Abstract
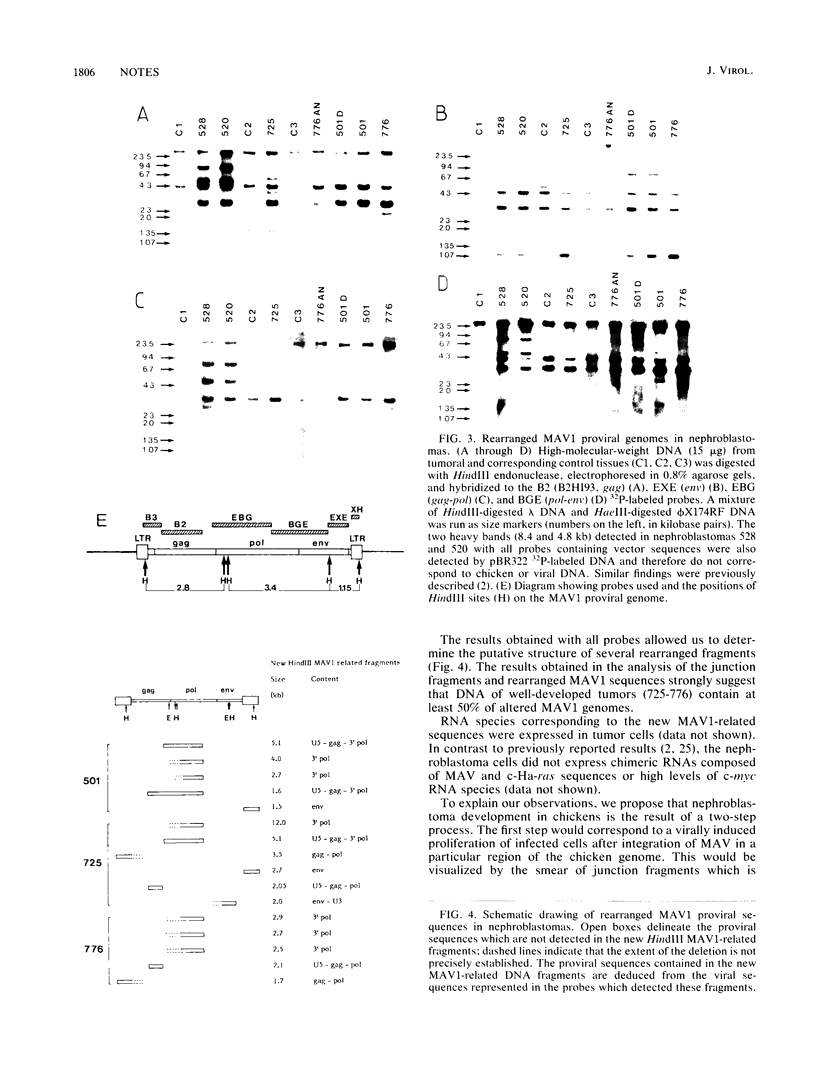
Myeloblastosis-associated virus type 1 (MAV1) derived from a molecular clone of infectious proviral DNA (B. Perbal, J. S. Lipsick, J. Svoboda, R. F. Silva, and M. A. Baluda, J. Virol. 56:240-244, 1985) was shown to specifically induce nephroblastoma in chickens and therefore belongs to the MAV-N class. We show that nephroblastomas are polyclonal tumors containing rearranged proviral genomes. Rearrangements occur preferentially in the gag-pol region of the MAV1 proviral genome, and similar rearrangements can be detected in well-developed independent tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banes A. J., Smith R. E. Biological characterization of avian osteopetrosis. Infect Immun. 1977 Jun;16(3):876–884. doi: 10.1128/iai.16.3.876-884.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Böni J., Ferdinand F. J., Franklin R. M. Developmental and molecular aspects of nephroblastomas induced by avian myeloblastosis-associated virus 2-O. J Virol. 1985 Jul;55(1):213–222. doi: 10.1128/jvi.55.1.213-222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Hauber J., Nelböck-Hochstetter P., Feldmann H. Nucleotide sequence and characteristics of a Ty element from yeast. Nucleic Acids Res. 1985 Apr 25;13(8):2745–2758. doi: 10.1093/nar/13.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Holland C. A., Hartley J. W., Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. H., Smith R. E. Rapid induction of osteopetrosis by subgroup E recombinant viruses. Virology. 1983 Sep;129(2):493–500. doi: 10.1016/0042-6822(83)90189-7. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Vogt P. K. Effects of genetic cellular resistance on cell transformation and virus replication in chicken hematopoietic cell cultures infected with avian myeloblastosis virus (BAI-A). Virology. 1968 Aug;35(4):487–497. doi: 10.1016/0042-6822(68)90278-x. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Hughes D., McFarlane R., Wilkie N. M., Onions D. E., Lees G., Jarrett O. Transduction and rearrangement of the myc gene by feline leukaemia virus in naturally occurring T-cell leukaemias. 1984 Apr 26-May 2Nature. 308(5962):814–820. doi: 10.1038/308814a0. [DOI] [PubMed] [Google Scholar]
- Noori-Daloii M. R., Swift R. A., Kung H. J., Crittenden L. B., Witter R. L. Specific integration of REV proviruses in avian bursal lymphomas. Nature. 1981 Dec 10;294(5841):574–576. doi: 10.1038/294574a0. [DOI] [PubMed] [Google Scholar]
- Ogura H., Gelderblom H., Bauer H. Isolation of avian nephroblastoma virus from avian myeloblastosis virus by the infectious DNA technique. Intervirology. 1974;4(2):69–76. doi: 10.1159/000149845. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Perbal B., Lipsick J. S., Svoboda J., Silva R. F., Baluda M. A. Biologically active proviral clone of myeloblastosis-associated virus type 1: implications for the genesis of avian myeloblastosis virus. J Virol. 1985 Oct;56(1):240–244. doi: 10.1128/jvi.56.1.240-244.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Schatz P. J., Jensen L. M., Tsichlis P. N., Coffin J. M., Robinson H. L. Sequences in the gag-pol-5'env region of avian leukosis viruses confer the ability to induce osteopetrosis. Virology. 1985 Aug;145(1):94–104. doi: 10.1016/0042-6822(85)90204-1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Davids L. J., Neiman P. E. Comparison of an avian osteopetrosis virus with an avian lymphomatosis virus by RNA-DNA hybridization. J Virol. 1975 Jan;17(1):160–167. doi: 10.1128/jvi.17.1.160-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Soret J., Kryceve-Martinerie C., Crochet J., Perbal B. Transformation of Brown Leghorn chicken embryo fibroblasts by avian myeloblastosis virus proviral DNA. J Virol. 1985 Jul;55(1):193–205. doi: 10.1128/jvi.55.1.193-205.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S. L., Smith R. E., Faras A. J. Avian nephroblastoma virus MAV-2(N) and avian osteopetrosis virus MAV-2(O) are genetically distinct. J Gen Virol. 1982 May;60(Pt 1):185–189. doi: 10.1099/0022-1317-60-1-185. [DOI] [PubMed] [Google Scholar]
- Watts S. L., Smith R. E. Pathology of chickens infected with avian nephoblastoma virus MAV-2(N). Infect Immun. 1980 Feb;27(2):501–512. doi: 10.1128/iai.27.2.501-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler O., Delain E., Lacour F. Studies on a viral nephroblastic nephroblastoma of the chicken: an electron microscope comparison of the sequence of development of the virions in different organs. Eur J Cancer. 1971 Dec;7(6):491–494. doi: 10.1016/0014-2964(71)90052-1. [DOI] [PubMed] [Google Scholar]
- Westaway D., Papkoff J., Moscovici C., Varmus H. E. Identification of a provirally activated c-Ha-ras oncogene in an avian nephroblastoma via a novel procedure: cDNA cloning of a chimaeric viral-host transcript. EMBO J. 1986 Feb;5(2):301–309. doi: 10.1002/j.1460-2075.1986.tb04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. M. Transposable elements in yeast. Int Rev Cytol. 1983;83:1–25. doi: 10.1016/s0074-7696(08)61684-8. [DOI] [PubMed] [Google Scholar]