Abstract
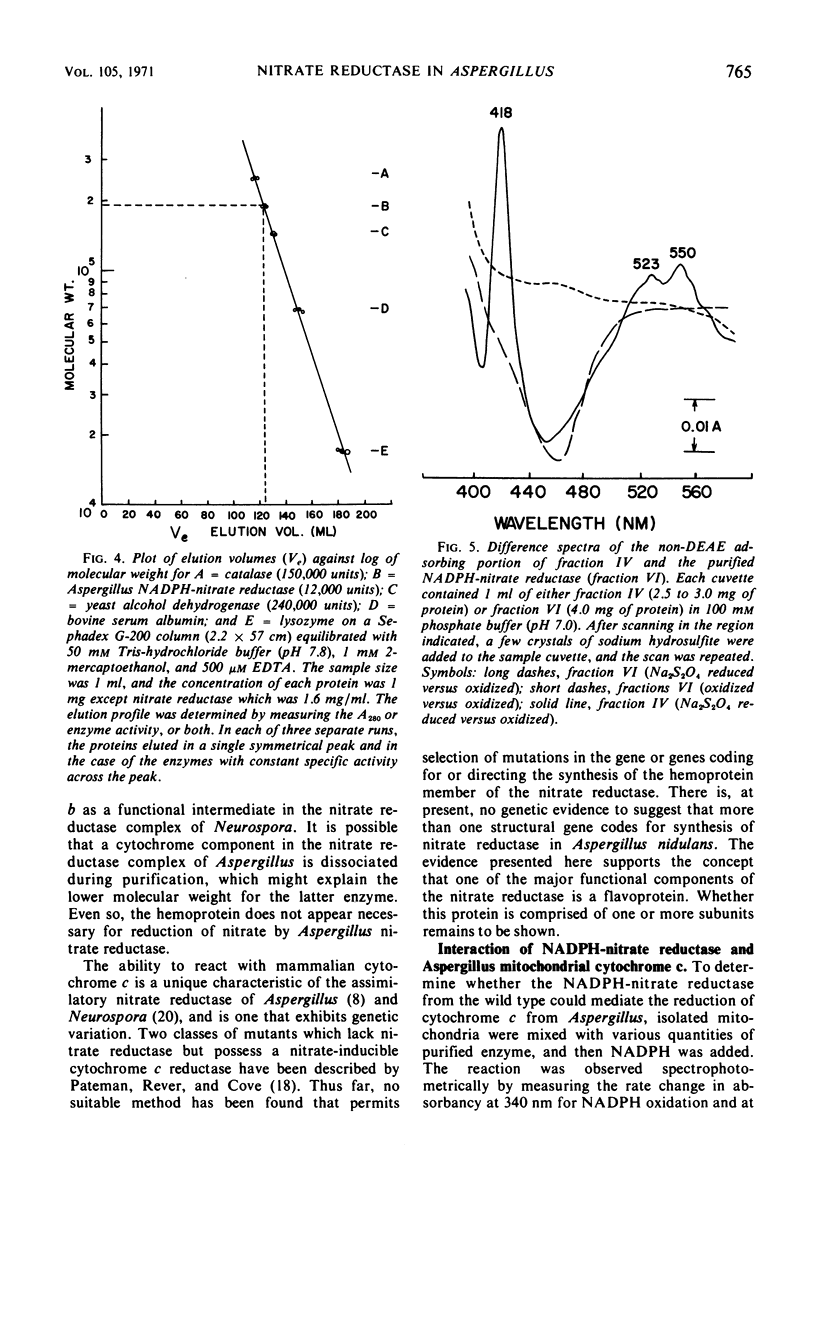
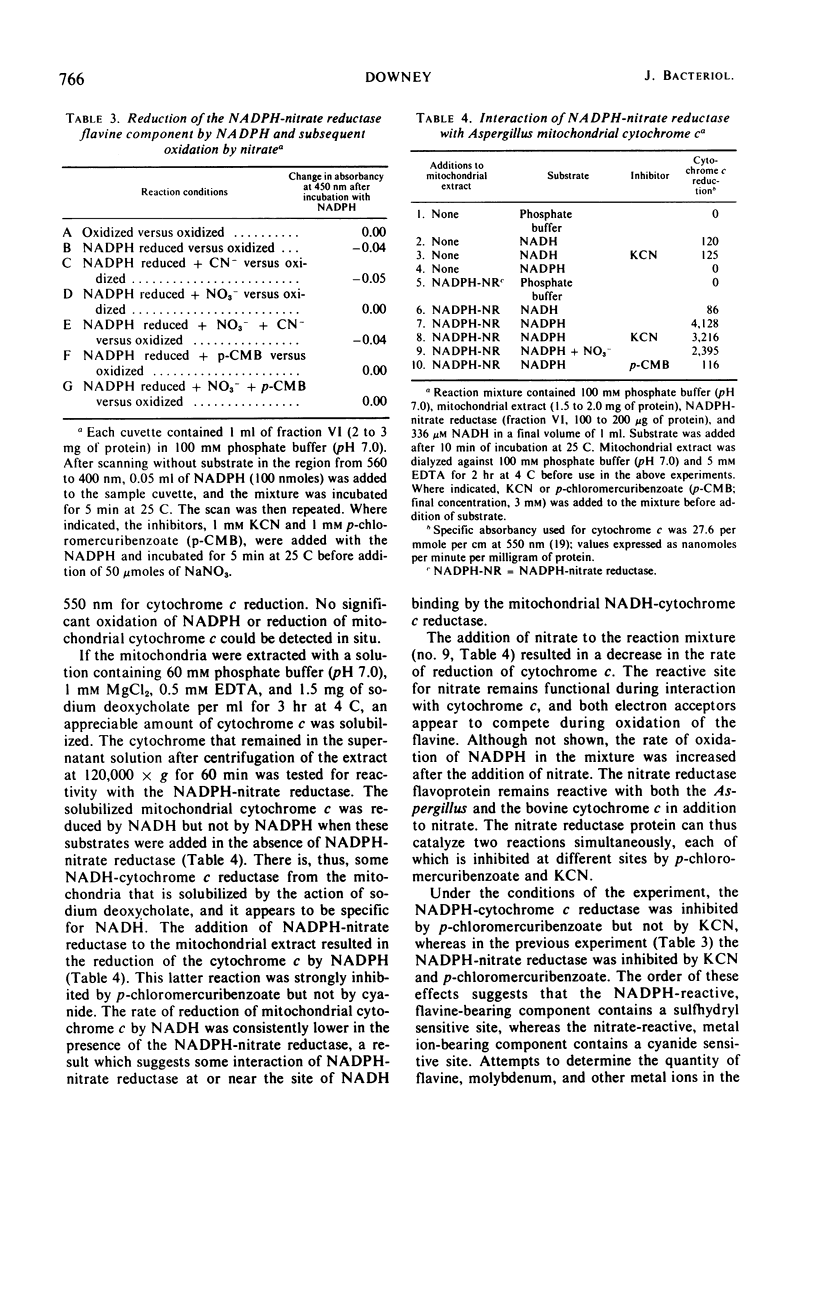
The reduced nicotinamide adenine dinucleotide phosphate (NADPH)-nitrate oxidoreductase (EC 1.6.6.2) from Aspergillus nidulans was purified over 200-fold by use of salt fractionation, gel filtration, and ion-exchange chromatography. The purified enzyme was specific for NADPH and catalyzed reduction of nitrate, cytochrome c from isolated mitochondria of Aspergillus, and mammalian cytochrome c. An S0.72520, w of 7.8 was derived with sucrose density gradient centrifugation, and a Stokes radius of 6.4 nm was derived by gel filtration on Sephadex G-200. From these values, a molecular weight of 197,000 was computed, assuming ¯v = 0.725 cm3/g. The spectral properties of the purified enzyme suggested a flavine component was present but revealed no pattern indicative of a hemoprotein. A cytochrome c, similar to the cytochrome c from isolated mitochondria, was found unassociated with the nitrate reductase after ion-exchange chromatography. No NADPH-nitrate reductase activity was detected in isolated mitochondria. Spectrally discernable reduction of the flavine component of the enzyme at 450 nm was noted after reaction with NADPH. This reduction was inhibited by p-chloromercuribenzoate but not by KCN. The addition of nitrate to NADPH reduced enzyme caused a reoxidation of the flavine component via a reaction which was inhibited by KCN but not by p-chloromercuribenzoate. The half-life of the purified enzyme at 37 C was 20 min for NADPH-nitrate reductase and 35 min for NADPH-cytochrome c reductase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVE D. J., PATEMAN J. A. Independently segregating genetic loci concerned with nitrate reductase activity in Aspergillus nidulans. Nature. 1963 Apr 20;198:262–263. doi: 10.1038/198262a0. [DOI] [PubMed] [Google Scholar]
- Cove D. J., Coddington A. Purification of nitrate reductase and cytochrome c reductase from Aspergillus nidulans. Biochim Biophys Acta. 1965 Nov 22;110(2):312–318. doi: 10.1016/s0926-6593(65)80038-8. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Kinetic studies of the induction of nitrate reductase and cytochrome c reductase in the fungus Aspergillus nidulans. Biochem J. 1967 Sep;104(3):1033–1039. doi: 10.1042/bj1041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J., Pateman J. A. Autoregulation of the synthesis of nitrate reductase in Aspergillus nidulans. J Bacteriol. 1969 Mar;97(3):1374–1378. doi: 10.1128/jb.97.3.1374-1378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Edelman M., Verma I. M., Littauer U. Z. Mitochondrial ribosomal RNA from Aspergillus nidulans: characterization of a novel molecular species. J Mol Biol. 1970 Apr 14;49(1):67–83. doi: 10.1016/0022-2836(70)90376-1. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Further purification and properties of Neurospora nitrate reductase. J Biol Chem. 1969 Jun 10;244(11):2870–2882. [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H., Noll H. Mitochondrial and cytoplasmic polysomes from Neurospora crassa. Nature. 1967 Sep 23;215(5108):1340–1345. doi: 10.1038/2151340a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NASON A., EVANS H. J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J Biol Chem. 1953 Jun;202(2):655–673. [PubMed] [Google Scholar]
- Pateman J. A., Rever B. M., Cove D. J. Genetic and biochemical studies of nitrate reduction in Aspergillus nidulans. Biochem J. 1967 Jul;104(1):103–111. doi: 10.1042/bj1040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEJTER A., GLAUSER S. C., GEORGE P., MARGOLIASH E. SPECTRA OF CYTOCHROME C MONOMER AND POLYMERS. Biochim Biophys Acta. 1963 Aug 6;73:641–643. doi: 10.1016/0006-3002(63)90334-2. [DOI] [PubMed] [Google Scholar]
- Sorger G. J. Nitrate reductase electron transport systems in mutant and in wild-type strains of Neurospora. Biochim Biophys Acta. 1966 Jun 15;118(3):484–494. doi: 10.1016/s0926-6593(66)80091-7. [DOI] [PubMed] [Google Scholar]
- Watson K., Smith J. E. Oxidative phosphorylation and respiratory control in mitochondria from Aspergillus niger. Biochem J. 1967 Aug;104(2):332–339. doi: 10.1042/bj1040332. [DOI] [PMC free article] [PubMed] [Google Scholar]