Abstract
Bacteriophage λ encodes a number of genes involved in the recombinational repair of DNA double-strand breaks. The product of one of these genes, rap, has been purified. Truncated Rap proteins that copurify with the full-length form are derived, at least in part, from a ρ-dependent transcription terminator located within its coding sequence. Full-length and certain truncated Rap polypeptides bind preferentially to branched DNA substrates, including synthetic Holliday junctions and D-loops. In the presence of manganese ions, Rap acts as an endonuclease that cleaves at the branch point of Holliday and D-loop substrates. It shows no obvious sequence preference or symmetry of cleavage on a Holliday junction. The biochemical analysis of Rap gives an insight into how recombinants could be generated by the nicking of a D-loop without the formation of a classical Holliday junction.
During the lytic cycle, phage λ switches off the host RecBCD recombinase and switches on the Red pathway, its own set of recombination enzymes required for processing DNA double-strand breaks (1, 2). Such breaks occur at the transition from θ to rolling-circle replication, as a result of cleavage by host restriction endonucleases or by λ terminase cutting at a cos site in advance of packaging (3). Redα (λ exonuclease) degrades the 5′-3′ strand of duplex DNA ends to provide a 3′ single-strand tail (4). The action of Redβ or cellular RecA results in the invasion of this strand tail into another [λ] chromosome to form a D-loop (5, 6). Recent experiments have established that RecT, a functional analog of Redβ, can promote this type of strand transfer reaction in vitro (7). Participation of the single-strand annealing properties of Redβ in D-loop formation has yet to be demonstrated. Another λ product, Orf (NinB), assists RecA-mediated strand exchange by contributing a function equivalent to the host RecFOR proteins (8, 9). Subsequent nicking and ligation of this 3-strand (D-loop) intermediate has been proposed as a means of producing a splice recombinant (4).
The rap (ninG) gene, located in the nonessential ninR region, has also been linked with phage recombination. Rap+ phages show an increase in RecBCD-dependent recombination between λ and a plasmid (10). In addition, they show a bias toward the formation of splice recombinants (11). The Rap function is also active in the Red pathway by apparently concentrating recombination events close to double-strand breaks induced at cos (T. Tarkowski and F. W. Stahl, personal communication). On the basis of the arrangement of adjacent genes, it was hypothesized that Rap is a functional analog of the Escherichia coli/phage 82 RusA Holliday junction resolvase (12). Although the rusA and rap genes show no sequence similarity, we cloned and purified the Rap gene product, and tested it on appropriate DNA substrates. Rap was found to be an endonuclease specifically targeted to branched DNA substrates. This activity could be responsible for the nicking of D-loops in λ recombination to generate splice recombinants. Phage λ therefore possesses an enzymatic system for both initiating and terminating homologous recombination.
MATERIALS AND METHODS
Cloning the rap Gene for Overproduction of Wild-Type (wt) and His-Tag Fusion Proteins.
A Rap-overproducing clone was constructed in pT7–7 by cloning PCR products from λ DNA. PCR followed standard procedures with Taq DNA polymerase (Perkin–Elmer Cetus). An oligonucleotide (5′-AGGAAGAAGCATATGGCTAA-3′) was designed to fuse the 5′ end of the rap gene to the NdeI site (underlined) in pT7–7, retaining the vector signals for ribosome binding. This was used in conjunction with a primer located immediately 3′ of the gene to create a HindIII (underlined) site suitable for cloning (5′-CATGTCTGGAAGCTTTTTTACTGA-3′). The insert from this clone (pGS812) was transferred to pGEM-7Zf(+) by using XbaI/HindIII, to confirm its nucleotide sequence by dideoxynucleotide sequencing of single-stranded DNA generated from JM101 with helper phage M13 KO7.
Using the NdeI and BamHI (HindIII) sites, we subcloned the insert from pGS823 [pGS812 insert in pGEM-7Zf(+)] into the expression vector pET-14b (Novagen) to generate an N-terminal Rap His-tag fusion (pGS875). The fusion adds 20 residues to Rap and includes 6 tandem histidines and a cleavage site for the thrombin site-specific protease.
Purification of wt Rap Protein.
Strain N3757 [BL21 (DE3) ΔruvAC eda-51∷Tn10 pLysS] carrying pGS812 was used for overproduction. This genetic background avoids potential contamination with the RuvABC Holliday-junction processing system (13). Cells were grown with aeration at 37°C in LB broth containing carbenicillin (125 μg/ml) and chloramphenicol (50 μg/ml). At an OD650 of 0.5, isopropyl β-d-thiogalactoside (IPTG) was added to 1 mM and incubation was continued for a further 3.5 h at 37°C. The cells were harvested by centrifugation and resuspended in 100 mM Tris⋅HCl, pH 8.0/2 mM EDTA/5% (vol/vol) glycerol and stored at −80°C.
Rap was purified from 1 liter of cells (3.51 g, wet weight). Cells were lysed by sonication, and the cell debris was separated from the supernatant by centrifugation at 15,000 rpm in a Sorvall RC 5B Plus for 20 min. The supernatant (fraction I, 13.5 ml, 7.8 mg/ml) was mixed with 5 ml of phosphocellulose slurry in buffer A [20 mM Tris⋅HCl, pH 8.0/1 mM EDTA/0.5 mM DTT, 10% (vol/vol) glycerol], and the column was packed with this mixture. Bound proteins were eluted with a 20-ml linear gradient of 0–1.25 M KCl in the same buffer. Fractions containing the ≈28-kDa Rap protein eluting at 1.25 M KCl were pooled and dialyzed against buffer A (fraction II, 3 ml, 1 mg/ml). Fraction II was loaded onto a 2-ml double-stranded-DNA-cellulose (Sigma) column and developed with a 20-ml linear gradient from 0 to 1 M KCl. Peak fractions at 400 mM KCl containing Rap were collected and dialyzed against buffer A (fraction III, 2 ml, 0.2 mg/ml). This fraction was applied to a 1-ml heparin column, and bound proteins were eluted with a 40-ml linear gradient of 0–1 M KCl in buffer A. Rap appeared in a broad peak between 600 and 800 mM KCl. These fractions were dialyzed against storage buffer [20 mM Tris⋅HCl, pH 8.0/1 mM EDTA/0.5 mM DTT/50% glycerol] and stored at −80°C (fraction IV, 1.2 ml, 0.1 mg/ml). The Rap protein retained some minor contaminants between 14 and 18 kDa in size.
Purification of His-Tagged Rap Fusion Protein.
An N-terminal His-tag fusion Rap protein was purified from strain N3757 carrying pGS875. A 500-ml batch of bacteria were grown, and gene expression was induced as before. Cells (2.75 g, wet weight) were resuspended in 15 ml of buffer B (20 mM Tris⋅HCl, pH 7.9/0.5 M NaCl/5 mM imidazole) and lysed by sonication. Cell debris was separated from the supernatant by centrifugation at 15,000 rpm for 20 min. The supernatant was applied to a 2-ml iminodiacetic acid agarose column (Sigma), previously charged with 10 ml of 50 mM NiSO4 and equilibrated in buffer B. The column was washed with 20 ml of buffer B and 18 ml of 20 mM Tris⋅HCl, pH 7.9/0.5 M NaCl/60 mM imidazole. Bound Rap His-tag fusion proteins were eluted with 18 ml of 20 mM Tris⋅HCl, pH 7.9/0.5 M NaCl/1 M imidazole. In addition to the full-length Rap, a variety of additional Rap proteins of 13–18 kDa were eluted from the column. To separate these species, the pooled Rap fractions (7 ml) were loaded directly on a 3.2 × 78 cm S200HR Sephacryl gel filtration column preequilibrated in buffer G (50 mM Tris⋅HCl, pH 8.0/1 mM EDTA/1 M KCl). Fractions containing the full-length Rap of ≈29 kDa (Rap 29K), and short Rap proteins of ≈17 kDa (Rap 17K) and ≈14 kDa (Rap 14K) were isolated. Molecular mass estimates are based on the migration of proteins in SDS/13.5% polyacrylamide gels with reference to appropriate size markers (Pharmacia). Pooled fractions were dialyzed against storage buffer and stored as aliquots at −80°C. Yield of protein was as follows: Rap 29K (2.8 ml, 0.5 mg/ml), Rap 17K (2.8 ml, 0.55 mg/ml), and Rap 14K (1.5 ml, 0.34 mg/ml). Protein concentrations were determined by a modified Bradford method, using a protein assay kit (Bio-Rad) and BSA as standard (Pharmacia). The molar concentration of the Rap 17K pool is an estimate because the molecular weights of the products it contains are unknown.
Antibodies.
Anti-Rap antibodies were raised in rabbits against Rap His-tag protein purified by nickel-affinity and heparin chromatography. The antibodies were purified by using standard techniques (14) employing Rap His-tag protein immobilized on cyanogen bromide-activated Sepharose 4 Fast Flow according to the manufacturer’s instructions (Pharmacia).
DNA Substrates.
λ cIind1 ts857 Sam7 DNA (BRL) was used as a substrate for PCR. Synthetic Holliday junction, D-loop, and duplex DNA substrates were generated by annealing 40- to 60-base oligonucleotides purified by denaturing 12–15% PAGE (15). The correctly annealed DNA forms were separated from other species and free oligonucleotide by electrophoresis on neutral 10% polyacrylamide gels followed by electroelution and dialysis. Oligonucleotides were labeled at the 5′ end with [γ-32P]ATP (Amersham) by using T4 polynucleotide kinase (BRL) prior to annealing. Oligonucleotides used to make J3, D-loop (substrate C), and linear duplex substrates have been published previously (16–18). Because of the instability of the D-loop, the substrate used for mapping cleavage sites was annealed and used in cleavage assays without further purification. The D-loop is therefore contaminated with a variety of unlabeled annealed DNA.
DNA-Binding Assays.
Bandshift reactions (20 μl) contained 0.4 ng of 32P-labeled J3 DNA or 0.3 ng of linear duplex and various amounts of Rap protein in binding buffer (50 mM Tris⋅HCl, pH 8.0/5 mM EDTA/1 mM DTT/5% glycerol containing BSA at 100 μg/ml). Samples were incubated on ice for 15 min before loading 12 μl on a 4% polyacrylamide gel in 6.7 mM Tris⋅HCl, pH 8.0/3.3 mM sodium acetate/2 mM EDTA. Electrophoresis was at 160 V for 2.5 h. Gels were dried and autoradiographed.
DNA Cleavage Assays.
Cleavage of 32P-labeled DNA by Rap was assayed at 37°C in cleavage buffer (50 mM Tris⋅HCl, pH 8.0/1 mM DTT/0.5 mM MnCl2 containing BSA at 100 μg/ml). Reactions (in 20 μl) were terminated by adding 5 μl of stop buffer (100 mM Tris⋅HCl, pH 8.0/2.5% SDS/100 mM EDTA containing proteinase K at 10 mg/ml) and incubated for a further 10 min at 37°C. A 15-μl sample was electrophoresed on 10% polyacrylamide gels in 90 mM Tris–borate/2 mM EDTA at 190 V for 2 h. Gels were dried and autoradiographed, and the amount of DNA cleavage was quantified by phosphorimaging (Molecular Dynamics).
Mapping Cleavage Sites.
Four J3 or three D-loop substrates, each 5′-32P-labeled in a different oligonucleotide, were incubated at 37°C for 60 min with 200 nM Rap in cleavage buffer. Reaction mixtures were deproteinized and precipitated with 0.4 vol of 10 M ammonium acetate, 0.02 vol of 0.1 μg/ml glycogen, and 3 vol of ethanol. Dried pellets were resuspended in loading dye (0.3% bromophenol blue/0.3% xylene cyanol/10 mM EDTA/97.5% formamide). Samples of 1–2 μl were boiled for 2 min and separated by gel electrophoresis in 15% polyacrylamide/7 M urea at 2,200 V for 4 h. A+G Maxam–Gilbert sequencing reactions (19) were performed on each oligonucleotide and the products were run on the same gel to provide markers. Cleavage sites were mapped by reference to the sequencing ladder with a 1.5-base allowance made to compensate for the nucleoside eliminated in the sequencing reaction. Gels were dried and analyzed by autoradiography and phosphorimaging.
Northern Analysis.
Two 5-ml samples of N3757 carrying pGS875 were grown to OD650 = 0.5. One was induced with IPTG (1 mM) for 3 h, while the other provided an uninduced control. Total RNA was isolated with the Purescript kit (Gentra Systems) from both induced and uninduced cells. A sample containing 5 μg of RNA was separated on a 2% agarose gel in 20 mM Mops, pH 7.0/5 mM sodium acetate/1 mM EDTA/5% formaldehyde at 100 V for 5 h. A 0.16- to 1.77-kb RNA ladder (BRL) was used as markers. The gel was blotted onto ZetaProbe GT membrane (Bio-Rad) and probed with the ≈700-bp NdeI/BamHI insert from pGS875 labeled with 32P by the random hexamer method using the Klenow fragment of DNA polymerase I (BRL). The membrane was washed in 0.5× SSC/0.1% SDS and analyzed by autoradiography and phosphorimaging.
RESULTS
Overproduction and Purification of Rap Protein.
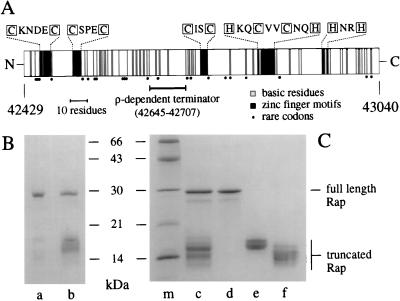
The rap gene was cloned by PCR into the pT7–7 vector for overexpression from the inducible φ10 promoter. Overproduction of Rap protein was poor (data not shown), probably because of a combination of tandem rare codons at the 5′ end and a ρ-dependent transcription terminator at the center of the coding region (Fig. 1A) (20). The highly basic nature of the protein (pI = 9.7) and the predicted zinc-binding motifs (Fig. 1A) suggested that the protein binds DNA, and a purification scheme was developed on that basis. Rap has a high affinity for phosphocellulose and was further purified by using double-stranded-DNA-cellulose and heparin matrices. The purified protein (≈28 kDa, designated Rap wt) is contaminated with proteins between 14 and 18 kDa in size (Fig. 1B, lane a). As these contaminants coeluted with Rap throughout purification, we suspected that they were actually truncated versions of the full-length Rap protein.
Figure 1.
Structure and purification of λ Rap protein. (A) The rap gene product is represented as a block with N and C termini. Basic residues (arginine and lysine) are indicated by shaded bars. Cysteine and histidine residues (boxed) that may form zinc-finger structures are marked as black bars with the relevant amino acid sequences shown above. Important elements of the gene structure are shown below the protein, namely the predicted location of the ρ-dependent terminator and the position of rare codons. Codons considered rare for E. coli were those with a relative synonymous codon usage of ≤0.04 (30). These codons are CTA (Leu), ATA (Ile), CCC (Pro), AGA, AGG, CGA, CGG (Arg), and GGA (Gly). The nucleotide values given at each end of the figure define the position of rap in the λ genome. (B) Purification of wt Rap. Purified wt Rap protein is in lane a, and His-tag Rap is in lane b. (C) Purification of His-tag Rap proteins by gel filtration. Lane c, Rap proteins eluted from Ni2+ column; lane c, pooled Rap samples separated by gel filtration; lane d, Rap 29K; lane e, Rap 17K; lane f, Rap 14K; and lane m, molecular masses marker proteins (Pharmacia). Proteins were resolved by SDS/PAGE on 13.5% gels and stained with Coomassie brilliant blue.
To obtain pure Rap we constructed an N-terminal histidine-tagged Rap fusion protein. Rap could then be purified in a single step by nickel chelation chromatography. However, in addition to the Rap protein of approximately 29 kDa, a number of proteins of 13–18 kDa were also released from the column (Fig. 1C, lane c). These small species are N-terminal Rap proteins, since the His tag could be removed with thrombin (data not shown). Gel filtration was used to separate the full-length Rap from the truncated Rap proteins. Three pools of protein were recovered and designated according to the approximate molecular masses of the products they contained (Rap 29K, Rap 17K, Rap 14K; Fig. 1C, lanes d, e, and f, respectively).
Northern Analysis of rap Transcripts.
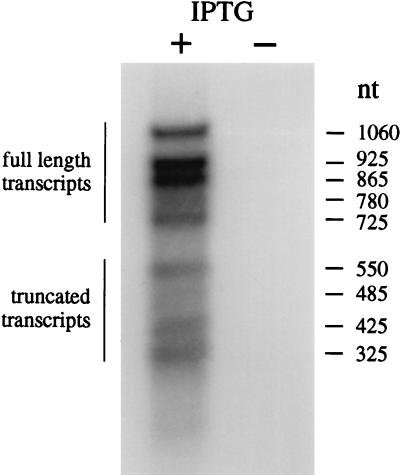
Northern analysis was carried out on transcripts generated by the overexpression clone pGS875 to determine the origin of the truncated Rap proteins. The data presented in Fig. 2 show that the majority (63%) of transcripts (725–1060 nucleotides in length) would yield full-length Rap protein. However, a significant proportion (37%) of truncated transcripts are generated that could lead to the formation of shortened Rap proteins. These transcripts of 325–550 nucleotides are consistent with termination downstream of the position of the known tR4 ρ-dependent terminator close to the center of the rap gene (20). Virtually no RNA was detected by the probe in the uninduced control, confirming that the induced transcripts were of rap origin (Fig. 2). Identical results were obtained after treatment of the RNA samples with DNase I (data not shown). The presence of a transcriptional terminator in the rap gene can, at least in part, explain the origin of the short Rap proteins, although we cannot exclude protease digestion as an additional factor in their formation.
Figure 2.
Northern analysis of Rap transcripts. Total RNA was recovered from N3757 carrying the Rap His-tag clone pGS875 with (+) or without (−) IPTG induction. RNA was separated on a 2% agarose gel and blotted as described in Materials and Methods. The membrane was probed with the insert from pGS875 labeled with 32P. The size of transcripts was determined by comparison with a defined RNA marker.
Binding to Synthetic Holliday Junctions.
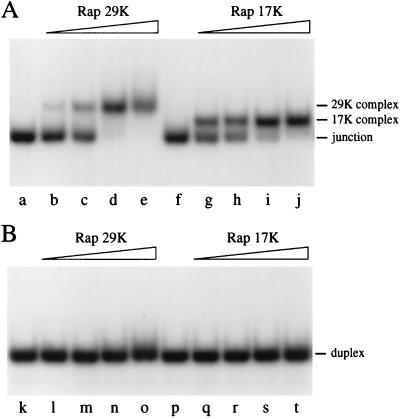
The prediction that Rap may be functionally equivalent to the RusA Holliday junction resolvase (12) led us to examine its binding to a synthetic Holliday junction. Purified Rap His-tag protein was incubated with a 50-bp synthetic X junction containing a 3-bp mobile core (designated as J3). Both Rap 29K and Rap 17K formed protein–DNA complexes with the junction (Fig. 3A). Rap 14K also bound the junction at high protein concentrations and gave a complex that migrated similarly to that obtained with Rap 17K (data not shown). It is likely that this was due to contamination with the junction-binding Rap protein present in the 17K pool. To confirm junction specificity we incubated Rap with a 50-bp linear duplex under the same conditions. Neither Rap 29K nor Rap 17K formed complexes with the duplex DNA (Fig. 3B, lanes l–o and q–t, respectively). Only at high Rap protein concentrations (>400 nM) was significant smearing of the linear duplex DNA detected with Rap 29K and 17K, indicating weak nonspecific DNA interactions (data not shown). Both Rap proteins are therefore highly specific for binding to a Holliday junction.
Figure 3.
Holliday junction-specific DNA binding by Rap. (A) Gel retardation assay showing binding of Rap 29K and Rap 17K to synthetic X junction. Reaction mixtures contained 0.4 ng of 32P-labeled J3 DNA and various amounts of Rap protein in binding buffer incubated on ice for 15 min. Protein–DNA complexes were resolved by 4% PAGE in low ionic strength buffer. Lanes b–e and g–j contained Rap protein at 6.25, 12.5, 25, and 50 nM. (B) Gel retardation assay with Rap 29K and Rap 17K on linear duplex DNA. Reaction mixtures contained 0.3 ng of 32P-labeled duplex DNA. Lanes l–o and q–t contained Rap protein at 6.25, 12.5, 25, and 50 nM. Concentrations of Rap 17K are approximate, as the molecular masses of the binding species are unknown.
Purified Rap wt was also examined for its binding to junction and duplex DNA. As expected, Rap wt bound to junction DNA, yielding two protein–DNA complexes as a result of binding by full-length and truncated Rap proteins. In contrast with the Rap His-tag protein, Rap wt yielded a number of complexes on duplex DNA (data not shown). This duplex binding is probably due to a contaminating factor, as Rap antibodies specifically inhibited the formation of Rap wt–J3 complexes but did not affect Rap wt–duplex complexes. In addition, Rap His-tag protein treated with thrombin to remove the first 17 residues of the fusion was unable to bind duplex DNA but retained junction-binding activity (data not shown).
Endonuclease Activity on Synthetic Holliday Junctions.
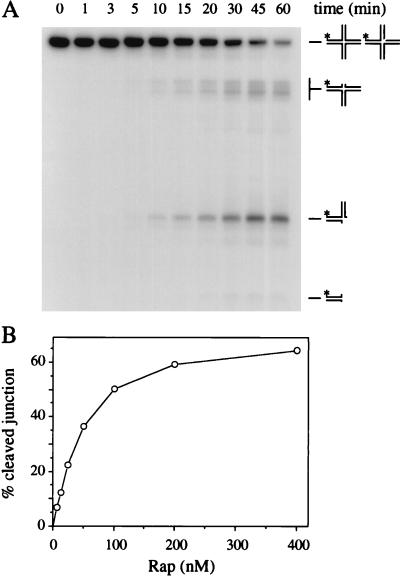
Rap 29K, Rap 17K, and Rap wt proteins were tested for the capacity to resolve a Holliday junction. Rap proteins were incubated with J3 at 37°C in the presence of either magnesium or manganese ions. Rap 29K displayed a nuclease activity that generated a range of DNA products (Fig. 4A). The activity requires manganese ions, although magnesium will support trace amounts of cleavage (G.J.S. and L.M.C., unpublished results). None of the cleavage products corresponded to the nicked duplex product associated with symmetrical cleavage by a Holliday junction resolvase like RuvC or RusA (13, 21). Instead the pattern of bands fits with multiple single-incision events around the center of the junction as depicted in Fig. 4A. The amount of product increased with time of incubation and protein concentration (Fig. 4). Very similar patterns and levels of cleavage were detected with Rap wt (data not shown). No nuclease activity was detected with the Rap 17K or 14K pools (data not shown).
Figure 4.
Holliday junction cleavage by Rap. (A) Time course of Rap junction cleavage. Bulk reaction mixtures (220 μl) contained 4.5 ng of 32P-labeled J3 DNA and 200 nM Rap 29K in cleavage buffer incubated at 37°C. Samples (20 μl) were removed at intervals and reactions were terminated. Products of the reaction were analyzed by 10% PAGE and visualized by autoradiography. The predicted products from the reaction are represented on the right. (B) Graph of junction cleavage with increasing Rap protein concentration. Reaction mixtures (20 μl) contained 0.1 ng of 32P-labeled J3 DNA and various amounts of Rap protein (6.25, 12.5, 25, 50, 100, 200, 400 nM) in cleavage buffer incubated at 37°C for 30 min. Reactions were stopped and the products were processed as in A and quantified by phosphorimaging. The data are the mean of four experiments.
Mapping Endonuclease Cut Sites on Holliday and D-Loop Substrates.
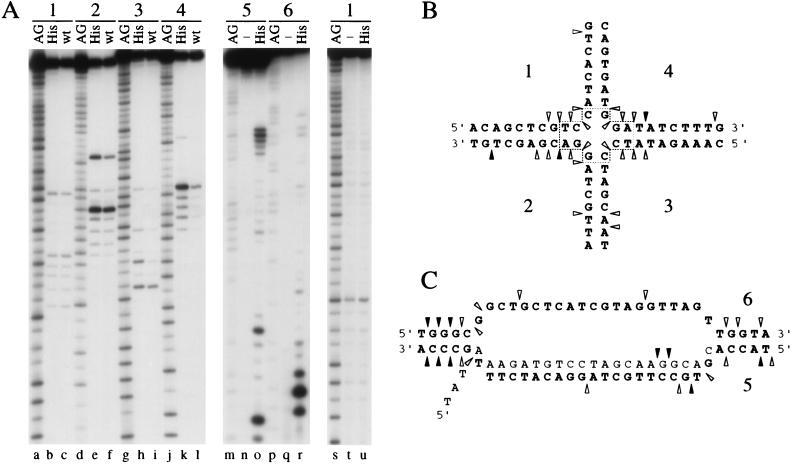
To establish that nuclease cleavage occurred at the center of the junction and that the sites were asymmetrical, we determined the location of cut sites by analyzing reaction products under denaturing conditions. Four J3 junctions, each 32P-labeled in a different strand, were made and incubated with Rap 29K His-tag and Rap wt proteins in cleavage buffer. DNA products were separated on a denaturing gel with A+G sequencing ladders as reference markers (Fig. 5A, lanes a–l). A very similar pattern of endonuclease cut sites was detected with wt and His-tag proteins, with incisions occurring only close to the branch point of the X junction. Moreover, the three major cleavage sites in strands 2 and 4 are not symmetrically related (Fig. 5 A and B). The cleavage sites of Rap wt on two different nonmobile junctions were also mapped (data not shown). Again cleavage was observed around the central core of the junctions with no obvious sequence specificity. No cut sites were detected on denaturing gels with single-stranded DNA incubated with 200 nM Rap 29K (Fig. 5A, lanes s–u) or with a duplex substrate after incubation with Rap 29K at 200 nM or Rap wt at 400 nM (data not shown). Inability to nick these substrates verifies the structure specificity of Rap.
Figure 5.
Mapping of Rap cleavage sites on Holliday and D-loop substrates. (A) Mapping Rap cut sites on J3 and a D-loop. Four X junctions (each 5′-32P-labeled in a different oligonucleotide; 1–4, lanes a–l), three D-loops (each labeled in a different strand; 5–7, lanes m–r), and a single-strand control consisting of oligonucleotide 1 (lanes s–u) were used as DNA substrates. Data for the invading strand 7 of the D-loop are not shown. DNA samples were incubated at 37°C for 60 min with 200 nM Rap 29K His-tag (His), with 200 nM Rap wt (wt), or without protein (−). A+G sequencing ladders (AG) were used as markers to identify cut site locations. Reaction products were analyzed on denaturing 15% PAGE, followed by phosphorimaging and autoradiography. Controls of J3 without protein were set up in parallel and showed no bands other than the full-length oligonucleotide (data not shown). (B) Diagram showing the location of Rap cleavage sites on Holliday junction J3 (A, lanes a–l). The positions of sites are indicated by arrowheads (black for major cut sites, shaded for minor sites). The 3-bp mobile core is boxed. (C) Diagram showing the location of Rap 29K cleavage sites on a D-loop substrate (A, lanes m–r and data not shown). Labeling is the same as in B. The invading strand is shaded. Only the relevant central regions of the Holliday junction and D-loop are shown in B and C. Additional minor cut sites visible by phosphorimaging are omitted for clarity. These sites were located around the core of the X junction and in the bubble structure at the center of the D-loop.
The introduction of single nicks in branched DNA molecules is predicted for the formation of splice recombinants in λ crosses (4, 22). To assess the potential involvement of Rap in this process we assayed Rap 29K His-tag for cleavage of a D-loop substrate that resembles the intermediate expected to form by RecA-mediated strand invasion. The 60-bp D-loop substrate is a duplex with a central unpaired region that has a third strand with a 5′ single-strand tail annealed (17). As with J3, junctions were made, each labeled in a different strand, and the Rap cleavage sites were mapped (Fig. 5A, lanes m–r and data not shown). A summary of the results is represented diagrammatically (Fig. 5C). Rap cleavage sites are located close to the branch points at the center of the structure, as was the case with the X junction. The major endonuclease cut sites are located in duplex regions immediately adjacent to the branch points of the D-loop. Some additional sites occur elsewhere in the center of the single-strand and double-strand DNA bubble section. The data confirm that Rap is a structure-specific endonuclease targeted to the branch points of DNA molecules.
DISCUSSION
Genetic analysis has implicated the phage λ rap gene product in the formation of splices in genetic recombination (11). The data presented here give an insight into the nature of the activity involved. Purified Rap specifically recognizes and cleaves synthetic Holliday junction and D-loop structures in vitro. Rap also cuts at the branch points of duplexes with single-strand arms, bubble structures, and D-loops with 3′ tails (G.J.S. and L.M.C., unpublished results). The endonuclease activity of Rap is clearly distinct from the known Holliday junction resolvases (T4 endo VII, T7 endo I, RusA, RuvC, and CCE1), all of which cleave by introducing symmetrically related nicks in four-way DNA (23). Rap has more in common with structure-specific endonucleases involved in eukaryotic nucleotide excision repair (e.g., XPG, ERCC1/XPF), recombination, and replication (e.g., FEN-1) and D-loop cleavage in E. coli. However, Rap differs from these enzymes by its ability to cut a variety of a branched DNA molecules without being restricted by strand polarity. Nucleotide excision repair endonucleases always target a specific side of bubble structures which are formed by enzyme-catalyzed unwinding at a DNA lesion (24). FEN-1 cleaves flap structures with a 5′ single-strand end and also has associated 5′-3′ exonuclease activity specific for double-stranded DNA. The enzyme functions to remove the RNA primers on Okazaki fragments and is also involved in certain recombination and repair reactions (25). The D-loop endonuclease identified in cell extracts from E. coli cleaves only on the displaced strand and 6–8 nucleotides away from the branch point (26).
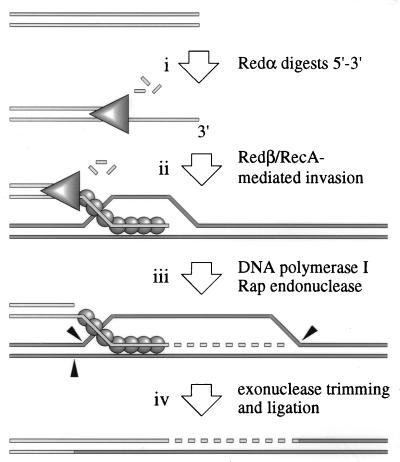
The properties of Rap endonuclease can be incorporated into a model for λ Red recombination initiated from a double-strand break (4, 22) (Fig. 6). Double chain breaks can occur in λ chromosomes by restriction cleavage, replication fork collapse to form rolling circles, and cutting at the cos site by terminase prior to packaging (3). Redα can bind to the broken duplex end and degrades the DNA to yield a 3′-ended single-strand tail (Fig. 6, step i). This tail is paired with another λ chromosome by Redβ or RecA, assisted by λ Orf (NinB), to create a D-loop (Fig. 6, step ii). The annealed 3′ end can prime DNA replication, and protein-free sections of the D-loop are a substrate for Rap cleavage (Fig. 6, step iii). Trimming of the DNA followed by ligation results in a splice recombinant (Fig. 6, step iv). The model assumes that Rap cannot attack the 3′ single-strand tail protected by Redβ/RecA or the strand upon which the DNA polymerase assembly is acting. Alternatively, Rap may be directed to specific sites on the D-loop by another phage protein potentially encoded by the ninR region.
Figure 6.
Model for λ Red recombination incorporating the action of Rap protein. The model depends on the observation that Rap is involved in Red pathway recombination at a double-strand break (T. Tarkowski and F. W. Stahl, personal communication). Step i, a double-strand break in λ DNA is a substrate for the Redα exonuclease. Redα (triangle) degrades the double-strand end to generate a 3′ tail. Step ii, this single-strand tail is paired with a homologous chromosome by either Redβ or RecA proteins (spheres). Step iii, the annealed 3′ end can prime DNA replication and protein-free sections of the D-loop are a substrate for Rap cleavage. Step iv, trimming of the DNA followed by ligation results in a splice product. See Discussion and Introduction for further details and references.
The structure-specific endonuclease of Rap may also account for the phenomenon of “cutting in trans” identified by Howard-Flanders and colleagues (27, 28). They observed the cleavage of covalent circular λ chromosomes after recombination with psoralen-damaged phage. This endonuclease could also be detected in vitro in extracts purified from an induced λ lysogen (29). Their results can be explained by the nicking of D-loop recombination intermediates by a specific endonuclease. Because their experiments involved wild-type λ phage it seems plausible that Rap could be the endonuclease responsible for the phenomenon rather than a host-encoded function.
In the absence of Rap, such as in a nin5 deletion phage, recombination can proceed efficiently by using host enzymes for the formation and resolution of Holliday junctions. Rap could in theory resolve a four-way Holliday junction, but this would require a two-step reaction. The inclusion of plasmids carrying rap in strains defective for Holliday junction processing enzymes (ruv or ruv recG) failed to improve their resistance to DNA-damaging agents (data not shown). Rap does not appear to be involved in end-annealing recombination by cleaving single-strand flaps because the ninR region is unnecessary for these events (6). However, this could be masked by a host 3′-5′ exonuclease or flap endonuclease.
The truncated Rap proteins that bind but fail to cleave junction structures may actually function to protect branched DNA early in the phage life cycle. They could prevent premature nucleolytic digestion by Rap or other nucleases. This system may therefore serve to regulate expression of endonuclease-active Rap until later in the life cycle when the tR4 terminator is bypassed and recombination intermediates are ready for processing.
Homologs of the RusA Holliday junction resolvase are present in a wide range of lambdoid phages, including 82, HK97, HK022 and Lactococcus lactis r1t (G.J.S., unpublished results). Rap can be found in the nin region of the phages 21, H-19B, and P22. This observation suggests that lambdoid phages have at least two alternative pathways for processing recombination intermediates. The RusA pathway promotes symmetrical resolution of a four-way (Holliday) junction in a manner similar to that of the host RuvC resolvase. The Rap pathway appears to be different, resolving at an earlier stage by nicking three-way junctions.
Acknowledgments
We thank T. Tarkowski and F. W. Stahl for communicating results prior to publication and R. G. Lloyd and P. M. McGlynn for advice and critical reading of the manuscript. This work was supported by the Biotechnology and Biological Sciences Research Council and the Royal Society.
ABBREVIATIONS
- wt
wild-type
- IPTG
isopropyl β-d-thiogalactoside
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Karu A E, Sakaki Y, Echols H, Linn S. J Biol Chem. 1975;250:7377–7387. [PubMed] [Google Scholar]
- 2.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaler D S, Stahl M M, Stahl F W. J Mol Biol. 1987;195:75–87. doi: 10.1016/0022-2836(87)90328-7. [DOI] [PubMed] [Google Scholar]
- 4.Stahl F W, Kobayashi I, Stahl M M. J Mol Biol. 1985;181:199–209. doi: 10.1016/0022-2836(85)90085-3. [DOI] [PubMed] [Google Scholar]
- 5.Muniyappa K, Radding C M. J Biol Chem. 1986;261:7472–7478. [PubMed] [Google Scholar]
- 6.Stahl M M, Thomason L, Poteete A R, Tarkowski T, Kuzminov A, Stahl F W. Genetics. 1997;147:961–977. doi: 10.1093/genetics/147.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noirot P, Kolodner R D. J Biol Chem. 1998;273:12274–12280. doi: 10.1074/jbc.273.20.12274. [DOI] [PubMed] [Google Scholar]
- 8.Sawitzke J A, Stahl F W. Genetics. 1992;130:7–16. doi: 10.1093/genetics/130.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb B L, Cox M M, Inman R B. Cell. 1997;91:347–356. doi: 10.1016/s0092-8674(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 10.Hollifield W, Kaplan E, Huang H. Mol Gen Genet. 1987;210:248–255. doi: 10.1007/BF00325690. [DOI] [PubMed] [Google Scholar]
- 11.Stahl F W, Shurvinton C E, Thomason L C, Hill S, Stahl M M. Genetics. 1995;139:1107–1121. doi: 10.1093/genetics/139.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahdi A A, Sharples G J, Mandal T N, Lloyd R G. J Mol Biol. 1996;257:561–573. doi: 10.1006/jmbi.1996.0185. [DOI] [PubMed] [Google Scholar]
- 13.Sharples G J, Chan S C, Mahdi A A, Whitby M C, Lloyd R G. EMBO J. 1994;13:6133–6142. doi: 10.1002/j.1460-2075.1994.tb06960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 15.Parsons C A, Kemper B, West S C. J Biol Chem. 1990;265:9285–9289. [PubMed] [Google Scholar]
- 16.Whitby M C, Bolt E L, Chan S N, Lloyd R G. J Mol Biol. 1996;264:878–890. doi: 10.1006/jmbi.1996.0684. [DOI] [PubMed] [Google Scholar]
- 17.McGlynn P, Al-Deib A A, Liu J, Marians K J, Lloyd R G. J Mol Biol. 1997;270:212–221. doi: 10.1006/jmbi.1997.1120. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd R G, Sharples G J. EMBO J. 1993;12:17–22. doi: 10.1002/j.1460-2075.1993.tb05627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxam A M, Gilbert W. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 20.Cheng S W, Court D L, Friedman D I. Genetics. 1995;140:875–887. doi: 10.1093/genetics/140.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunderdale H J, Benson F E, Parsons C A, Sharples G J, Lloyd R G, West S C. Nature (London) 1991;354:506–510. doi: 10.1038/354506a0. [DOI] [PubMed] [Google Scholar]
- 22.Hill S A, Stahl M M, Stahl F W. Proc Natl Acad Sci USA. 1997;94:2951–2956. doi: 10.1073/pnas.94.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White M F, Giraud-Panis M-J E, Pöhler J R, Lilley D M J. J Mol Biol. 1997;269:647–664. doi: 10.1006/jmbi.1997.1097. [DOI] [PubMed] [Google Scholar]
- 24.Wood R D. Ann Rev Biochem. 1996;65:135–167. doi: 10.1146/annurev.bi.65.070196.001031. [DOI] [PubMed] [Google Scholar]
- 25.Lieber M R. BioEssays. 1997;19:233–240. doi: 10.1002/bies.950190309. [DOI] [PubMed] [Google Scholar]
- 26.Chiu S K, Low K B, Yuan A, Radding C M. Proc Natl Acad Sci USA. 1997;94:6079–6083. doi: 10.1073/pnas.94.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross P, Howard-Flanders P. J Mol Biol. 1977;117:137–158. doi: 10.1016/0022-2836(77)90028-6. [DOI] [PubMed] [Google Scholar]
- 28.Ross P, Howard-Flanders P. J Mol Biol. 1977;117:159–174. doi: 10.1016/0022-2836(77)90029-8. [DOI] [PubMed] [Google Scholar]
- 29.Cassuto E, Mursalim J, Howard-Flanders P. Proc Natl Acad Sci USA. 1978;75:620–624. doi: 10.1073/pnas.75.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp P M, Burgess C J, Lloyd A T, Mitchell K J. In: Transfer RNA in Protein Synthesis. Hatfield D L, Lee B J, Pirtle R M, editors. Boca Raton, FL: CRC; 1992. pp. 397–425. [Google Scholar]