Abstract
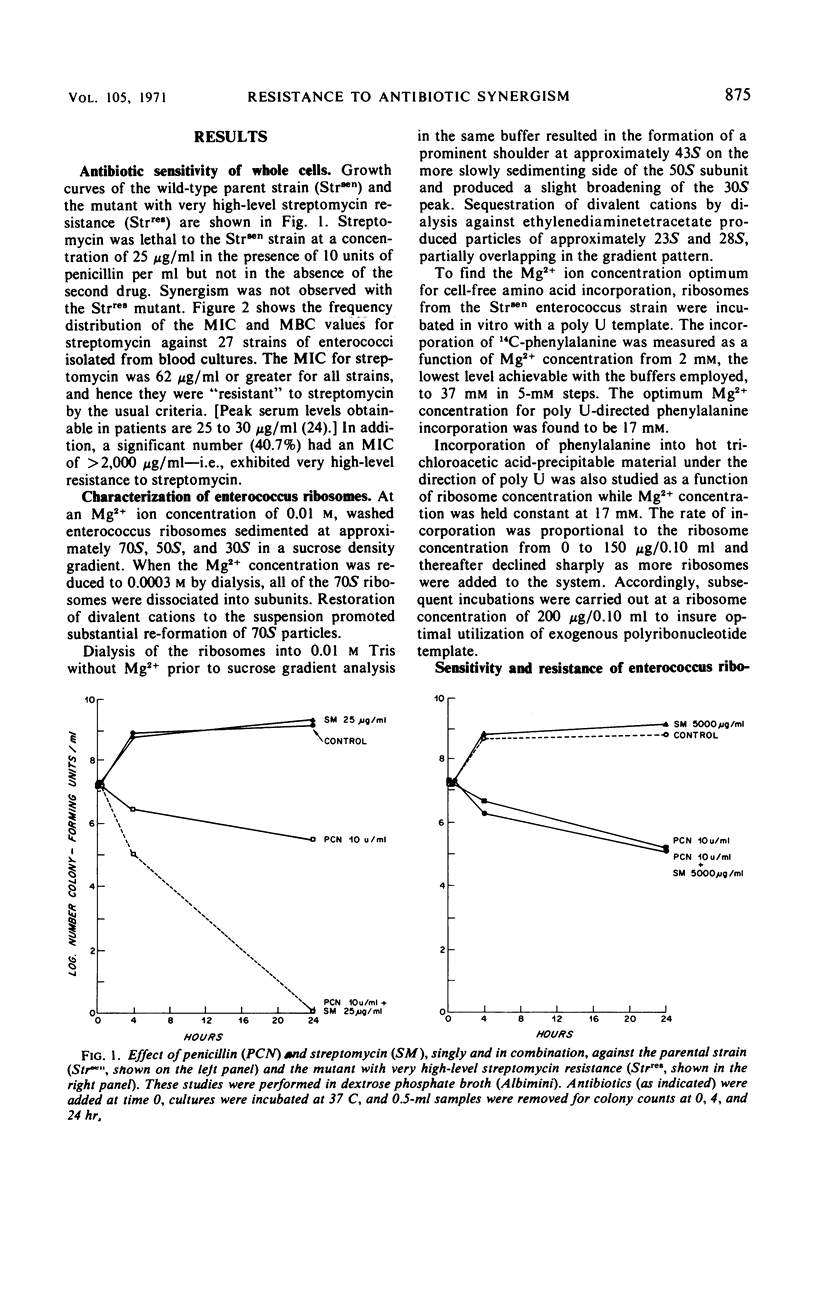
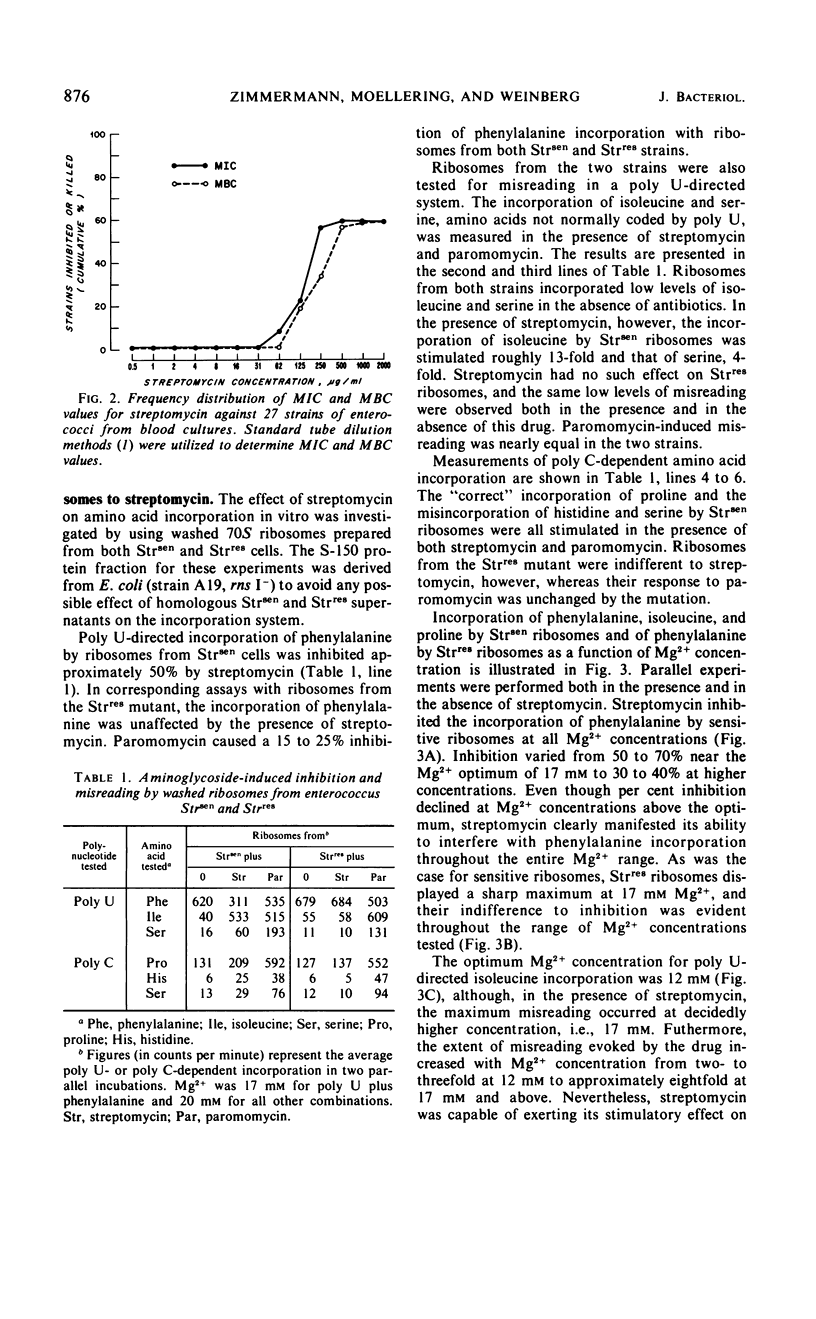
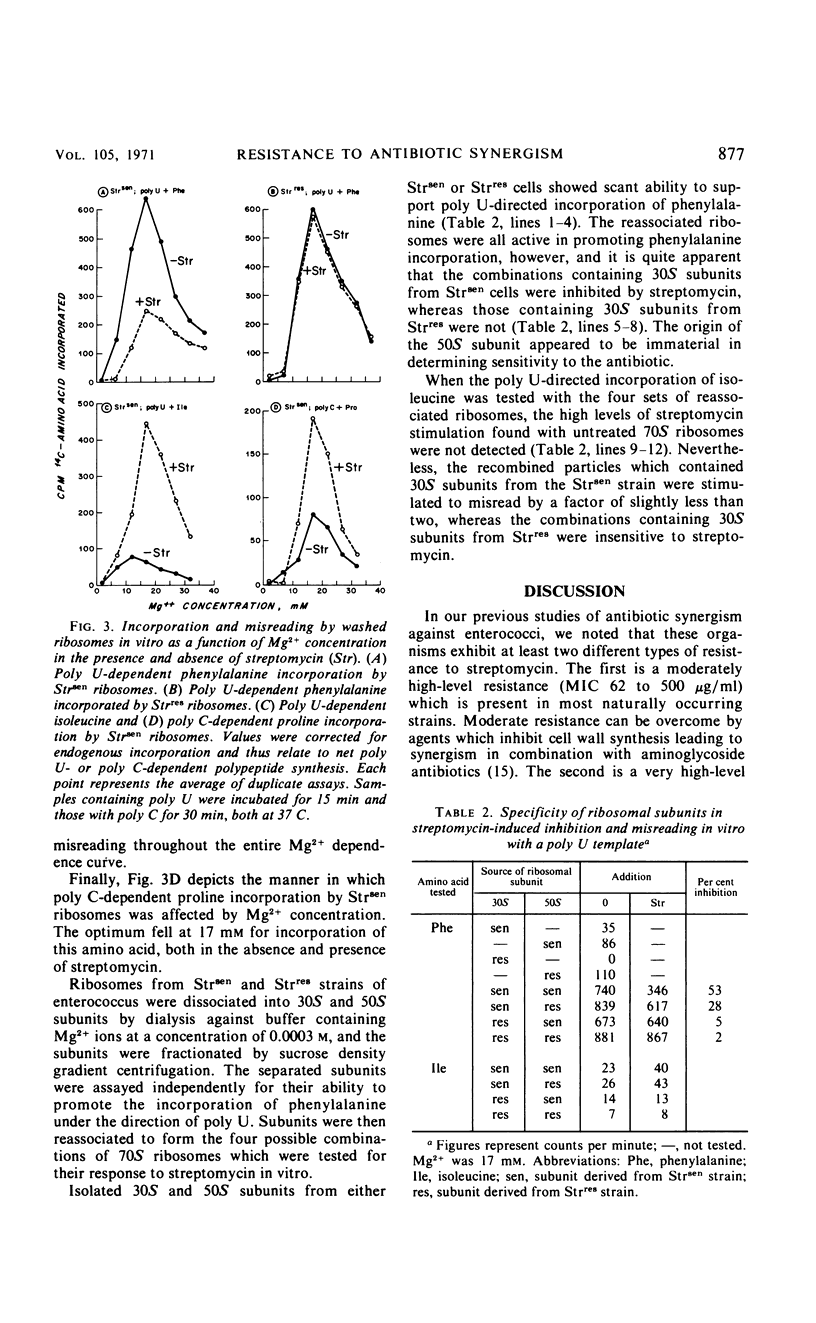
Enterococci exhibit two types of resistance to streptomycin. Moderately high-level resistance is observed in most naturally occurring strains and can be overcome by simultaneous exposure to penicillin. In addition, very high-level resistance is found in those strains against which penicillin plus streptomycin fail to produce synergism in vitro. To study the mechanism of streptomycin resistance in enterococci, ribosomes from a wild-type strain and from a highly streptomycin-resistant mutant were isolated, characterized, and studied in an in vitro amino acid incorporation system. The ribosomes from the organism with moderately high-level streptomycin resistance were sensitive to streptomycin in vitro, suggesting that this type of resistance is caused by failure of streptomycin to reach the ribosomes. Very high-level resistance (and lack of penicillin-streptomycin synergism), on the other hand, appears to be due to ribosomally mediated streptomycin resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX E. C., WHITE J. R., FLAKS J. G. STREPTOMYCIN ACTION AND THE RIBOSOME. Proc Natl Acad Sci U S A. 1964 Apr;51:703–709. doi: 10.1073/pnas.51.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., HANCOCK R., DAVIS B. D. THE SEQUENCE OF SOME EFFECTS OF STREPTOMYCIN IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 13;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- Davies J., Gorini L., Davis B. D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol. 1965 Jul;1(1):93–106. [PubMed] [Google Scholar]
- ERDOS T., ULLMANN A. Effect of streptomycin on the incorporation of amino-acids labelled with carbon-14 into ribonucleic acid and protein in a cell-free system of a Mycobacterium. Nature. 1959 Feb 28;183(4661):618–619. doi: 10.1038/183618a0. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WHITE J. R. Inhibition of polypeptide synthesis by streptomycin. Biochem Biophys Res Commun. 1962 May 11;7:385–389. doi: 10.1016/0006-291x(62)90320-0. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WITTING M. L., WHITE J. R. Polypeptide synthesis with ribosomes from streptomycin-resistant and dependent E. coli. Biochem Biophys Res Commun. 1962 May 11;7:390–393. doi: 10.1016/0006-291x(62)90321-2. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Ivanov D. A., Spirin A. S. Studies on the structure of ribosomes. 3. Stepwise unfolding of the 50 s particles without loss of ribosomal protein. J Mol Biol. 1966 Apr;16(2):473–489. doi: 10.1016/s0022-2836(66)80186-9. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J Mol Biol. 1966 Jul;18(2):356–371. doi: 10.1016/s0022-2836(66)80253-x. [DOI] [PubMed] [Google Scholar]
- Herson D. S., Pon C. L. Protein synthesis in cell-free extracts of Streptococcus faecalis. J Bacteriol. 1969 Dec;100(3):1350–1354. doi: 10.1128/jb.100.3.1350-1354.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawetz E. Combined antibiotic action: some definitions and correlations between laboratory and clinical results. Antimicrob Agents Chemother (Bethesda) 1967;7:203–209. doi: 10.1128/AAC.7.2.203. [DOI] [PubMed] [Google Scholar]
- Moore L. D., Umbreit W. W. Membrane-associated protein synthesis in Streptococcus faecalis. Biochim Biophys Acta. 1965 Jul 15;103(3):466–477. doi: 10.1016/0005-2787(65)90139-5. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Smith D. H. R factors for aminoglycoside antibiotics. J Infect Dis. 1969 Apr-May;119(4):378–380. doi: 10.1093/infdis/119.4-5.378. [DOI] [PubMed] [Google Scholar]
- TISSIERES A., WATSON J. D. Ribonucleoprotein particles from Escherichia coli. Nature. 1958 Sep 20;182(4638):778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]



