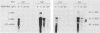Abstract
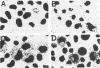
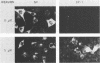
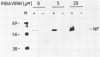
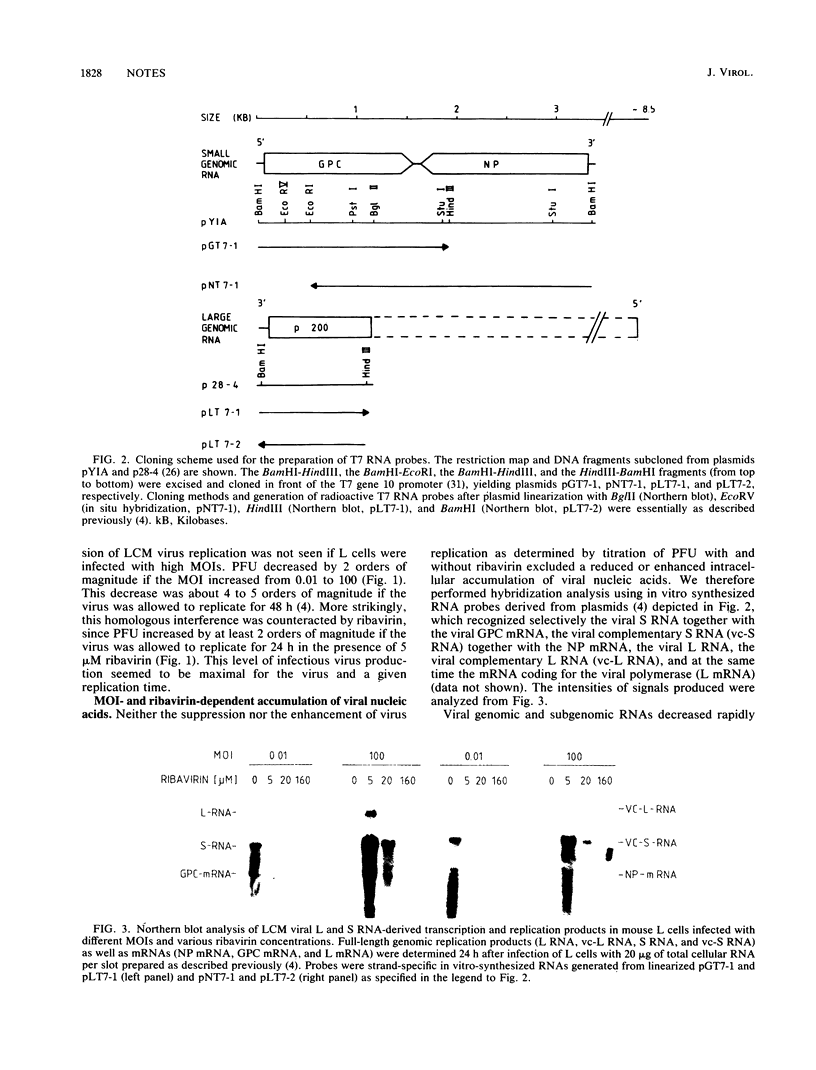
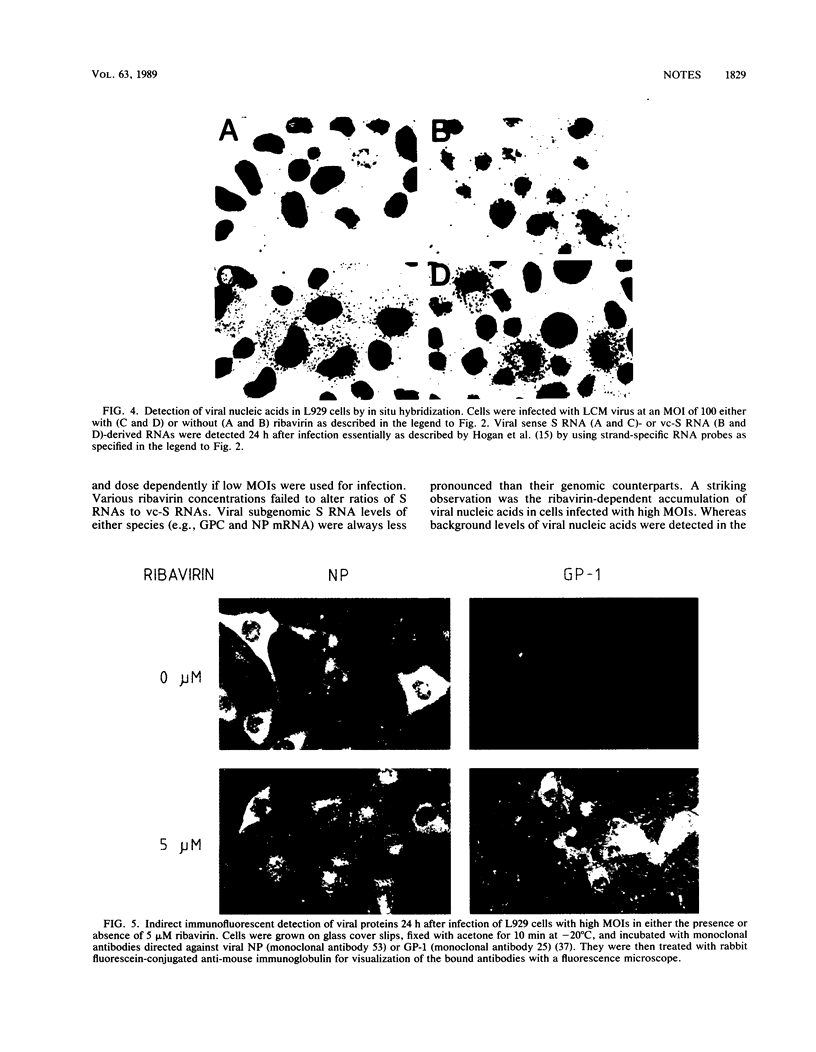
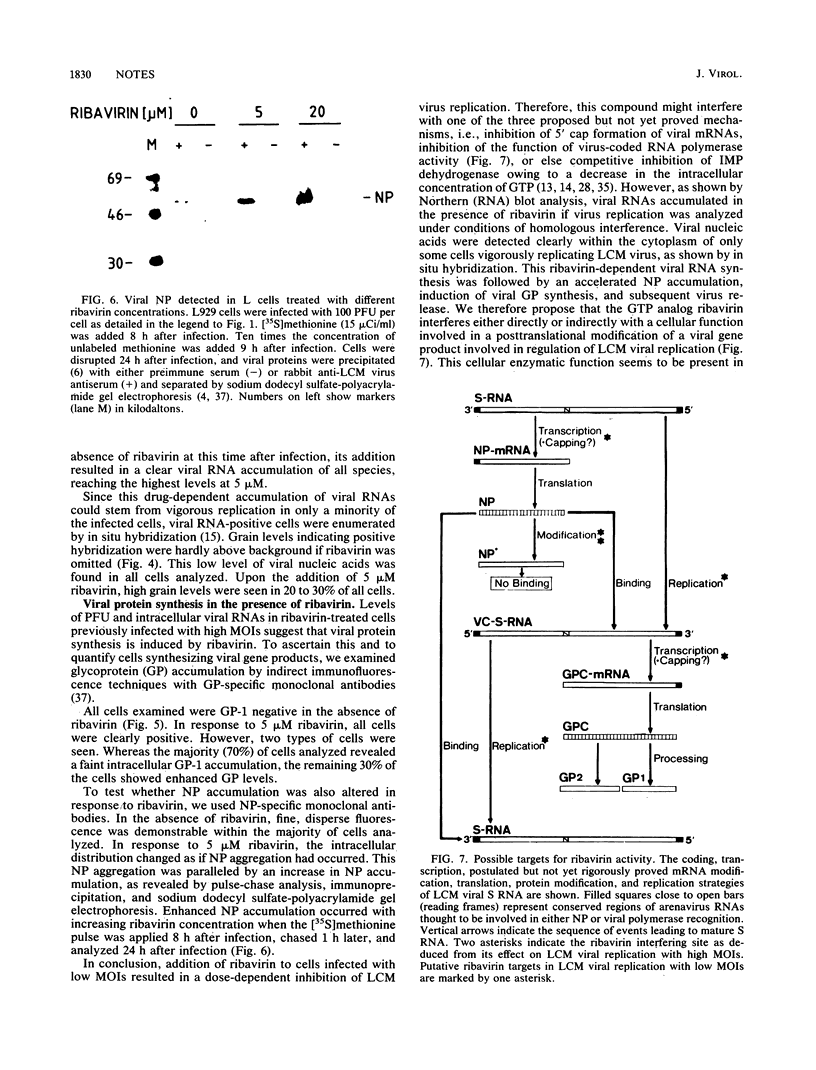
Depending on the multiplicity of infection (MOI), infection of L929 cells results in either productive lymphocytic choriomeningitis virus replication or homologous interference M. Bruns, A. Gessner, H. Lother, and F. Lehmann-Grube, Virology 166:133-139, 1988). As shown in this communication, productive lymphocytic choriomeningitis virus replication as observed at a low MOI was effectively inhibited by ribavirin. In contrast, virus yields increased if cells were infected with a high MOI and in the presence of 5 microM of the antiviral compound. This drug-dependent release of infectious virus was preceded by enhanced nucleoprotein (NP) synthesis, a change in intracellular NP distribution, and by an onset of glycoprotein synthesis. It is therefore proposed that this block in viral replication is brought about by a posttranslational effect on a viral gene product, probably the NP, present in reasonably large quantities both during homologous interference as well as persistent infection.
Full text
PDF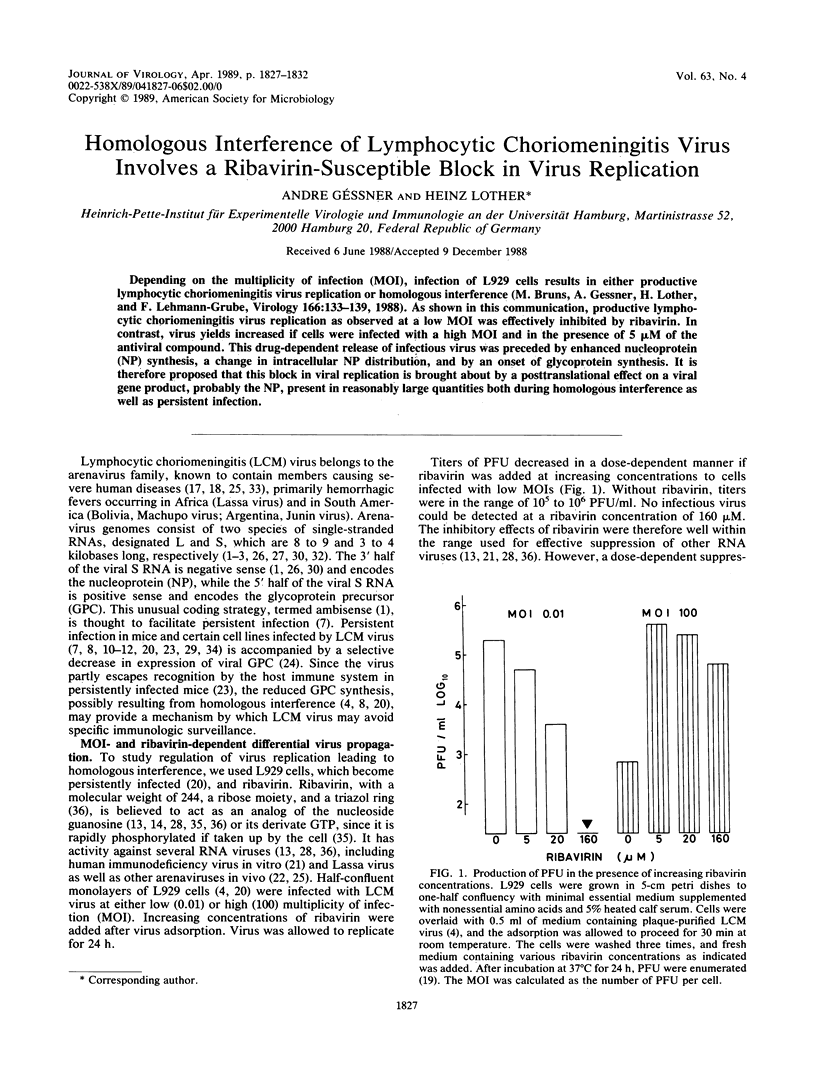


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D. D., Romanowski V., Galinski M., Bishop D. H. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J Virol. 1984 Dec;52(3):897–904. doi: 10.1128/jvi.52.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H. Ambisense RNA genomes of arenaviruses and phleboviruses. Adv Virus Res. 1986;31:1–51. doi: 10.1016/S0065-3527(08)60261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Auperin D. D. Arenavirus gene structure and organization. Curr Top Microbiol Immunol. 1987;133:5–17. doi: 10.1007/978-3-642-71683-6_2. [DOI] [PubMed] [Google Scholar]
- Bruns M., Gessner A., Lother H., Lehmann-Grube F. Host cell-dependent homologous interference in lymphocytic choriomeningitis virus infection. Virology. 1988 Sep;166(1):133–139. doi: 10.1016/0042-6822(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Bruns M., Zeller W., Rohdewohld H., Lehmann-Grube F. Lymphocytic choriomeningitis virus. IX. Properties of the nucleocapsid. Virology. 1986 May;151(1):77–85. doi: 10.1016/0042-6822(86)90105-4. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Elder J. H., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: identification of the virus structural and cell associated polypeptides. Virology. 1978 Aug;89(1):133–145. doi: 10.1016/0042-6822(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Parekh B. S. Protein structure and expression among arenaviruses. Curr Top Microbiol Immunol. 1987;133:41–57. doi: 10.1007/978-3-642-71683-6_4. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Pfau C. J. Arenavirus defective interfering particles mask the cell-killing potential of standard virus. J Gen Virol. 1978 Feb;38(2):195–208. doi: 10.1099/0022-1317-38-2-195. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Francis S. J., Southern P. J. Molecular analysis of viral RNAs in mice persistently infected with lymphocytic choriomeningitis virus. J Virol. 1988 Apr;62(4):1251–1257. doi: 10.1128/jvi.62.4.1251-1257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S. J., Southern P. J., Valsamakis A., Oldstone M. B. State of viral genome and proteins during persistent lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 1987;133:67–88. doi: 10.1007/978-3-642-71683-6_6. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F. V., Southern P. J. Temporal analysis of transcription and replication during acute infection with lymphocytic choriomeningitis virus. Virology. 1988 Jan;162(1):260–263. doi: 10.1016/0042-6822(88)90419-9. [DOI] [PubMed] [Google Scholar]
- Gilbert B. E., Knight V. Biochemistry and clinical applications of ribavirin. Antimicrob Agents Chemother. 1986 Aug;30(2):201–205. doi: 10.1128/aac.30.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B. B., Borek E., Sharma O. K., Fujitaki J., Smith R. A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979 Aug 13;89(3):830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- Howard C. R., Buchmeier M. J. A protein kinase activity in lymphocytic choriomeningitis virus and identification of the phosphorylated product using monoclonal antibody. Virology. 1983 Apr 30;126(2):538–547. doi: 10.1016/s0042-6822(83)80011-7. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Ambrassat J. A new method to detect lymphocytic choriomeningitis virus-specific antibody in human sera. J Gen Virol. 1977 Oct;37(1):85–92. doi: 10.1099/0022-1317-37-1-85. [DOI] [PubMed] [Google Scholar]
- McCormick J. B., Getchell J. P., Mitchell S. W., Hicks D. R. Ribavirin suppresses replication of lymphadenopathy-associated virus in cultures of human adult T lymphocytes. Lancet. 1984 Dec 15;2(8416):1367–1369. doi: 10.1016/s0140-6736(84)92060-9. [DOI] [PubMed] [Google Scholar]
- McCormick J. B., King I. J., Webb P. A., Scribner C. L., Craven R. B., Johnson K. M., Elliott L. H., Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986 Jan 2;314(1):20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Immunotherapy for virus infection. Curr Top Microbiol Immunol. 1987;134:211–229. doi: 10.1007/978-3-642-71726-0_9. [DOI] [PubMed] [Google Scholar]
- Peters C. J., Jahrling P. B., Liu C. T., Kenyon R. H., McKee K. T., Jr, Barrera Oro J. G. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- Romanowski V., Matsuura Y., Bishop D. H. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 1985 Sep;3(2):101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Singh M. K., Fuller-Pace F. V., Buchmeier M. J., Southern P. J. Analysis of the genomic L RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Dec;161(2):448–456. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Blount P., Oldstone M. B. Analysis of persistent virus infections by in situ hybridization to whole-mouse sections. Nature. 1984 Dec 6;312(5994):555–558. doi: 10.1038/312555a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Singh M. K., Riviere Y., Jacoby D. R., Buchmeier M. J., Oldstone M. B. Molecular characterization of the genomic S RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Mar;157(1):145–155. doi: 10.1016/0042-6822(87)90323-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Clewley J. P., Gard G. P., Abraham N. Z., Compans R. W., Bishop D. H. Virion RNA species of the arenaviruses Pichinde, Tacaribe, and Tamiami. J Virol. 1978 May;26(2):485–497. doi: 10.1128/jvi.26.2.485-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Murphy F. A. Pathology and pathogenesis of arenavirus infections. Curr Top Microbiol Immunol. 1987;133:89–113. doi: 10.1007/978-3-642-71683-6_7. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Carson D. A., Seegmiller J. E. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3042–3044. doi: 10.1073/pnas.75.7.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski J. T., Robins R. K., Sidwell R. W., Simon L. N. Design, synthesis, and broad spectrum antiviral activity of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J Med Chem. 1972 Nov;15(11):1150–1154. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- Zeller W., Bruns M., Lehmann-Grube F. Lymphocytic choriomeningitis virus. X. Demonstration of nucleoprotein on the surface of infected cells. Virology. 1988 Jan;162(1):90–97. doi: 10.1016/0042-6822(88)90397-2. [DOI] [PubMed] [Google Scholar]