Abstract
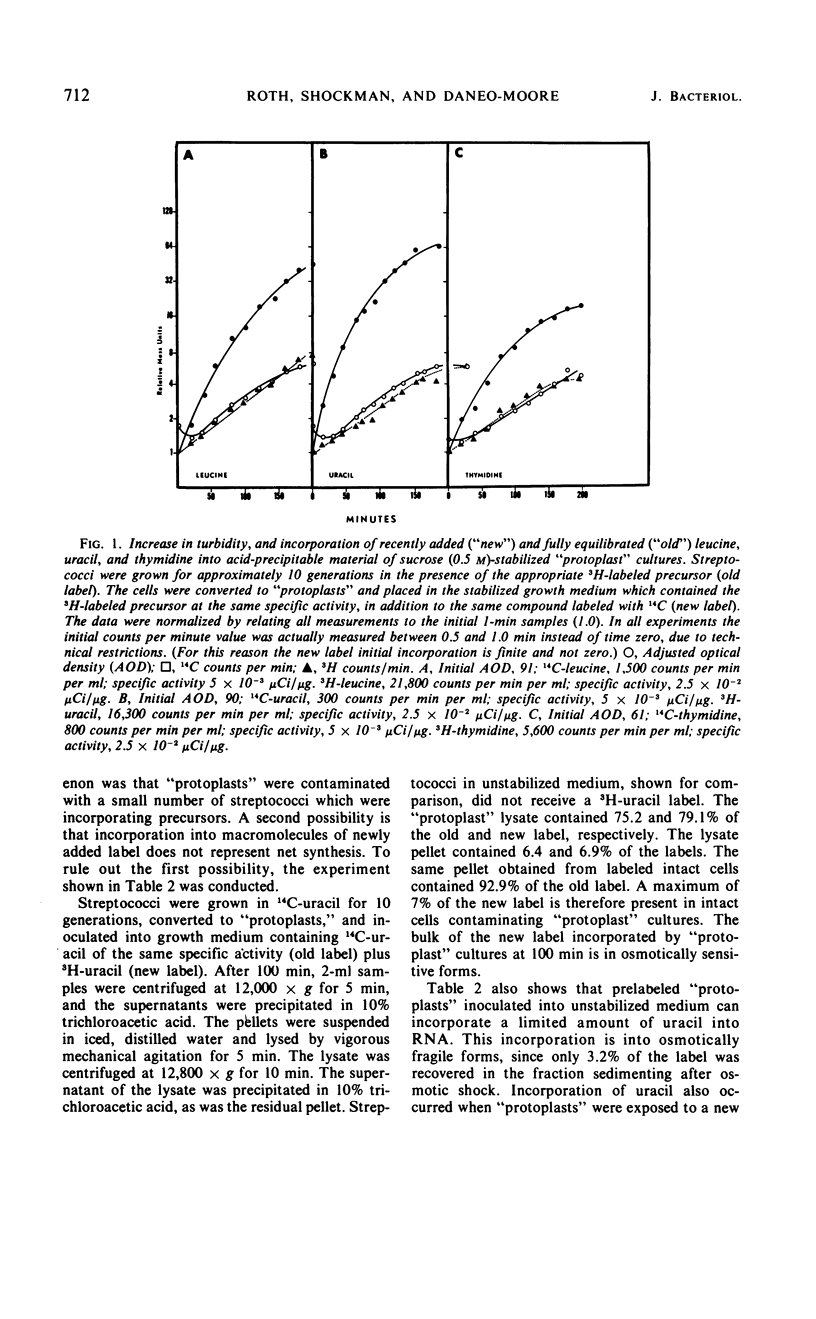
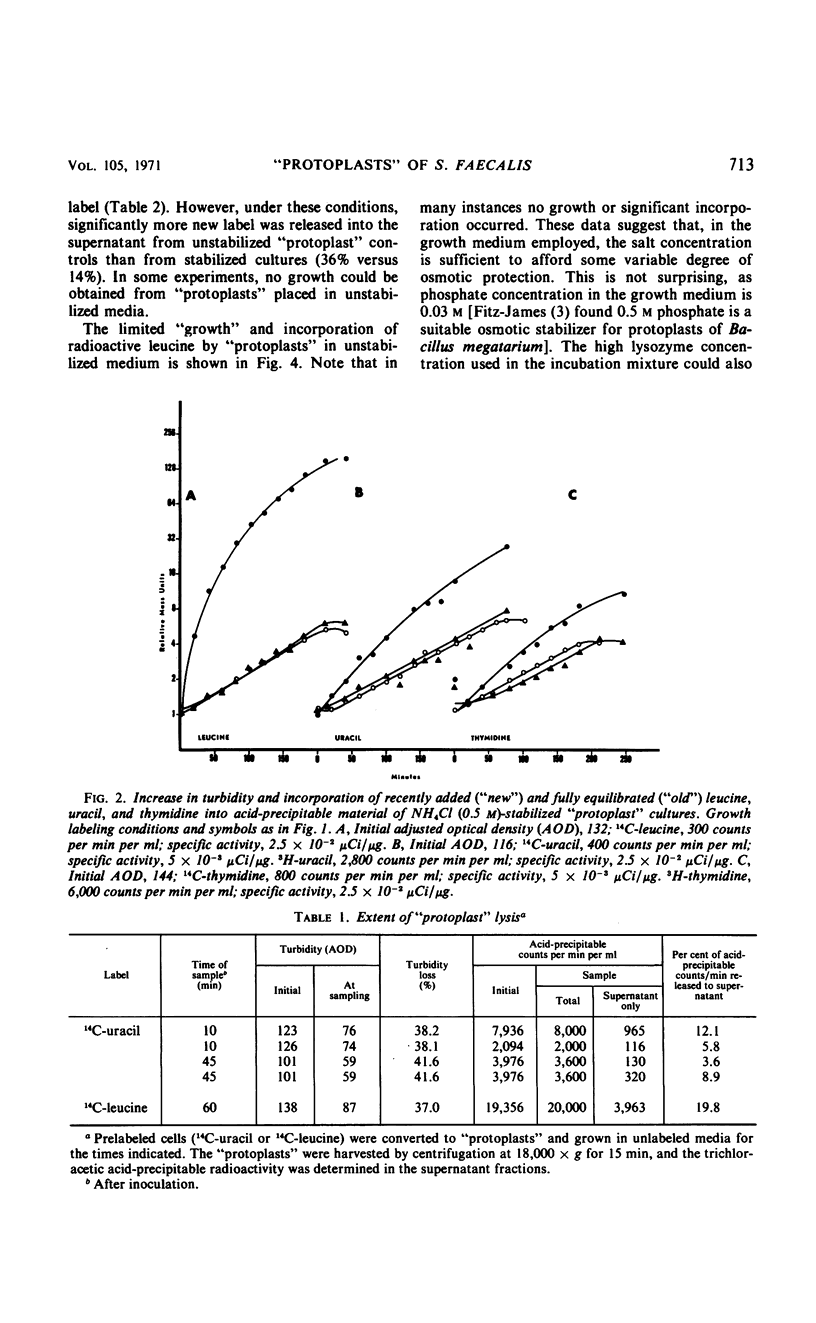
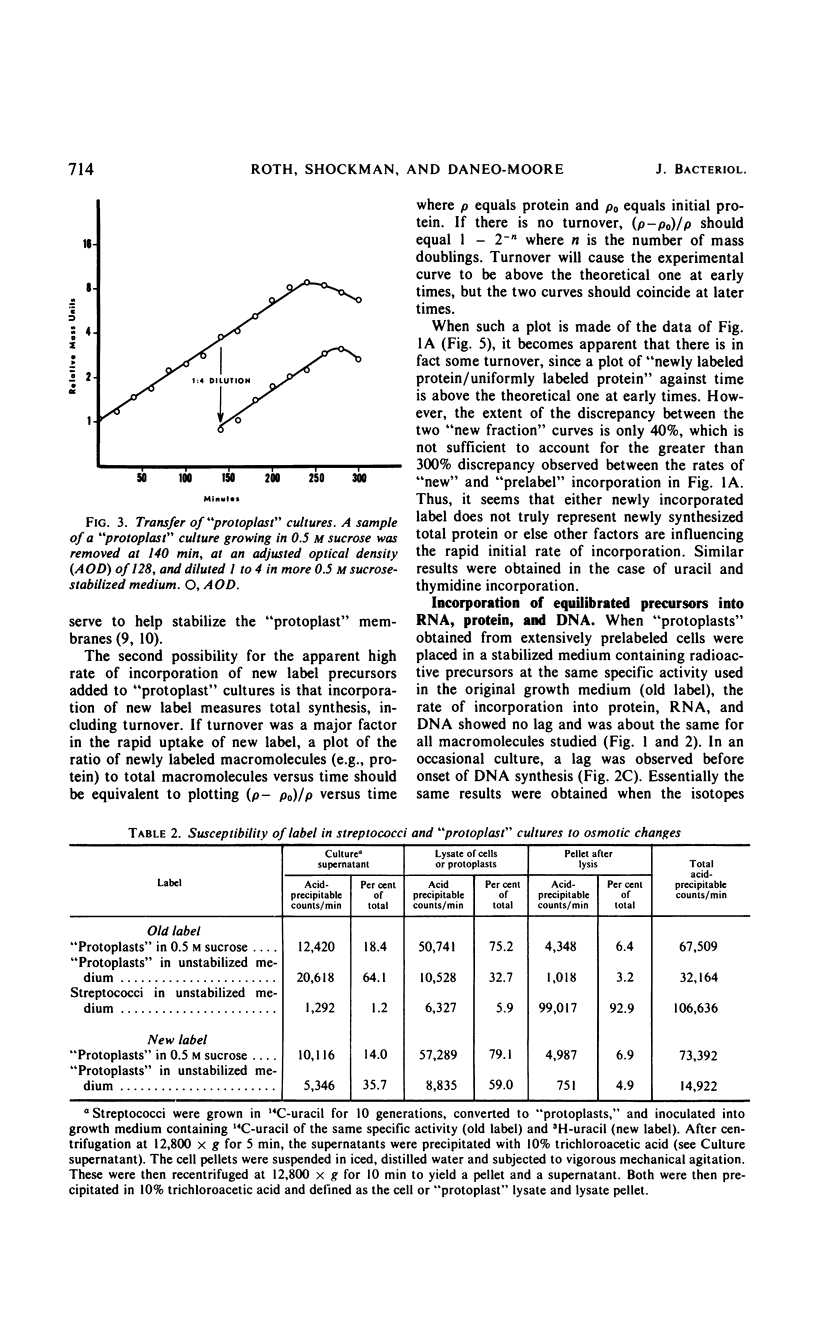
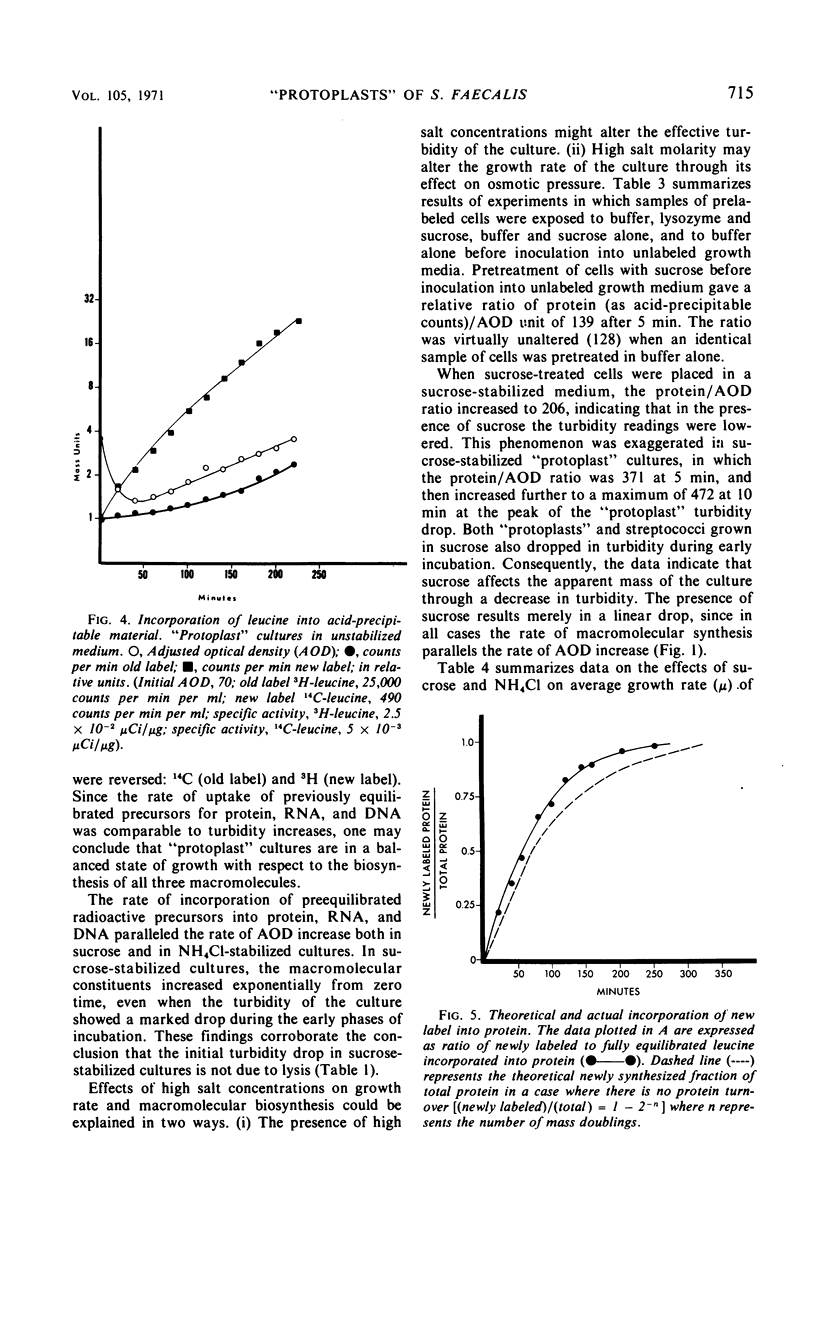
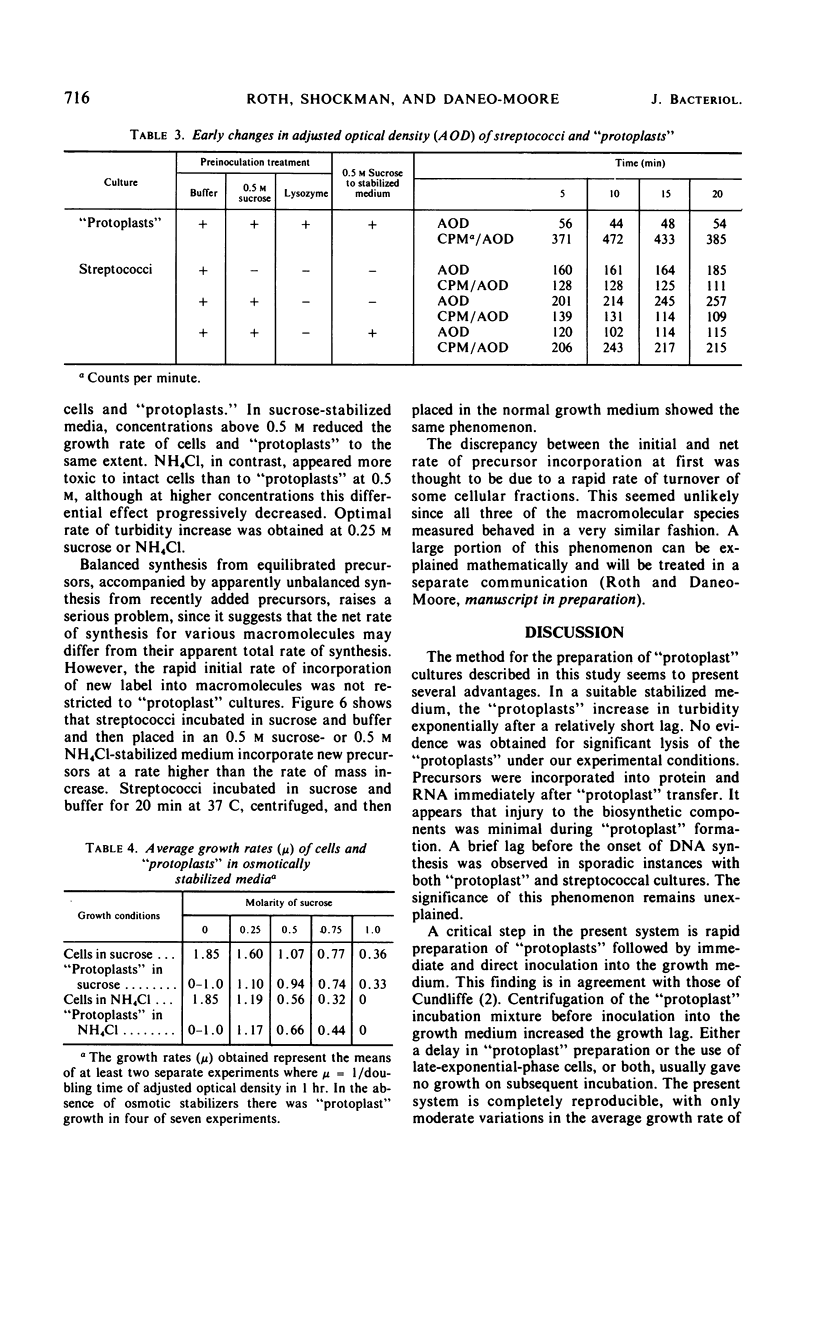
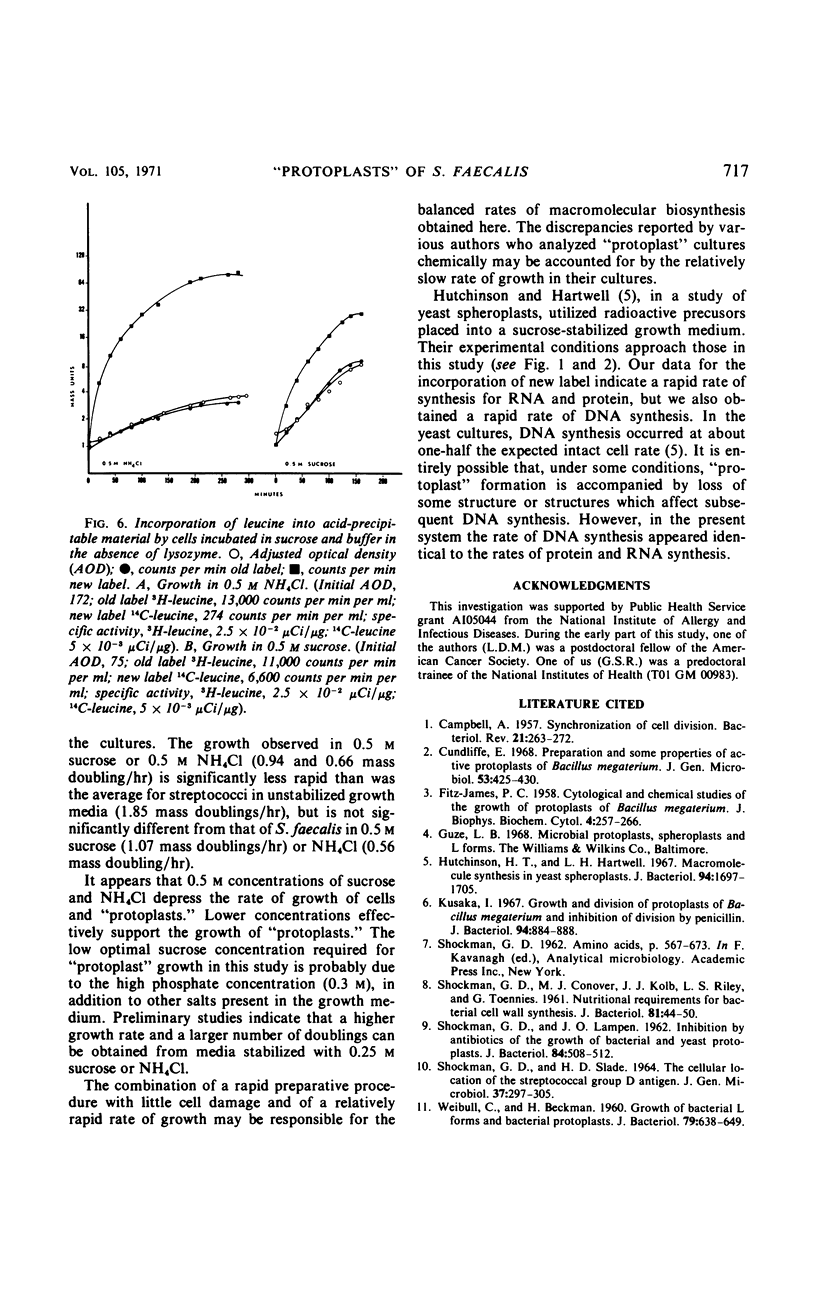
Osmotically fragile forms of Streptococcus faecalis 9790 were grown in 0.5 m sucrose- or 0.5 m NH4Cl-stabilized medium. The “protoplast” cultures exhibit an average growth rate constant of 0.66 to 0.94 mass doublings/hr. In a variety of experiments, turbidity and the net content of protein, ribonucleic acid (RNA) and deoxyribonucleic acid increase at the same rate, indicating balanced macromolecular biosynthesis. A total of two to three mass doublings was obtained, with no evidence of cell division. After osmotic shock, “protoplast” cultures released 93 to 94% of their RNA content in a form not sedimentable at 12,800 × g for 15 min, in contrast to streptococci, which released 7% of their RNA content after the same treatment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMPBELL A. Synchronization of cell division. Bacteriol Rev. 1957 Dec;21(4):263–272. doi: 10.1128/br.21.4.263-272.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. Preparation and some properties of active protoplasts of Bacillus megaterium. J Gen Microbiol. 1968 Oct;53(3):425–430. doi: 10.1099/00221287-53-3-425. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Cytological and chemical studies of the growth of protoplasts of Bacillus megaterium. J Biophys Biochem Cytol. 1958 May 25;4(3):257–266. doi: 10.1083/jcb.4.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I. Growth and division of protoplasts of Bacillus megaterium and inhibition of division by penicillin. J Bacteriol. 1967 Oct;94(4):884–888. doi: 10.1128/jb.94.4.884-888.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D., LAMPEN J. O. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J Bacteriol. 1962 Sep;84:508–512. doi: 10.1128/jb.84.3.508-512.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D., SLADE H. D. THE CELLULAR LOCATION OF THE STREPTOCOCCAL GROUP D ANTIGEN. J Gen Microbiol. 1964 Dec;37:297–305. doi: 10.1099/00221287-37-3-297. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BECKMAN H. Growth of bacterial L forms and bacterial protoplasts. J Bacteriol. 1960 May;79:638–649. doi: 10.1128/jb.79.5.638-649.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



