Mg2+-dependent 2′-deoxycytidine triphosphate (dCTP) deaminase from S. typhimurium suggests that the combined action of dCTP deaminase and 2′-deoxyuridine triphosphate pyrophosphatase accounts for 70% of the dUMP, and therefore the dTMP, synthesized in vivo. The introduction of a thymine requirement (i.e., a block in thymidylate synthetase) into DP-55 results in a 100-fold increase in the size of the dUMP pool. However, the relative contribution of the uridine and cytidine pathways to dUMP synthesis is unaltered. The high dUMP pool is accompanied by extensive catabolism of dUMP to uracil. Partial thymine starvation of the cells results in a significant increase in the dUMP and dCTP pools. Moreover, an increase in the contribution of the dCTP pathway to dUMP synthesis is observed. As a result of these changes the catabolism of dUMP to uracil is augmented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- BERTANI L. E., HAEGGMARK A., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. II. FORMATION AND INTERCONVERSION OF DEOXYURIDINE PHOSPHATES. J Biol Chem. 1963 Oct;238:3407–3413. [PubMed] [Google Scholar]
- Biswas C., Hardy J., Beck W. S. Release of repressor control of ribonucleotide reductase by thymine starvation. J Biol Chem. 1965 Sep;240(9):3631–3640. [PubMed] [Google Scholar]
- Eisenstark A., Eisenstark R., Cunningham S. Genetic analysis of thymineless(thy) mutants in Salmonella typhimurium. Genetics. 1968 Apr;58(4):493–506. doi: 10.1093/genetics/58.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG G. R., SOMERVILLE R. L. Deoxyuridylate kinase activity and deoxyuridinetriphosphatase in Escherichia coli. Proc Natl Acad Sci U S A. 1962 Feb;48:247–257. doi: 10.1073/pnas.48.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- KECK K., MAHLER H. R., FRASER D. Synthesis of deoxycytidine-5'-phosphate deaminase in Escherichia coli infected by T2 bacteriophage. Arch Biochem Biophys. 1960 Jan;86:85–88. doi: 10.1016/0003-9861(60)90373-8. [DOI] [PubMed] [Google Scholar]
- Karlström O., Larsson A. Significance of ribonucleotide reduction in the biosynthesis of deoxyribonucleotides in Escherichia coli. Eur J Biochem. 1967 Dec;3(2):164–170. doi: 10.1111/j.1432-1033.1967.tb19512.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., NEUHARD J. STUDIES ON THE ACID-SOLUBLE NUCLEOTIDE POOL IN THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI DURING THYMINE STARVATION. I. ACCUMULATION OF DEOXYADENOSINE TRIPHOSPHATE IN ESCHERICHIA COLI 15 T-A-U-. Biochim Biophys Acta. 1964 Apr 27;80:542–551. doi: 10.1016/0926-6550(64)90298-1. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Maley F. Regulatory properties and subunit structure of chick embryo deoxycytidylate deaminase. J Biol Chem. 1968 Sep 10;243(17):4506–4512. [PubMed] [Google Scholar]
- Munch-Petersen A. Deoxyribonucleoside catabolism and thymine incorporation in mutants of Escherichia coli lacking deoxyriboaldolase. Eur J Biochem. 1970 Jul;15(1):191–202. doi: 10.1111/j.1432-1033.1970.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A. Thymidine breakdown and thymine uptake in different mutants of Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):228–237. doi: 10.1016/0005-2787(67)90530-8. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Munch-Petersen A. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. II. Changes in the amounts of deoxycytidine triphosphate and deoxyadenosine triphosphate in Escherichia coli 15 T-A-U. Biochim Biophys Acta. 1966 Jan 18;114(1):61–71. doi: 10.1016/0005-2787(66)90253-x. [DOI] [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J. Studies on the acid-soluble nucleotide pool in Escherichia coli. IV. Effects of hydroxyurea. Biochim Biophys Acta. 1967 Aug 22;145(1):1–6. doi: 10.1016/0005-2787(67)90647-8. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XIV. Separation of nucleotide sugars and nucleoside monophosphates on PEI-cellulose. Anal Biochem. 1965 Dec;13(3):575–579. doi: 10.1016/0003-2697(65)90356-8. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarano E., Geraci G., Rossi M. Deoxycytidylate aminohydrolase. II. Kinetic properties. The activatory effect of deoxycytidine triphosphate and the inhibitory effect of deoxythymidine triphosphate. Biochemistry. 1967 Jan;6(1):192–201. doi: 10.1021/bi00853a031. [DOI] [PubMed] [Google Scholar]
- Tomita F., Takahashi I. A novel enzyme, dCTP deaminase, found in Bacillus subtilis infected with phage PBS I. Biochim Biophys Acta. 1969 Mar 18;179(1):18–27. doi: 10.1016/0005-2787(69)90117-8. [DOI] [PubMed] [Google Scholar]
- WAHBA A. J., FRIEDKIN M. The enzymatic synthesis of thymidylate. I. Early steps in the purification of thymidylate synthetase of Escherichia coli. J Biol Chem. 1962 Dec;237:3794–3801. [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Evidence for a dual role for the bacteriophage T4-induced deoxycytidine triphosphate nucleotidohydrolase. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1233–1240. doi: 10.1073/pnas.56.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN S. B., KORNBERG A. Deoxycytidine di- and triphosphate cleavage by an enzyme formed in bacteriophage-infected Eschrichia coli. J Biol Chem. 1961 May;236:1480–1486. [PubMed] [Google Scholar]



