Abstract
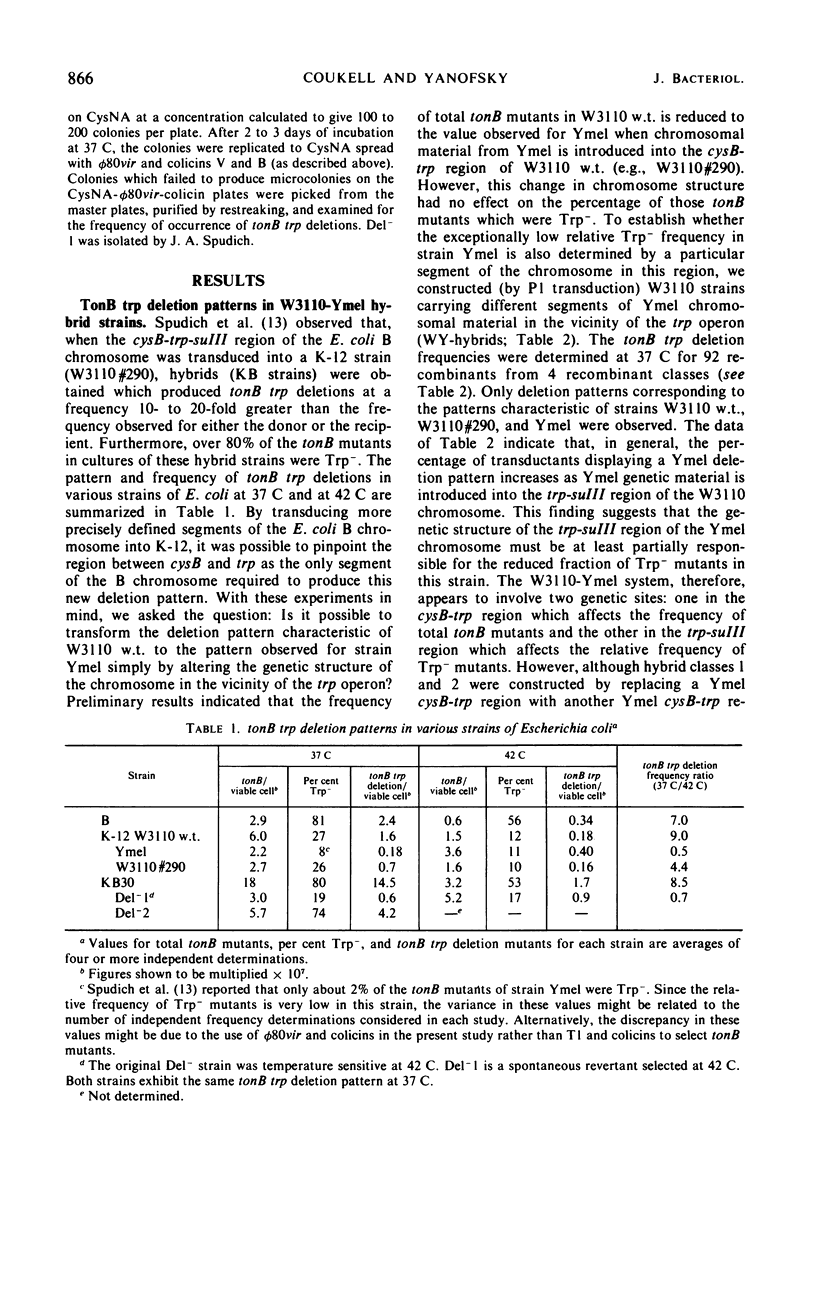
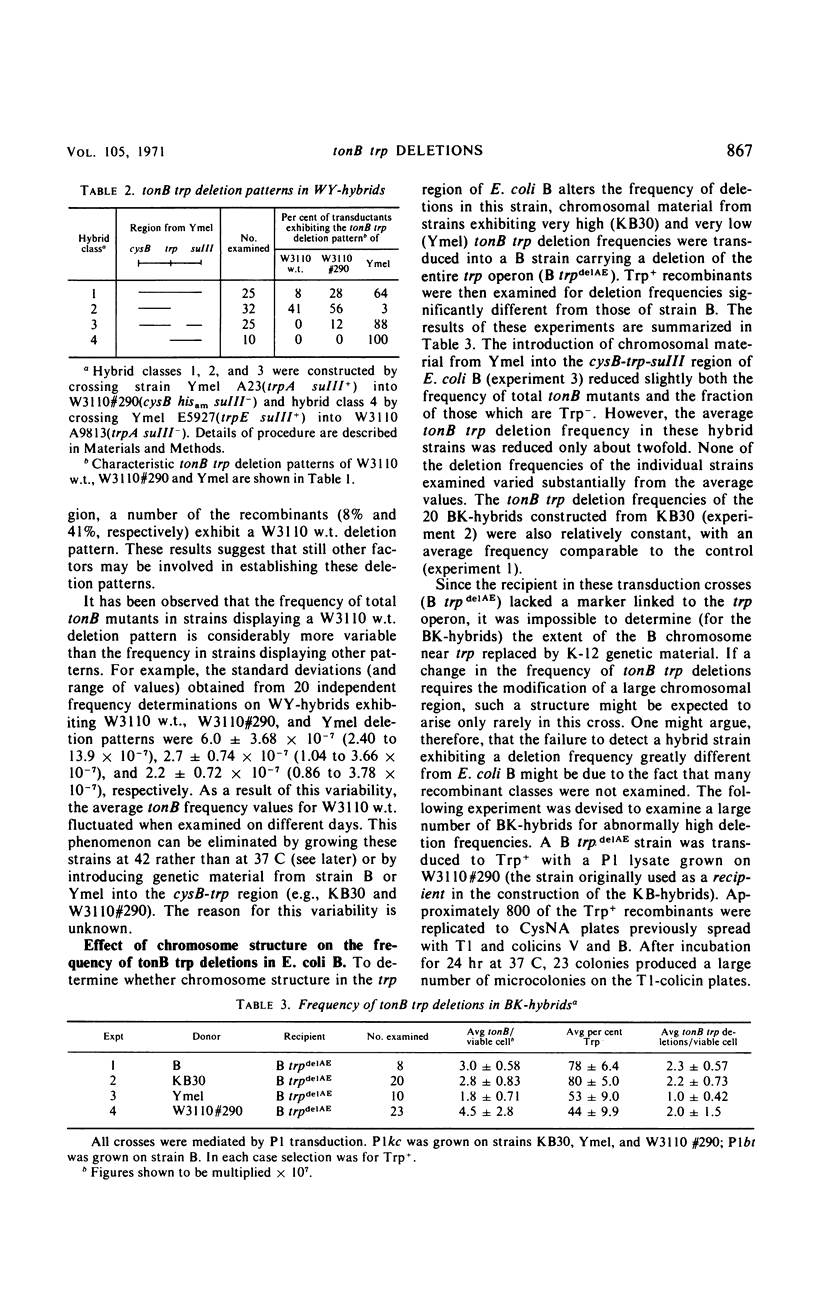
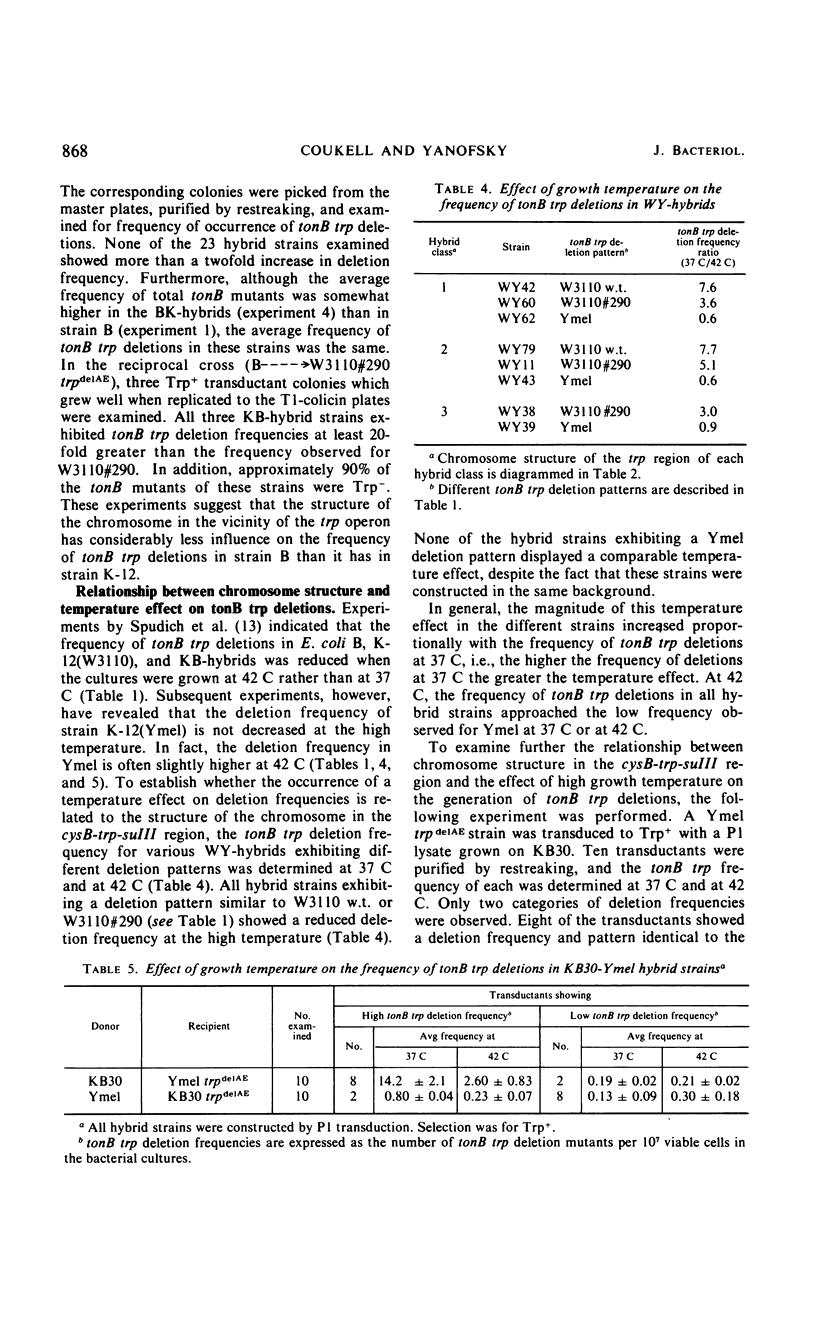
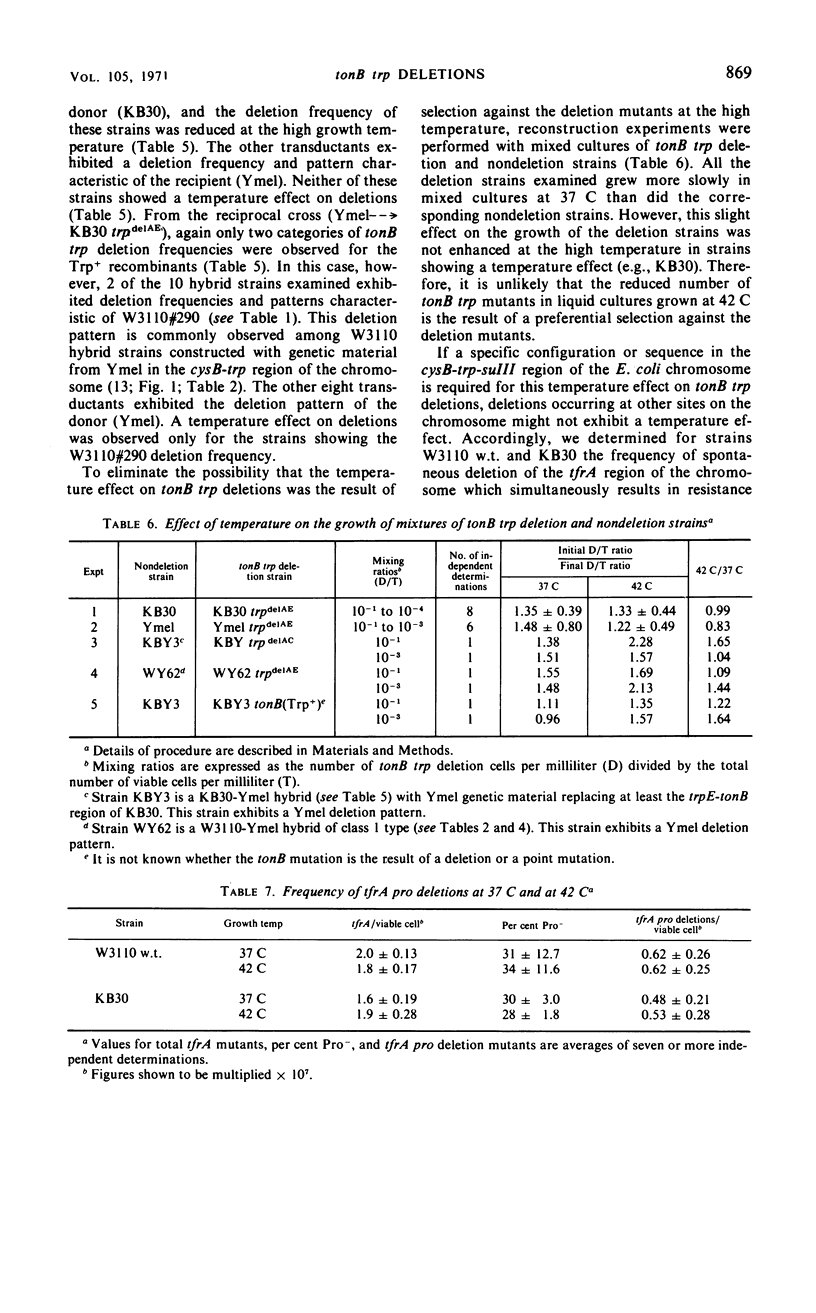
The frequency of tonB trp deletions varies in different strains and substrains of Escherichia coli. Studies with chromosomal hybrids constructed by transducing various segments of the cysB-trp-suIII region from K-12(Ymel) into K-12(W3110) indicate that the characteristic low deletion frequency of K-12(Ymel) is determined largely by the (genetic) structure of the trp-suIII region of the chromosome. Transduction of the trp region from K-12(W3110) or K-12(Ymel) into strain B has little effect on the frequency of tonB trp deletions in that strain. When tonB trp deletions occur at 42 C rather than at 37 C, there is a significant reduction in the frequency of deletions in all strains examined except K-12(Ymel) and hybrids exhibiting a Ymel deletion pattern. The magnitude of this temperature effect in different K-12 strains increases proportionally with the frequency of tonB trp deletions at 37 C. At 42 C the frequency of tonB trp deletions in all K-12 strains approaches the low frequency observed for Ymel at 37 or 42 C. In contrast, spontaneous deletions in another region of the genome which simultaneously result in resistance to phages T7 and λ and in proline auxotrophy (tfrA pro deletions) occur at a constant frequency regardless of growth temperature or the structure of the chromosome in the trp region. Two mutants of strain KB30 obtained after treatment with nitrosoguanidine show very low tonB trp deletion frequencies. The alterations in both mutants map in the trp region of the chromosome. These studies indicate that the structure of the cysB-trp-suIII region is responsible for many of the characteristic deletion frequencies observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W. Spontaneous deletion formation in several classes of Escherichia coli mutants deficient in recombination ability. Mutat Res. 1970 Feb;9(2):155–165. doi: 10.1016/0027-5107(70)90054-0. [DOI] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R. Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J Mol Biol. 1966 Aug;19(2):254–265. doi: 10.1016/s0022-2836(66)80003-7. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Extraordinary recombinational events in Escherichia coli. Their independence of the rec+ function. Genetics. 1967 Apr;55(4):699–707. doi: 10.1093/genetics/55.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRATIA J. P. R'ESISTANCE 'A LA COLICINE B CHEZ E. COLI. RELATIONS DE SP'ECIFICIT'E ENTRE COLICINES B, I ET V ET PHAGE T-4. ETUDE G'EN'ETIQUE. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:151. [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Formation of deletion mutations in recombination-deficient mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1266–1267. doi: 10.1128/jb.94.4.1266-1267.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Horn V., Yanofsky C. On the production of deletions in the chromosome of Escherichia coli. J Mol Biol. 1970 Oct 14;53(1):49–67. doi: 10.1016/0022-2836(70)90045-8. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


