Abstract
Contraction and electrophysiological effects of 5-methylfurmethiodide (MFI), a selective muscarinic agonist in mammals, were tested on Ascaris suum muscle strips. In a contraction assay, MFI produced weak contraction and was less potent than levamisole and acetylcholine. Atropine (3 µM) a non-selective muscarinic antagonist in mammalian preparations, did not affect contractions produced by MFI. Mecamylamine (3 µM) a nicotinic antagonist in A. suum preparations, blocked the MFI contractions indicating that MFI had weak nicotinic agonist actions. In two-micropipette current-clamp experiments MFI, at concentrations greater than 10 µM, produced concentration-dependent depolarizations and small increases in membrane conductance. The depolarizing effects were not abolished by perfusing the preparation in a calcium-free Ascaris Ringer solution to block synaptic transmission, suggesting that MFI effects were mediated by receptors on the muscle and were calcium-independent. A high concentration of mecamylamine, 30 µM, only reduced the depolarizing responses by 42%, indicating that MFI also had effects on non-nicotinic receptors. Three non-nicotinic effects in the presence of 30 µM mecamylamine were identified using voltage-clamp techniques: i) MFI produced opening of mecamylamine-resistant non-selective-cation channel currents; ii) MFI inhibited opening of voltage-activated potassium currents; and iii) MFI increased the threshold of voltage-activated calcium currents. We suggest that a drug that is more selective for voltage-activated potassium currents, without effects on other channels like MIF, may be exploited pharmacologically as a novel anthelmintic or as an agent to potentiate the action of levamisole. In a larval migration assay we demonstrated that 4-aminopyridine (4-AP: a potassium channel blocker) potentiated the effects of levamisole but MFI did not.
Keywords: Methylfurmethiodide, Ascaris sum, Electrophysiology, Muscarinic receptors, Nicotinic receptors, Potassium channels, Calcium channels Levamisole, 4-aminopyridine
1. Introduction
The development of novel anthelmintics has been limited, so it is important to identify novel therapeutic lead compounds as well as methods for enhancing the potency of existing compounds that might counter drug resistance. The methods may include pharmacological agents that increase responses to existing anthelmintics. Previously our studies have focused on levamisole and related drugs. Levamisole belongs to the ionotropic cholinergic agonist group of anthelmintics that includes pyrantel, and that selectively produces muscle cell depolarization and spastic paralysis in parasitic nematodes (Aceves et al., 1970; Aubry et al., 1970). We have shown, using current-clamp, voltage-clamp and patch-clamp, that electrophysiological responses to these anthelmintics can be observed in body muscle cells of the nematode parasites Ascaris suum and Oesophagostomum dentatum (Martin, 1982; Pennington and Martin, 1990; Robertson and Martin, 1993; Robertson et al., 1994, 2002; Dale and Martin, 1995; Evans and Martin, 1996; Martin et al., 2002; Trailovic et al., 2002). These studies have identified nematode nicotinic acetylcholine gated receptor channels (nAChRs) over the nematode muscle cell surface that is selectively and directly gated by these anthelmintics. These studies have described, down to the single-channel level, the agonist action and channel-blocking action of the anthelmintics.
If it were possible to find a drug that could stimulate nematode metabotropic cholinergic (G-protein coupled, muscarinic-like) receptors, it might be developed as a stand alone anthelmintic, or it might be used with ionotropic cholinergic anthelmintics to increase their potency. There is evidence in A. suum of metabotropic cholinergic eceptors on muscle cells. Colquhoun et al. (1991) have tested a range of cholinergic agonists on the electrophysiology of A. suum muscle cells and found furtrethonium to be one of the more potent muscarinic agonists. Segerberg and Stretton (1993) have also found evidence of muscarinic-like receptors on A. suum muscle mediating depolarization and contraction; and Martin and Valkanov (1996) have described effects of acetylcholine potentiating the opening of mecamylamine-resistant non-selective cation channel currents (Ibcat) in isolated A. suum muscle bags. In Caenorhabditis elegans three G-protein coupled acetylcholine receptors (GAR-1, GAR-2 and GAR-3) have been reported (Hwang et al., 1999; Lee et al., 2000). GAR-3 is pharmacologically similar to mammalian muscarinic receptors.. GAR-1 and GAR-2 are pharmacologically unlike mammalian muscarinic receptors and are not antagonized by usual concentrations of muscarinic antagonists.
Colquhoun et al. (1991) tested effects on membrane potential of A. suum, using a number of mammalian cholinergic agonists including furtrethonium, muscarine, arecoline, pilocarpine, McN A343, bethanocol and oxtremorine. They found that furtrethonium was the most potent. We therefore selected 5-methylfurmethiodide (MFI, Fig. 1A) which is a potent mammalian muscarinic agonist (Newberry and Priestley, 1987) for further testing. In this paper we show that MFI in A. suum has four effects: i) it is a weak nicotinic agonist; ii) it opens mecamylamine-resistant non-selective cation channels; iii) it inhibits opening of voltage-activated potassium channels; and iv) it increases the threshold for activation of calcium channels. We discuss the significance of mecamylamine-resistant channels and receptor operated channels (ROCs: ROCs are part of a group of channels also known as TRPs). We also illustrate the use of inhibition of voltage-sensitive potassium channels as a method for potentiation of cholinergic anthelmintics.
Fig. 1.
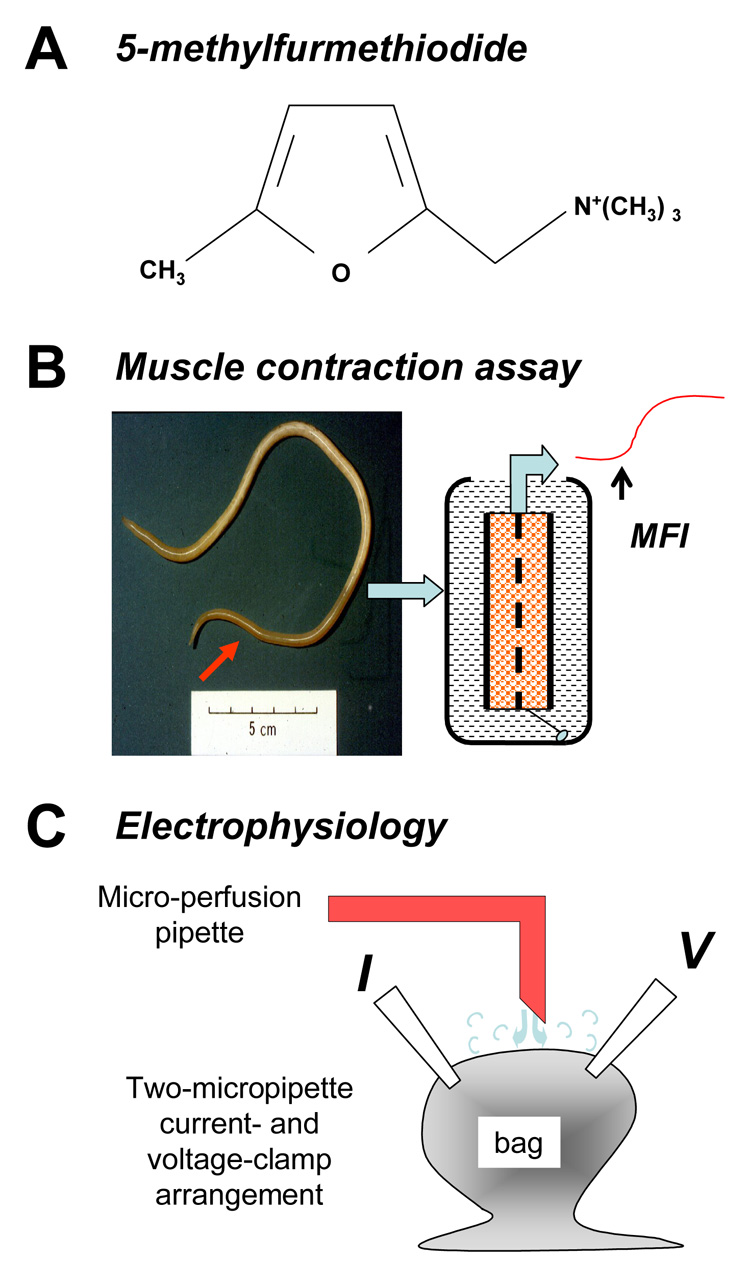
Different methods were used to investigate the pharmacology of 5-methylfurmethiodide (MFI). A) The chemical structure of MFI. B) Ascaris suum muscle strip preparations are dissected from the anterior region of the worm and mounted in a water bath for the contraction assay. C) Diagram of the two micro-pipette current-clamp and voltage-clamp recording from the bag region of the A. suum muscle cell and the application of solutions by microperfusion. The current-injecting micropipette (I) and voltage-sensing micropipette (V) are illustrated.
2. Materials and methods
2.1. Collection of worms
Adult A. suum (Fig. 1B), were obtained weekly from the Tyson pork packing plant at Storm Lake, Iowa. Worms were maintained in Locke’s solution [NaCl (155 mM), KCl (5 mM), CaCl2 (2 mM), NaHCO3 (1.5 mM) and glucose (5 mM)] at 32 °C. The Locke’s solution was changed daily.
2.2. Muscle contraction assay
Ascaris suum were used for the contraction studies within 72 h of collection, since the ability to contract vigorously to cholinergic agonists declined after this period. Two 1-cm body-flap preparations, one dorsal and one ventral, were made from each A. suum female from the region anterior to the genital pore (Fig. 1B). Each flap was monitored isometrically by attaching a force transducer in an experimental bath maintained at 37 °C containing 10 ml Ascaris Ringer solution: NaCl (23 mM), Na-acetate (110 mM), KCl (24 mM), CaCl2 (6 mM), MgCl2 (5 mM), glucose (11 mM), HEPES (5 mM), pH 7.6, adjusted with NaOH and bubbled with nitrogen. Eight baths were used simultaneously. After dissection, the preparations were allowed to equilibrate for 15 min under an initial tension of 2.0 g. The antagonist was then added to the preparation 15 min before the application of the first concentration of the agonist. We ran two controls (no antagonist) and three replicate antagonist concentrations during each experiment (eight preparations). The dorsal and ventral flaps were assigned randomly in the experiments to reduce any potential error associated with differences in receptor populations. The agonists were added cumulatively with 2–3 min intervals between applications and the responses were steady changes in tension. Results for individual flaps were rejected if the maximum change in tension, in response to agonist, did not exceed 0.5 g. The responses for each concentration were measured in grams and expressed in grams or as a % of the maximum tension produced by each individual flap preparation.
Changes in isometric muscle tension responses were monitored using a PowerLab System (AD Instruments) that consists of the PowerLab hardware unit and Chart for Windows software. The system allows recording, display and analysis of experimental data. Sigmoid dose-response curves for each individual flap preparation at each concentration of antagonist were described by the Hill equation and fitted using GraphPad Prism 4 software. The fits allowed estimation of EC50s (the concentrations producing 50% of the maximum response) with 95% confidence intervals.
2.3. Electrophysiology
A 1 cm long muscle tissue flap was prepared by dissecting the anterior part of the worm, 2–3 cm caudal to the head. The body muscle flap preparation was then pinned onto a 3 ml Sylgard lined Petri-dish. The intestine was removed to expose the muscle bags. The preparation (Fig. 1C) was continuously perfused, unless otherwise stated, with Ascaris Ringer solution. The preparation was maintained in the experimental chamber at 34 °C using a Warner Heating collar [DH 35] and heating the incoming perfusate with a Warner instruments (TC 324B) in-line heating system (Hamden, CT, USA). The perfusate was applied at 4–6 ml/min through a 19-gauge needle placed directly over the muscle bag from which recordings data were recorded. Calcium-free experiments were performed using calcium-free Ascaris Ringer solution (composition in mM: NaCl 23, Na-acetate 110, KCl 24, MgCl2 11, glucose 11, HEPES 5 and EGTA 0.5; NaOH was used to adjust the pH to 7.6). The sodium and calcium substitution experiments were conducted using n-methyl-d-glucamine Ringer (NMDG Ringer) solution (composition in mM: NMDG Cl 23, NMDG-acetate 110, KCl 24, MgCl2 11, glucose 11, HEPES 5 and 5 EGTA; CsOH was used to adjust the pH to 7.6). Calcium currents were recorded using modified Ascaris Ringer (calcium-Ringer) solution with an additional 5 mM 4-aminopyridine (4-AP) to reduce potassium currents.
Two-micropipette voltage-clamp and current-clamp techniques were employed to investigate whole cell currents in A. suum. Borosilicate capillary glass (Harvard Apparatus, Holliston, MA, USA) micropipettes were pulled on a P-97 Flaming Brown Micropipette puller (Sutter Instrument Co., Novato, CA, USA). The voltage-sensing and current-injecting micropipettes for the current–clamp and the voltage-sensing micropipettes for the voltage-clamp had resistances in the range 20–30 MΩ. The current-injecting micropipette for the voltage-clamp had a resistance of 4–6 MΩ to keep the gain high and to facilitate clamp quality. The recordings were obtained by impaling both electrodes in the bag region of the A. suum muscle. All experiments were performed using an Axoclamp 2B amplifier, 1320A Digidata interface and Clampex 8.2 software (Axon Instruments, Sunnywale, CA). All data were displayed and analyzed on a Pentium IV-based desktop computer. The amplifier gain for the voltage-clamp was more than 100 in all recordings. The phase lag was set to 1.5 ms in all experiments. In addition, muscles closer to nerve cord were impaled for experimental study as they were rounder with shorter arms. This allowed us to keep the membrane capacitance load low and to maintain the space-clamp. Only cells with stable membrane potentials more negative than −30 mV and input conductance less than 2.5 microsemen (µS) were selected for recording. To investigate the effects of MFI on currents at hyperpolarized and depolarized potentials at the same times, a ramped voltage protocol was used from a holding potential of −35mV. The voltage was stepped to −65 mV and over a period of 5 s brought to −15 mV before stepping back to −35 mV. Leak subtraction was not used for the ramped-voltage experiments.
To investigate effects on inward and outward voltage-activated currents, voltage-steps of 5 mV each were used. The step potentials were: −25 mV, −20 mV, −15 mV, −10 mV, −5 mV, 0 mV, +5 mV, +10 mV, +15 mV and + 20 mV. Step potentials up to 150 ms were used for observing the outward potassium currents; step potential up to 50 ms were used for the inward calcium currents. The holding potential was −35 mV and leak subtraction was used to display the voltage-activated currents (Verma et al., 2007). Outward potassium currents were observed using 3 M potassium acetate in the recording micropipettes. Calcium currents were observed using a combination of 1.5 M potassium acetate and 1.5 M cesium acetate in recording micropipettes; the cesium acetate was included in pipette solution to block outward potassium currents. For all voltage-clamp experiments, the drug applications were done in a current-clamp for a minimum of 2 min before moving onto a voltage-clamp. For current-clamp experiments a hyperpolarizing pulse of 40 Nan amps (nA) was injected for 500 ms at 0.28 Hz frequency through the current-injecting micropipette and the other micropipette recorded the change in membrane potential.
2.4. Larval migration studies
Levamisole-sensitive O. dentatum were originally supplied by the Royal Veterinary and Agricultural School, Frederiksberg, Copenhagen and then reproduced at 6–9 month intervals by passage in pigs at Iowa State University, Ames, Iowa. The L3 isolates were maintained between passages in tap water refrigerated at 11 °C (changed every 2–4 months). The pigs were infected and, following collection of the parasites, killed humanely: all animal procedures complied with and were governed by Institutional Animal Care and Use Committee (IACUC) regulations under U.S. Federal laws and policies. The policies include formal written prior approval by the IACUC committee of the procedures as well as veterinary supervision of the animals.
Between 1,500–3,000 L3s were ex-sheathed by 5–10 min incubation in 1.5% sodium hypochlorite solution. The larvae were then washed three times in migration buffer (composition: 0.85% NaCl, 5 mM Tris-HCl, pH to 7.0 with 1 M NaOH) with the aid of centrifugation (5 min 1,000 g). Approximately 150 larvae were collected with a pipette and placed in each of the drug concentrations to be tested for 2 h at 37°C After this incubation, the larvae were re-suspended in fresh test solutions. A migration apparatus consisting of two tightly fitting plastic tubes (~ 10 mm length) that secured a 20 µm nylon filter (Small Parts Inc. Miami Lakes, Fl.) was placed in each test solution of a 24-well plate. The re-suspended larvae were slowly added to the top of each filter, allowed to migrate through the filters and into the wells during a 2 h incubation at 37 °C. At the end of the incubation period, the number of larvae remaining within each of the filter tubes was counted and the number of larvae entering into the 24 well plates were counted. We then calculated the percentage of larvae not migrating for each of the concentrations. The relationship between the concentration of levamisole and the percentage of inhibited larvae was then examined by fitting sigmoidal dose-response curves.
2.5. Drugs
A MFI, (Tocris Bioscience, Ellisville, Missouri) 3 mM stock solution was prepared every week and kept in 1.5 ml micro-tubes at −20 °C. The MFI stock solution was thawed just before use for experiments. All other drugs were obtained from Sigma-Aldrich, St. Louis, MO. All the stock solutions were made using double distilled water.
2.6. Statistical analysis
Currents were plotted as a function of step potential to generate I–V curves. All statistical analysis was done using Graph Pad Prism software (version 4.0, San Diego, CA, USA). Data were fitted with Boltzmann sigmoidal equation: I = Imax / {1 + exp [(V50 –V)/Kslope]}. The EC50 concentrations for levamisole, acetylcholine and MFI were determined by fitting the standard sigmoidal dose-response relationship (slope = 1) using Graph Pad Prism software. Paired t-tests were used to test significant differences in responses with control and test recordings in the same preparation. Observations given are mean ± S.E.M. Minitab was used for analysis of variance using the general linear model to test the significance of effects of MFI and 4-AP in the larval migration studies. P values < 0.05 were considered significant.
3. Results
3.1. Contraction
MFI was less potent than levamisole and acetylcholine in the contraction assay. Fig. 2A shows the contraction-concentration plots for levamisole, acetylcholine (with 3 µM neostigmine to block cholinesterase) and MFI. The EC50s (with 95% confidence intervals) were: levamisole: 0.7 (0.5 – 1.1, n = 18 preparations) µM; acetylcholine 3.1 (1.9 – 4.9, n = 24 preparations) µM; and MFI 221 (120– 410, n = 18 preparations) µM. MFI was not a full agonist and produced a maximum contraction that was approximately 30% of that produced by levamisole.
Fig. 2.
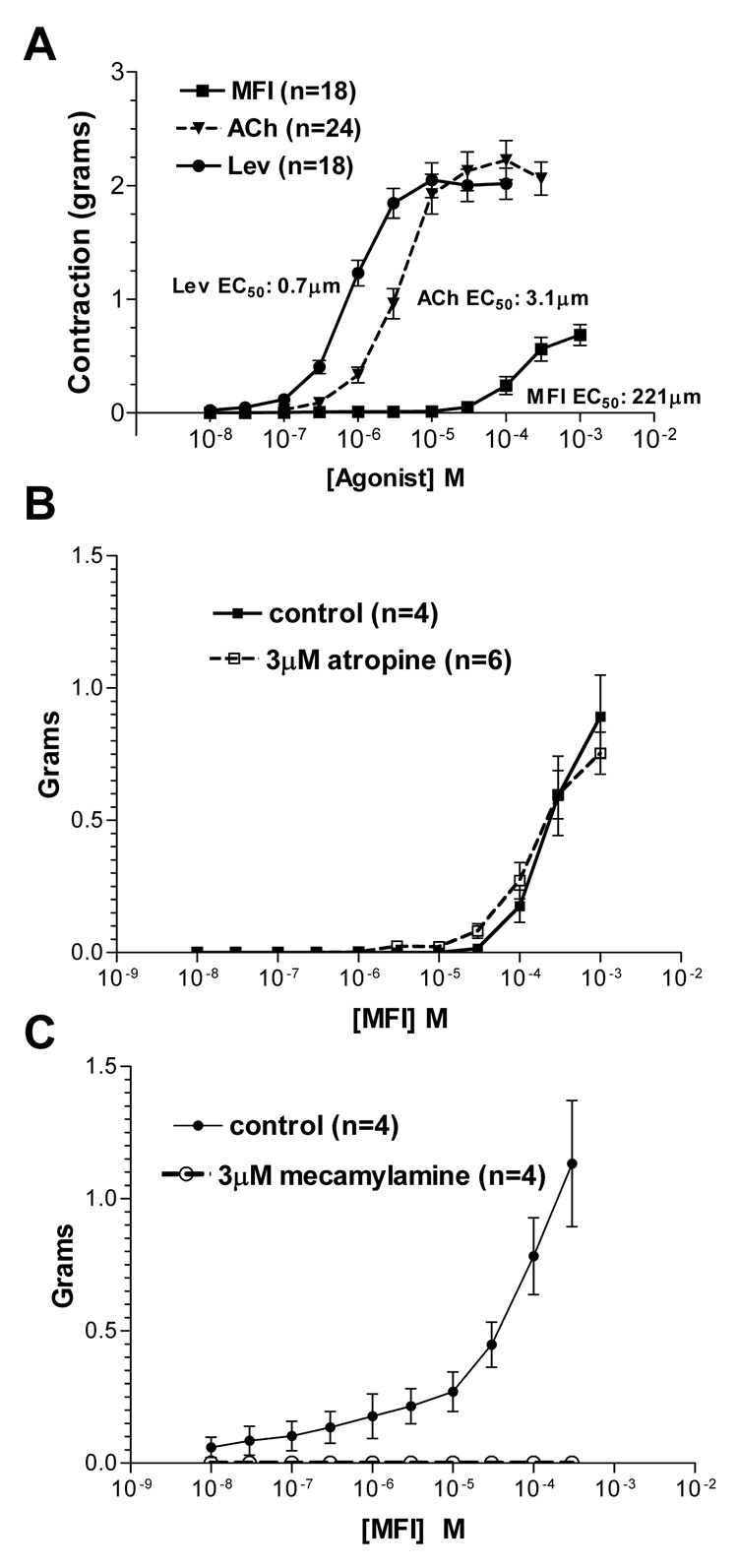
The effects of 5-methylfurmethiodide (MFI) on Ascaris suum muscle flap contraction were measured in the presence of different pharmacological antagonists A) Effect of MFI (■), acetylcholine (ACh) with 3 µM neostigmine to block cholinesterase (▼)and levamisole (Lev) (●) on contraction of Ascaris suum muscle strips. EC50s: MFI 221µM; acetylcholine, 3.1 µM, levamisole, 0.7 µM. Mean ± standard error of maximum contractions are shown. MFI is not a full agonist and does not produce a maximum contraction. B) Atropine (3 µM applied 10 min before and maintained in the presence of the agonist) had no inhibitory effect on MFI muscle contractions. Control (■); 3 µM atropine (□). C) Mecamylamine (3 µM) completely antagonizes the contractions of MFI. Control (●); 3 µM atropine (○).
Atropine (3 µM) did not inhibit (control: n = 4 preparations; atropine n = 6 preparations) the contractile effects of MFI (Fig. 2B) showing that the effects were not mediated by classic muscarinic receptors. However 3 µM mecamylamine (Fig. 2C), a nicotinic antagonist, completely blocked (n = 4 preparations) contractions produced by MFI, showing that MFI has weak nicotinic effects, sensitive to low concentrations of mecamylamine.
3.2. MFI depolarizations (current-clamp)
Fig. 3A shows a representative recording of the effect of application, by rapid microperfusion, of 300 µM MFI on the membrane potential and input conductance of an A. suum muscle cell. The dark central trace is the membrane potential which had a resting value of −35 mV; the downward deflection is the voltage response to the injected current, showing that the resting input conductance was 2.4 µS; the upward deflection is the anode break spike that followed the end of the 40 nA hyperpolarizing current pulse. The response, despite rapid MFI application, was a slow depolarization of 13 mV associated with a very small conductance increase of less than 0.1 µS; the response took 75 s from onset to reach its peak and was reversible on washing. The slow nature of the response to rapidly-applied MFI by A. suum muscle suggests that most of the response may not be mediated directly on ligand-gated channels (Purcell et al., 2002). The anode break spikes increased in amplitude during the application of the MFI as shown in Fig. 3A where there was an increase from 11 mV to 17 mV. Similar effects on anode break spikes were observed in the other preparations. One possible explanation for the effects on anode break spikes is an effect of MFI on voltage-sensitive currents associated with the spiking mechanism and this possibility was investigated (see sections 3.6, section 3.7 and section 3.8).
Fig. 3.
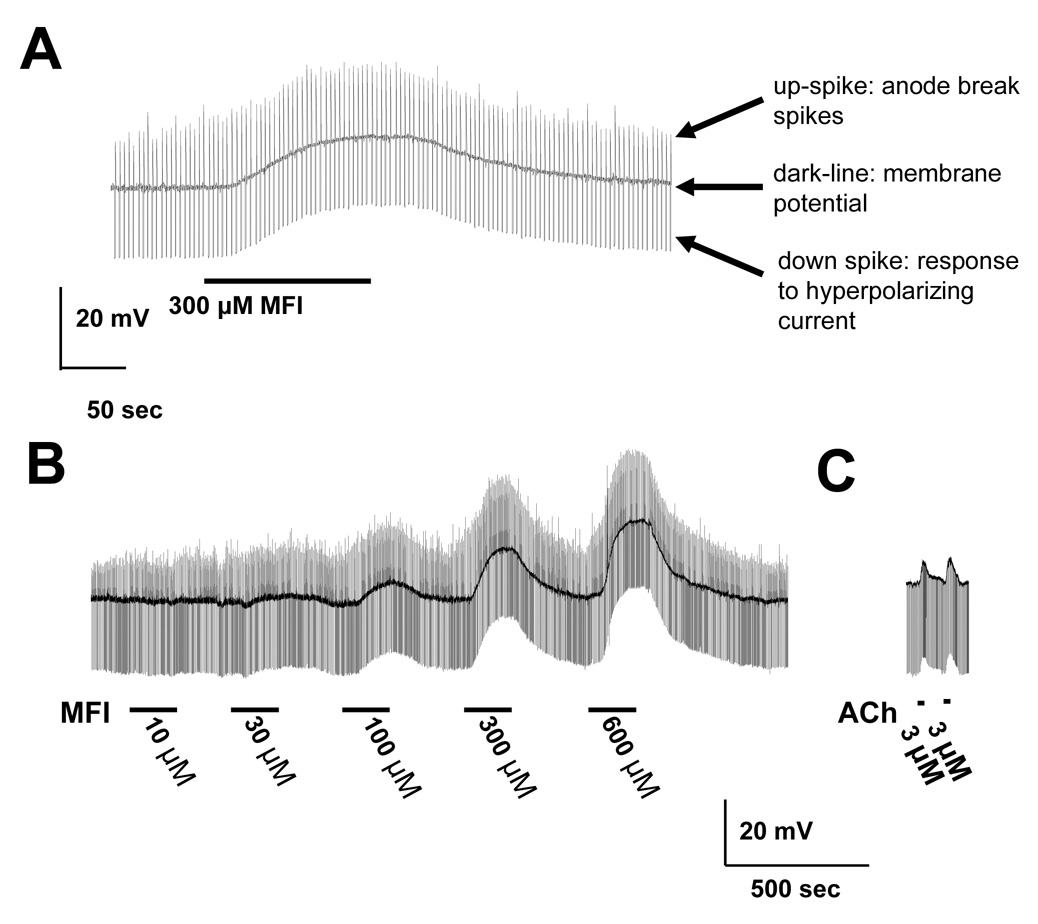
Effect of 5-methylfurmethiodide (MFI) on membrane potential and conductance. A) Effect of 300 µM MFI in the presence of 6 mM calcium. A 2-min application of MFI produces a 10 mV depolarization and a small change in input conductance (downward pulses) but a clear increase in the anode break spikes was produced at the end of each hyperpolarizing potential response to the injected current pulse. The central trace is the membrane potential, −35 mV; the downward deflection is the voltage response to injected current. The resting input conductance was 2.4 µS; and the upward deflection is the anode break spike that followed the end of the 40 nA hyperpolarizing current pulse. There was a slow 13 mV depolarization associated with a very small conductance increase of 0.1 µS; the response took 75 s form onset to reach its peak. The anode break spikes increased in amplitude during MFI application from 11 mV to 17 mV. B) Lower time-resolution recording of a representative recording of applying increasing concentrations of MFI on the membrane potential and resting conductance of the muscle cell. There was little effect at 10 µM MFI but a noticeable effect at 30 µM MFI; again there was a very slow onset and offset of the response. C) Representative recordings of the effect of acetylcholine (ACh) on membrane potential. Note that the time course is faster than that of MFI. The faster time-course (onset and offset) of the depolarizing response to 3 µM acetylcholine with the peak occurring with our application method within 9 s after onset.
Fig. 3B shows a representative recording of the effect of different concentrations of MFI on the membrane potential and resting conductance of the muscle cell. There was little effect at 10 µM MFI but a more noticeable effect at 30 µM MFI; again there was a very slow onset and offset of the response. The depolarizations were concentration-dependent and reversible on washing. Fig. 3C shows, for comparison, a recording from another preparation, the faster time-course (onset and offset) of the depolarizing response to 3 µM acetylcholine with the peak occurring in less that 9 s; the MFI response was slower, suggesting a different mechanism of action. The peak MFI concentration-depolarization relationships are shown in Fig. 4; the relationships were determined in normal Ascaris Ringer solution and in Ca-free Ascaris Ringer solution and were not distinguishable. The concentration-conductance relationships (Fig 4 inset) in normal Ascaris solution and in Ca-free Ascaris Ringer solution, however, suggested that the presence of calcium could increase the MFI-induced conductance change, but we did not analyze this effect further. The fact that removal of calcium did not abolish the MFI response suggests that the effects of MFI are direct on muscle receptors and are not mediated trans-synaptically because calcium is required for synaptic transmission. The EC50 in the presence of calcium was 151 µM (95% confidence intervals 77 – 262 µM, degrees of freedom (df) 23, six preparations) which was not significantly different in calcium-free Ascaris Ringer solution, 191 µM (95% confidence intervals 76 – 540 µM, df 23, six preparations). We also determined conductance-concentration plots in the presence and absence of calcium (Fig. 4 inset). The conductance changes associated with the depolarization responses were small, ranging up to 0.1 µS over the concentration range. The input conductance changes associated with nicotinic effects of acetylcholine are usually larger, up to 2.5 µS in A. suum muscle (Colquhoun et al., 1991), suggesting again that the receptor population stimulated by MFI is not entirely nicotinic. The MFI conductance EC50 in the presence of calcium was 71 µM and in calcium-free Ringer solution it was 58 µM. The lack of major effects of calcium removal on membrane potential suggests that calcium is not the major charge carrier mediating the depolarization.
Fig. 4.
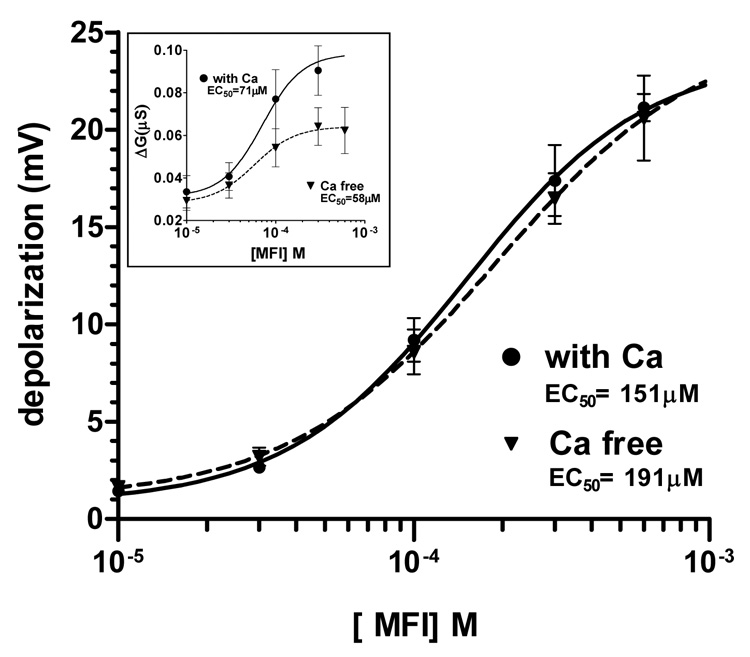
Peak 5-methylfurmethiodide (MFI) depolarization-concentration relationships. The relationships were determined in normal Ascaris Ringer solution and in calcium (Ca)-free Ascaris Ringer solution. The EC50 in the presence of calcium was 151 µM and in calcium-free solution, it was 191µM. Number of preparations, n = 6. Inset: MFI-conductance-concentration plots in the presence and absence of calcium. The conductance change associated with the depolarization responses was small, up to 0.1 µS over the concentration range. The MFI EC50 in the presence of calcium was 71 µM and in calcium-free solution it was 58 µM.
3.3. Effect of atropine and mecamylamine on MFI depolarizations
Next, we tested effects of a high concentration of atropine, 30 µM, on the MFI depolarization response and found that it only had a small, but statistically significant, inhibitory effect (Fig. 5A). The mean response to 100 µM MFI was 9.8 ± 0.8 mV (n = 4) and in the presence of 30 µM atropine it was 7.4 ± 0.8 mV (n = 4, P < 0.001, paired t-test). These observations suggest that MFI did not mediate most of its effect on membrane potential by activating muscarinic (atropine-sensitive) receptors.
Fig. 5.
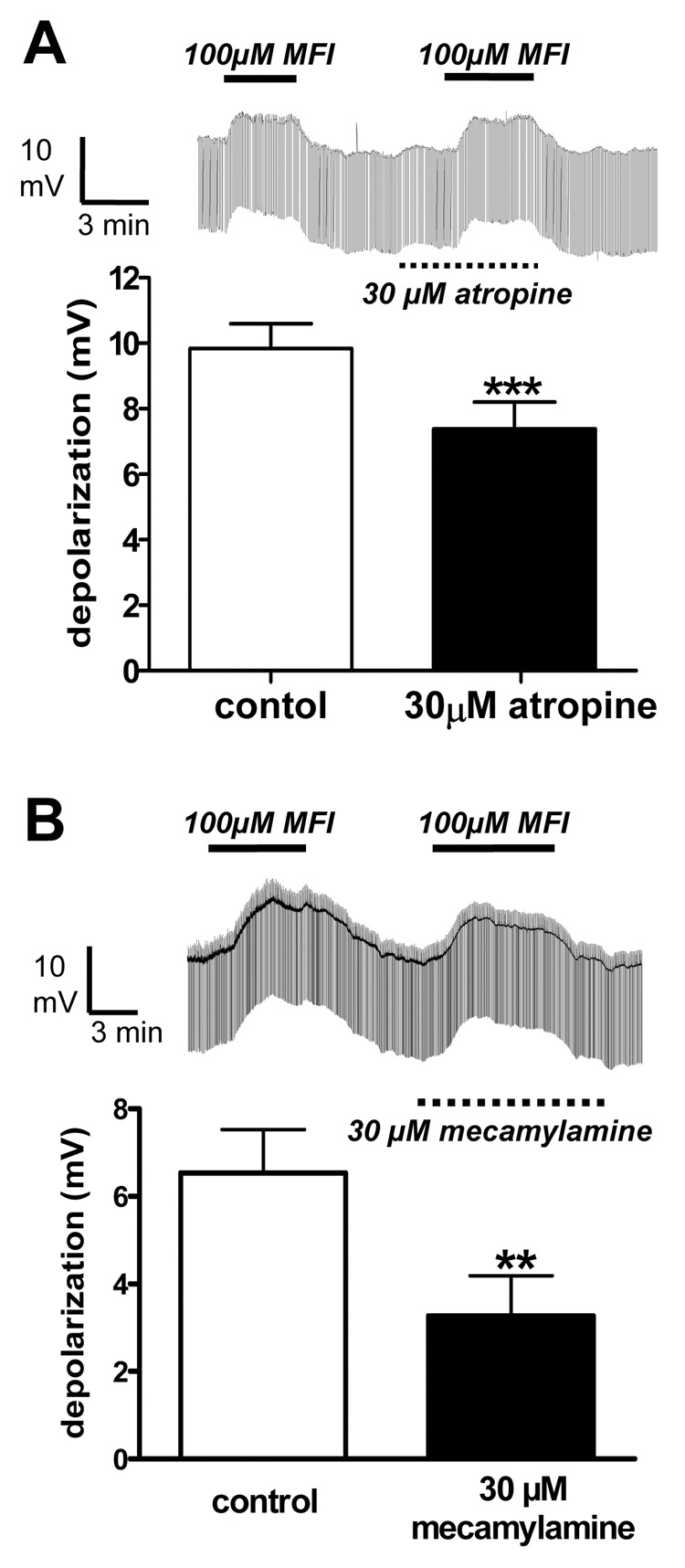
Effects of atropine and mecamylamine on the membrane potential and conductance changes produced by 5-methylfurmethiodide (MFI). A) Thirty µM atropine has a small but significant effect on the depolarizing responses to 100 µM MFI. Representative trace and bar chart (n = 8). Control, 9.8 ± 0.8 mV; + atropine, 7.4 ± 0.8 mV. B) Thirty µM mecamylamine reduces, but does not completely block, depolarizing responses to 100 µM MFI. Representative trace and bar chart (n = 4). Control, 6.5 ± 1.0 mV; + mecamylamine, 3.3 ± 1.0 mV.
When we tested the effects of a high concentration of mecamylamine (30 µM), it reduced but did not block depolarizing responses to 100 µM MFI (Fig. 5B); the mean response before application of mecamylamine was 6.5 ± 1.0 mV (n = 4); in the presence of mecamylamine the mean response was 3.3 ± 0.9 (n = 4, P < 0.01, paired t-test). Since mecamylamine is a potent nicotinic antagonist in A. suum (Colquhoun et al., 1991), the modest antagonism of mecamylamine suggests that only part of the MFI response is mediated by nicotinic receptor channels; most of the response is mediated by other mecamylamine-resistant receptor ion-channel effects. In order to isolate and characterize the ionic mechanisms involved we conducted voltage-clamp experiments to examine effects on specific voltage-activated currents.
3.4. Ramp voltage-clamp experiments
Initial voltage-clamp experiments showed that 100 µM MFI induced a steady inward current at a holding potential of −35 mV (mean 57 ± 9 nA) and that this was significantly reduced, but not blocked, (mean 42 ± 5 nA) in the presence of 30 µM mecamylamine (P < 0.001, paired t-test, n = 7). We subsequently used 30 µM mecamylamine in the voltage-clamp experiments to block nicotinic effects and isolate the non-nicotinic effects of MFI. Fig. 6 shows sections of a recording made during the ramp voltage-clamp experiments. The top trace (Fig. 6A) shows the ramp protocol that was used: the potential was held at −35 mV between the ramp-voltages, near to the normal resting membrane potential; there was then a step to −65 mV followed by a ramped depolarization over a 5 s period to −15 mV; the membrane potential was then stepped back to −35 mV. These ramp pulses were repeated after 5 s. The middle trace (Fig. 6B) shows current traces: i) control, just before the application of MFI; and ii) during the plateau phase of the MFI response. Following washout of MFI, the currents returned towards control levels (data not shown).
Fig. 6.
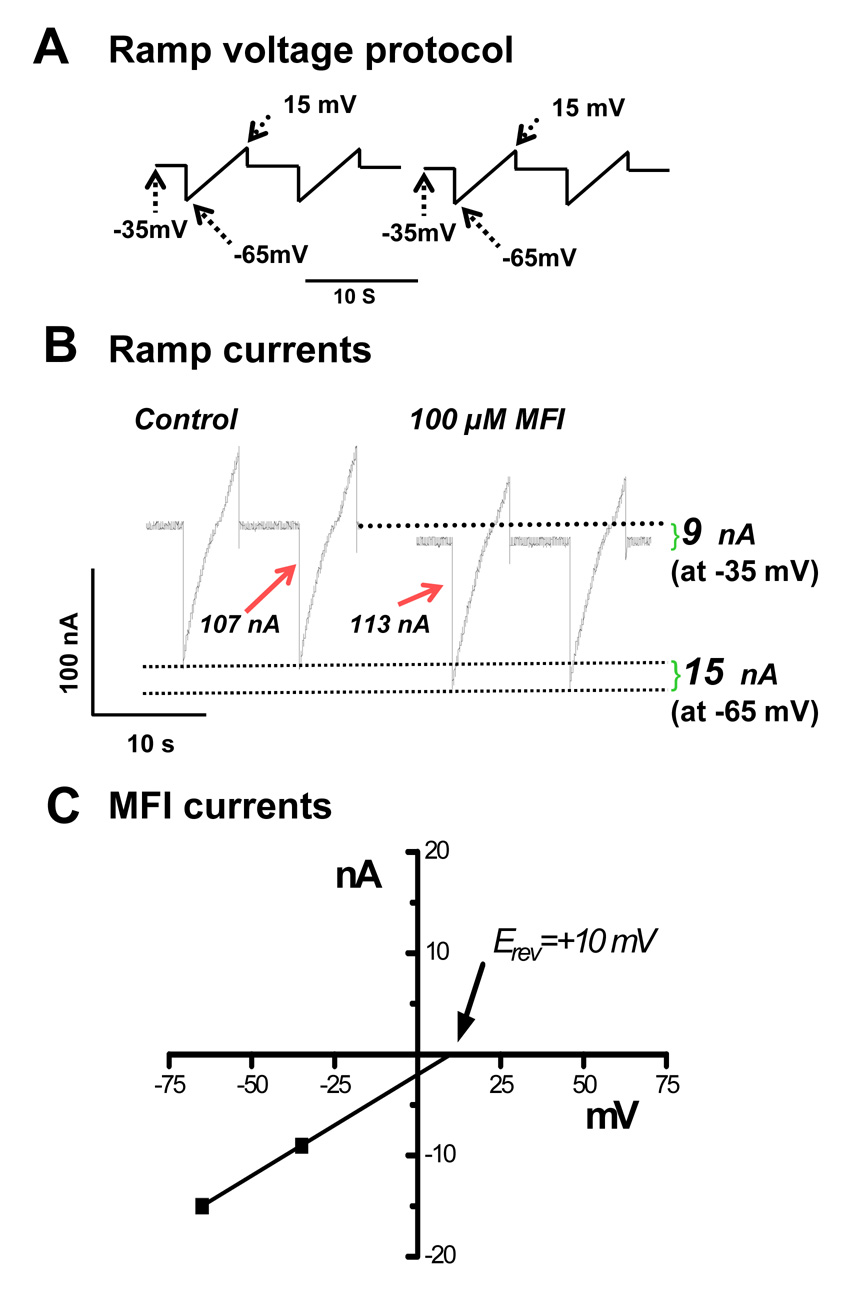
Ramp-voltage clamp experiments used to investigate the effects of 5-methylfurmethiodide (MFI). A) Ramp-voltage protocol used to view effects of MFI on bag currents. The holding potential was −35 mV; the ramp began at −65 mV, increased to −15 mV and then returned to −35 mV. B) Effect of 100 µM MFI on voltage-ramp activated currents under voltage-clamp in the presence of 30 µM mecamylamine to block nicotinic effects. The holding current increased by −9 Nan amps (nA) at −35 mV and by −15 nA at −65 mV. The current record in B shows that the current-step associated with the voltage-step, −35mV to −65mV, increased from 107 nA in the control to 113 nA in the presence of 100 µM MFI, showing that MFI increased the membrane conductance at −35 mV. At −15 mV the current-step associated with the −15 mV to −35mV voltage-step decreased from 58 nA in the control to 46 nA in the presence of MFI, showing that MFI decreased the conductance of the membrane at −15 mV that could be associated with potassium channel block. The effect was reversible on washout (data not shown). C) Estimation of the reversal potential (Erev) of the MFI current induced at potential more negative than −35 mV. Extrapolation of the MFI-induced currents at −35 and −65 mV gave +10mV as the reversal potential. Because of the composition of the intracellular and extracellular ions, the reversal potential is consistent with the current occurring due to opening of a non-selective cation channel. The high concentration of mecamylamine suggests that this is not due to a nicotinic channel.
3.5. MFI opens a mecamylamine-resistant non-selective cation channel
Fig. 6B shows the ramp currents that were displayed without leakage correction. At −35 mV during the application of MFI, there was an increase in the inward holding current of 9 nA (follow the upper dashed line in Fig. 6B). At −65 mV during the application of MFI, the inward current increased by 15 nA (follow the lower dashed line). The increases in current are plotted in Fig. 6C and extrapolated to estimate the reversal potential (Erev: +10 mV) of the MFI current. The mean reversal potential in experiments on separate preparations was −6.0 ± 5.8 mV (n = 5). Additionally, the current associated with the −35 mV to −65 mV voltage-step increased from −107 nA (control) to 113 nA in the presence of 100 µM MFI and 30 µM mecamylamine. In similar experiments using five different preparations, the mean increase in the current step was 12.2 ± 3.6 nA and it was statistically significant (paired t-test, P < 0.05, n = 5). For a 30 mV step, the increased current step seen in the ramp experiments corresponds to a mean membrane conductance increase of 0.4 µS. The increased conductance and mean Erev of −6 mV can be explained by MFI opening non-selective cation channels (Martin and Valkanov, 1996) which are mecamylamine-resistant. We also considered the possibility that the effect could be mediated by an increase in chloride channel conductance but this is unlikely since the estimated reversal potential would be more negative than −32 mV (based on an intracellular chloride concentration less than 20 mM (Brading and Caldwell 1971), and an extracellular concentration of 69 mM as used in our experiments). Thus one effect of MFI is to open mecamylamine-resistant, non-selective cation channels.
3.6. MFI also inhibits voltage-activated channel currents
Further inspection of the ramp currents (Fig. 7A) shows that the current step associated with the −15 mV to −35mV voltage-step decreased from 58 nA in the control to 46 nA in the presence of MFI. In experiments using five separate preparations, the mean reduction in the current step was 9.0 ± 1.1 nA and it was statistically significant (paired t-test, P < 0.05, n = 5). For the 20 mV step, the reduction in current step corresponds to a reduction in membrane conductance of 0.45 µS. Depolarization is known to activate voltage-sensitive potassium and voltage-sensitive calcium channels in A. suum muscle (Martin et al., 1992). The reduced current step (decreased membrane conductance) from −15 mV suggests that additional effects of MFI are to inhibit opening of voltage-sensitive potassium and/or voltage-sensitive calcium channels. We investigated this possibility in the next series of experiments.
Fig. 7.
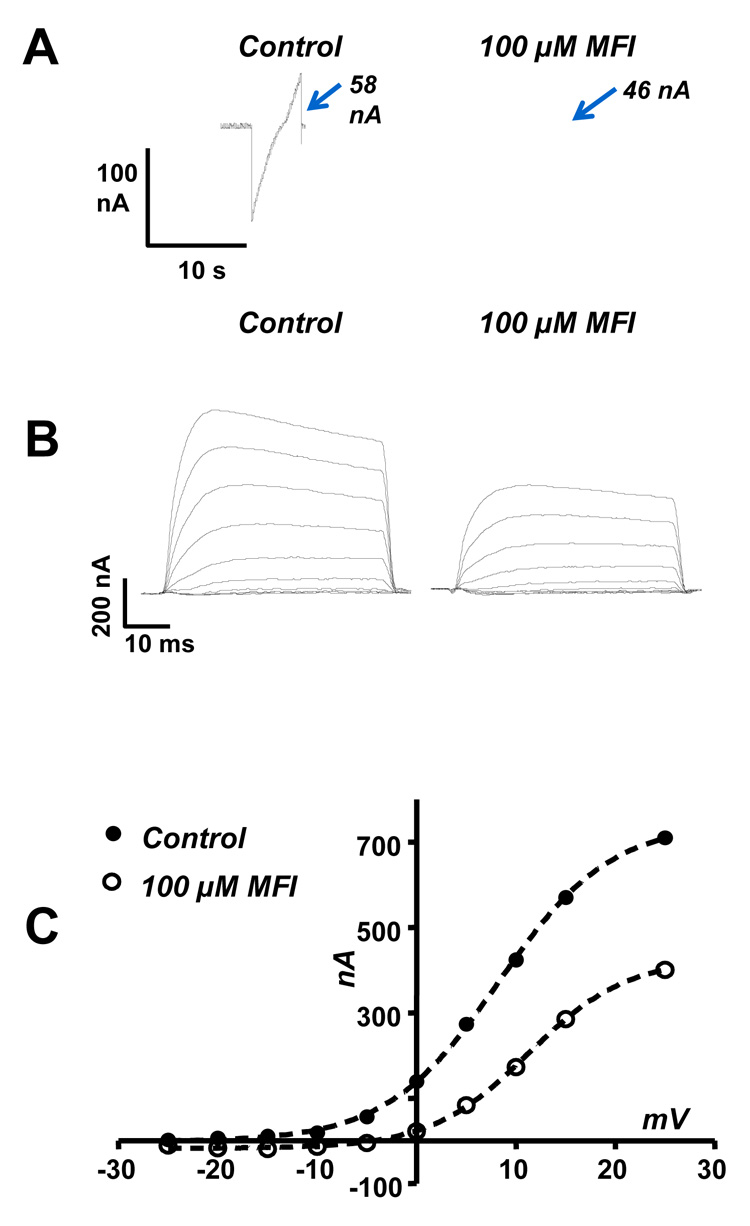
Effect of 5-methylfurmethiodide (MFI) on depolarization-activated potassium currents in n-methyl-d-glucamine (NMDG) Ringer solution. A) Ramp currents from the experiment shown in Fig. 6B. Note that there is a reduction from 58 Nan amps (nA) to 46 nA in the current step associated with the voltage step −15 to −35 mV indicating that 100 µM MFI limits opening of voltage-activated currents at depolarized potentials. B) The muscle cell was held at −35 mV and rectangular depolarizing steps were made to different potentials in 5 mV steps to activate the potential dependent potassium channels. The control currents reduced in the presence of 100 µM MFI and increased again on wash. C) Peak outward current-voltage plots: control (●) and in the presence of 100 µM MFI (○). The plots are fitted with the Boltzmann equation (equation-1) to obtain estimates of the constants (see Table 1). MFI inhibits the outward current by reducing the maximum current and decreasing the half-activation potential.
3.7. MFI inhibits opening of voltage-activated potassium currents
To isolate effects of MFI on voltage-activated potassium currents, we used a NMDG-Ringer to block voltage-activated inward currents (Martin et al., 1992; Verma et al., 2007) in the presence of 30 µM mecamylamine. Fig. 7B shows the inhibitory effect of MFI on voltage-activated potassium currents activated by rectangular depolarizing step potential from a holding potential of −35 mV. Fig. 7C plots peak outward currents against the step potential for the control and in the presence of 100 µM MFI. The plots were described by the Boltzmann equation (1):
| (1) |
where I the peak current, Imax the maximum current V50 is the potential that produced the half-maximum effect, V is the step potential and Kslope is the slope of the plot. Table 1 shows the values estimated for the Boltzmann equation for the control and in the presence of MFI for the experiment shown if Fig. 7C. When the data from five different preparations were pooled we found that: i) the V50 significantly increased (P < 0.05, paired t-test n = 5) from a mean of 7.7 ± 0.9 mV to 11.1 ± 1.2 mV; ii) the Imax significantly decreased (P < 0.05, paired t-test n = 5) from a mean of 495 ± 138 to 311 ± 114 nA; and iii) that Slope decreased significantly (P < 0.05, paired t-test, n = 5) from 6.4 ± 0.4 nA.mV−1 to 4.9 ± 0.6 nA.mV−1. Imax V50 and Kslope were all changed by MFI.
Table 1.
The constant values for equation (1), the Boltzmann equation, obtained by fitting the activation curve for the voltage-activated potassium currents.
| Control | 100 µM MFI | |
|---|---|---|
| Imax | 745 nA | 427 nA |
| V50 | 8.3 mV | 11.3 mV |
| Kslope | 5.6 nA mV−1 | 4.9 nA mV−1 |
Note that 100 µM 5-methylfurmethiodide (MFI) reduced the maximum activated current and raised the V50 threshold for activation of the current.
3.8. MFI also raises the threshold for activation of voltage-activated calcium currents
Fig. 8A shows the reversible effect of 100 µM MFI on voltage-activated calcium currents. Fig. 8B shows the current-voltage relationships of the calcium currents and that the effect of 100 µM MFI was to increase the threshold for activation of the calcium current so that a greater depolarization was normally required to activate the same currents. Notice that between −20 mV and −5 mV the calcium current is reduced by MFI but at 0 mV the current is the same. Similar increases in the threshold for activation of the calcium currents produced by MFI were observed in experiments using four separate preparations. The mean voltage-activated calcium current activated by a step from a holding potential of −35 mV to a step potential of −5 mV was −99.9 ± 10.6 nA; in the presence of 100 µM MFI it was reduced significantly (P < 0.05, paired t-test, n = 4) to −75.1 ± 13.1 nA (Fig. 8C) and this returned to −97.6 ± 11.0 nA on washout.
Fig. 8.
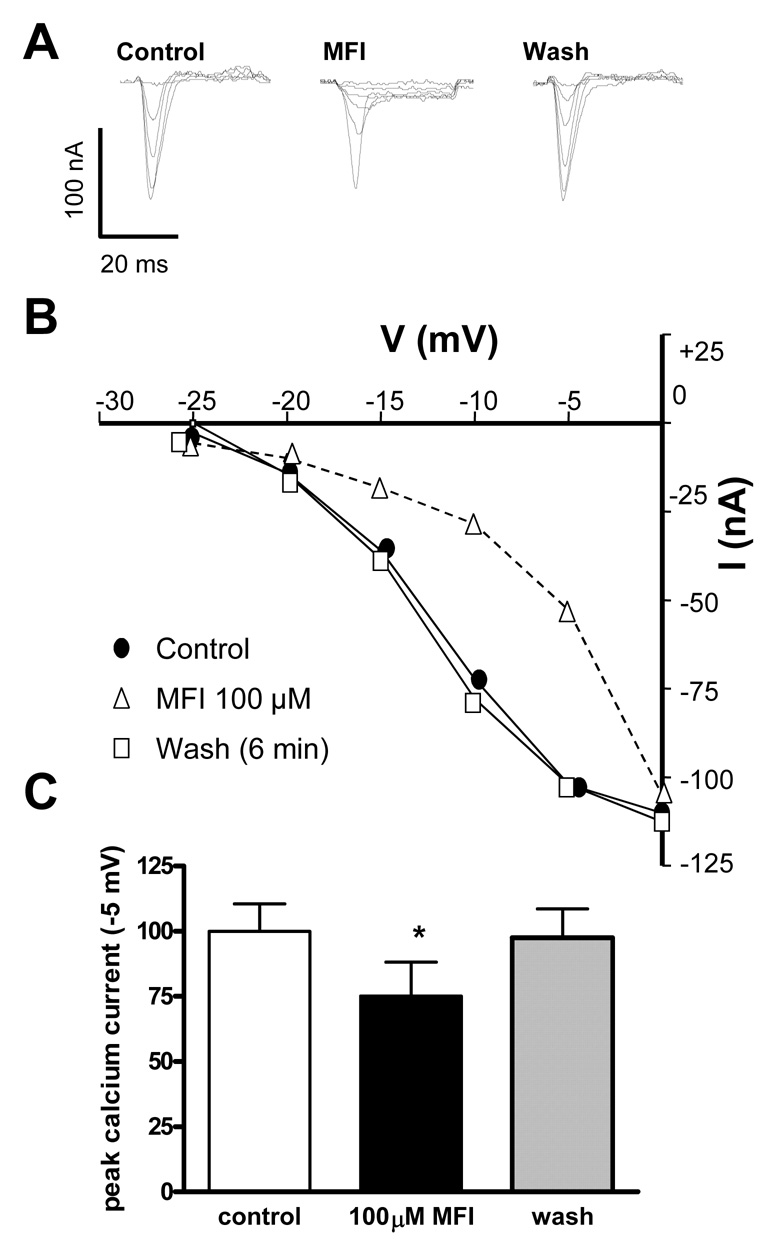
One hundred µM 5-methylfurmethiodide (MFI) increases the threshold for activation of voltage-activated calcium currents. A) Control currents (left) activated by step potentials from a holding potential of −35 mV. Currents in the presence of 100 µM MFI (middle) and following washout (right). B) Plot of the peak inward calcium current at different step potentials. Control (●), 100 µM MFI (Δ), wash (□). Note that it takes a greater depolarization step to activate the current. C) Bar chart of the peak calcium current at −5 mV in the control, presence of 100 µM MFI and following washout. MFI had a significant (P < 0.05 paired t-test n = 4) effect compared with control and wash.
3.9. Effects of MFI and 4-AP on levamisole inhibition of larval migration
One of our longer-term aims has been to identify pharmacological mechanisms that could be used to design novel therapeutic agents or to potentiate the effects of levamisole. However, MFI produced a number of effects on channel currents which were mutually antagonistic: the effect on inhibiting potassium channels would be excitatory, but the effects on calcium currents would be inhibitory. We were therefore interested to with see if the anthelmintic effects of levamisole could be potentiated by addition of a selective potassium channel blocker and to compare effects of MFI. Fig. 9 shows the effects of 100 µM MFI and 1 mM 4-AP (selective potassium channel blocker) on levamisole concentration-response plot in larval migration assays. The effect of MFI was statistically significant: it inhibited the effects of levamisole (P < 0.001, f-test, df 1, 62). The effects of 4-AP were also statistically significant and it potentiated the effect of levamisole (P < 0.001, f-test, df 1, 62). The levamisole control had an EC50 of 173 µM (134 – 222 µM: 95% confidence interval, n = 3 separate experiments); in the presence of MFI the levamisole EC50 was 1,062 µM (531 – 2,124 µM: 95% confidence interval n = 3 separate experiments); in the presence of 1 mM 4-AP, the levamisole EC50 was 47 µM (29 – 75 µM 95% confidence interval, n = 3 separate experiments). The EC50s were significantly different, with non-overlapping confidence intervals. We found that 1 mM 4-AP was near threshold for inhibitory effects on migration. At low concentrations of levamisole, MFI had little effect but at higher levamisole concentrations it acted as a levamisole antagonist. The antagonism may be due to MFI acting as a partial (weak) nicotinic agonist or due to inhibition of calcium currents. These actions appear to limit the ability of MFI to potentiate the actions of levamisole. However, it was clear that 4-AP potentiated the effects of levamisole. Addition of 4-AP made levamisole 3.7 times more potent when comparing EC50s due to antagonism of the potassium current.
Fig. 9.
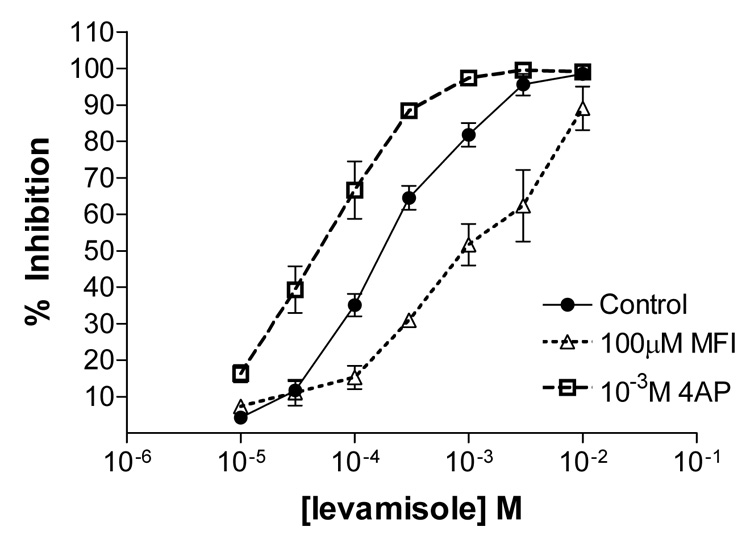
Potentiation effect of 100 µM 4-aminopyridine (4-AP) and antagonistic effect of 100 µM 5-methylfurmethiodide (MFI) on levamisole inhibition of larval migration. Oesophagostomum dentatum ex-sheathed L3 larval migration assay. Control, levamisole response (●); levamisole with 1 mM 4-AP (□); levamisole with 100 µM MFI (Δ). MFI reduces the effect of levamisole at higher doses; n = 3.
4. Discussion
We found evidence of effects of MFI in A. suum on four types of ion-channels, summarized in Fig. 10. In our muscle strip experiments we observed that MFI was a weak agonist producing contraction with a relative potency of 0.014 of acetylcholine. MFI did not produce a maximum contraction like acetylcholine or levamisole. The contraction appears to be mediated by nicotinic cholinergic receptors rather than muscarinic receptors because it was inhibited by a low concentration of mecamylamine (3 µM) in A. suum (Colquhoun et al., 1991).
Fig. 10.
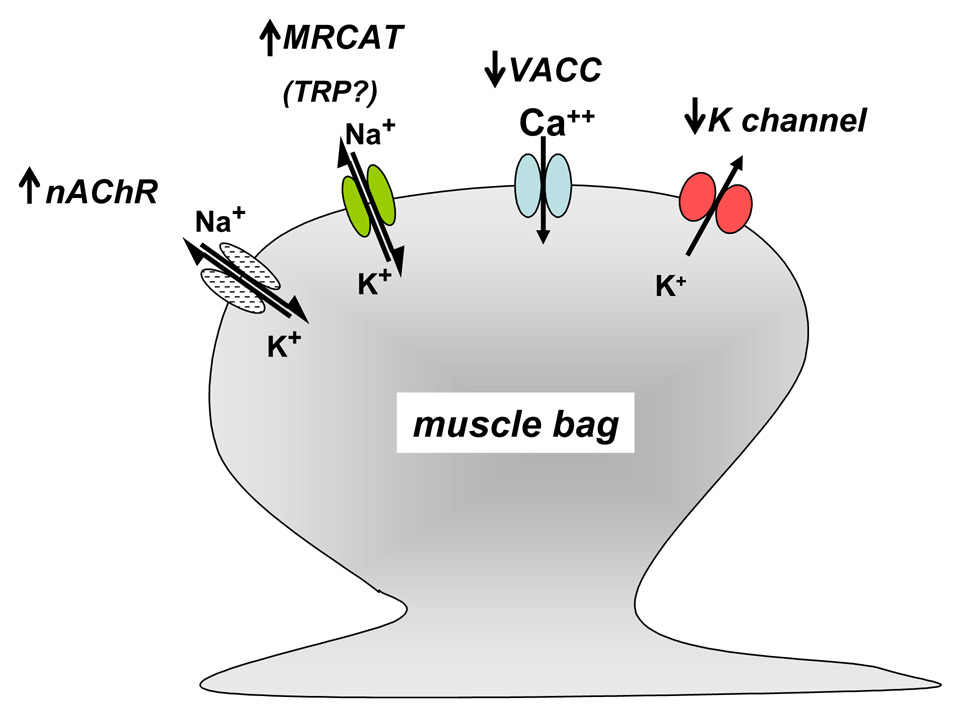
Summary diagram of the effects of 5-methylfurmethiodide (MFI) on the membrane ion-channels of Ascaris suum muscle. MFI is a weak agonist of the nicotinic acetylcholine channels on the bag (up arrow: nAChR). MFI activates mecamylamine-resistant non-selective cation channels (up arrow: MRCAT: TRP?). MFI raises the threshold of voltage-activated calcium currents (down arrow: VACC). MFI inhibits opening of voltage-activated potassium currents (down arrow: K channels)
MFI also activated a mecamylamine-resistant non-selective cation channel current, inhibited voltage-activated potassium channel currents and raised the threshold of voltage-activated calcium channel currents (Fig. 10). The nicotinic channel currents, the non-selective cation channel currents and inhibition of the potassium current would contribute to depolarization and contraction, but the increase in the calcium current threshold would not. The calcium current effect probably limited the contractile effects of MFI in the muscle strip experiments.
Three subtypes of nicotinic acetylcholine receptor channels have been described in A. suum (Robertson et al., 2002; Qian et al., 2006). There is the N-type, which is activated preferentially by nicotine; there is the L-type, which is activated preferentially by levamisole; and there is the B-type, which is activated preferentially by bephenium. It is possible that one nicotinic receptor subtype was not inhibited by mecamylamine. This is unlikely because of the potency of mecamylamine as a nicotinic antagonist in Ascaris (Colquhoun et al., 1991). We checked the potency of mecamylamine as an antagonist of nicotine, levamisole and bephenium using our contraction assay to obtain contraction concentration plots in the absence of mecamylamine and then 0.03, 0.3 and 3.0 µM mecamylamine (at least four preparations for each agonist) were obtained. The negative logarithm of the molar concentration of antagonist required to produce an agonist dose-ratio equal to 2 (pA2) was calculated using standard methods (Robertson et al., 2002). Table 2 shows the pA2 and 50% inhibitory concentration (IC50) (µM) values. The potency of mecamylamine against the three agonists makes it unlikely that the mecamylamine-resistant cation channels are due to a nicotinic receptor subtype.
Table 2.
The negative logarithm of the molar concentration of antagonist required to produce an agonist dose-ratio equal to 2 (pA2) and 50% inhibitory concentration (IC50) (µM) values obtained for mecamylamine with nicotine, levamisole or bephenium as agonist.
| Agonist | pA2 | µM value of pA2 |
|---|---|---|
| nicotine | 7.2 | 0.062 |
| levamisole | 6.6 | 0.235 |
| bephenium | 6.7 | 0.191 |
Agonist contraction concentration plots in the absence of mecamylamine and then in the presence of 0.03, 0.3 and 3.0 µM mecamylamine (four or more preparations were used for each agonist) were obtained.
The significance and function of the nicotinic cholinergic channels that mediate muscle contraction and the effects of ionotropic cholinergic anthelmintics like levamisole have been described (Martin and Robertson, 2007), but the function of the MFI-activated mecamylamine-resistant cation channels is uncertain. Martin and Valkanov (1996) have described activation by acetylcholine of an atropine-resistant, mecamylamine-resistant non-selective cation channel current (Ibcat) which has slow kinetics and that this current may underlie the slow depolarization waves seen in A. suum muscle (Weisblat et al., 1976). ROCs which are non-selective cation channels are activated by a variety of ligands including muscarinic agonists and belong to the TRP group of channels (Albert and Large, 2006; Ramsey et al., 2006). The permeability properties of the mecamylamine-resistant non-selective cation channel, channels of the slow waves, Ibcat (Martin and Valkanov, 1996) and TRP-channels have some similarities. These similarities give rise to the possibility that the slow waves and Ibcat are conducted by a TRP-like channel (Zholos et al., 2004; Takai et al., 2004) that is activated by MFI. Interestingly, Arevalo and Saz (1992) have already demonstrated the activation of phospholipase C and production of diacylglycerol by acetylcholine in A. suum which activate ROCs (Beech et al., 2004).
We have examined, in A. suum muscle, the effects of MFI, which is known to activate M1 receptors in rat cervical ganglion neurons. Mammalian muscarinic acetylcholine receptors are seven transmembrane receptors of the rhodopsin-like family where the natural agonist is acetylcholine (Caulfield et al., 1994). They are divided into five pharmacological subtypes, M1 – M5. MFI produces depolarization in rat cervical ganglion cells (Newberry and Priestley, 1987; Roberts and Newberry, 1990) by stimulation of receptors that produce closure of an inwardly rectifying potassium channel current known as the M-current (Caulfield et al., 1994). We have shown here that MFI also produces depolarization and inhibition of voltage-activated potassium currents in A. suum.
In other mammalian preparations, stimulation of the M1 receptor can also inhibit N- and R-type calcium currents (Melliti et al., 2001). We saw both inhibition of potassium currents and an increase in the threshold for calcium currents in A. suum muscle cells. It is not clear why MFI produces mutually opposing effects in A. suum. It is possible that the extrasynaptic location of MFI application is stimulating a mixture of cholinergic receptors that would not occur physiologically with synaptic release of acetylcholine. We also point out that we have not tested for any role of G-proteins in this study and it remains a possibility that the effect of MFI in A. suum may be via a direct action of MFI on the potassium or calcium channels. The slow speed of the MFI response, however, suggests an indirect action on the ion-channels mediated by a second messenger (Purcell et al., 2002).
In the model nematode C. elegans, three main categories of G-protein linked acetylcholine receptors have been described that are known as GARs (Hwang et al., 1999; Lee et al., 2000). GAR-1, GAR-2 and GAR-3 are molecular homologues of mammalian muscarinic acetylcholine receptors (Lee et al 2000). GAR-1 and GAR-2 are pharmacologically separable from mammalian muscarinic receptors: they are not sensitive to all muscarinic antagonists. GAR-3 is molecularly and pharmacologically more like mammalian muscarinic receptors and is sensitive to atropine. In C. elegans the GAR-3 receptor is coupled to and inhibits opening of a potassium channel and enhances nicotinic receptor function via a Gαq protein in the male spicule muscle (Liu et al., 2007) but in the pharyngeal muscle GAR-3 stimulation enhances a calcium current (Steger and Avery, 2004).
There is much less molecular information available concerning A. suum GARs but homologues to GAR-1 are in expressed sequence tag (EST) databases (BM281808 http://www.ncbi.nlm.nih.gov/sites/entrez?db=nucest&cmd=search&term=BM281808 and BM281722 http://www.ncbi.nlm.nih.gov/sites/entrez?db=nucest&cmd=search&term=BM281722: NCBI) and their function and pharmacology remains to be characterized. At this stage we do not have enough information to determine which G-protein coupled acetylcholine receptors are coupled to potassium or other channels in A. suum.
There are a number of physiological and pharmacological reports indicating the presence of muscarinic-like receptors on A. suum body muscle. Colquhoun et al. (1999) have described the relative potency of a range of selective muscarinic agonists in A. suum: furmethiodide (furtrethonium) had a relative potency of 0.007 (compared with acetylcholine); arecoline had a relative potency of 0.001 but oxtremorine was without effect. In an isolated muscle bag preparation of A. suum, Martin and Valkanov (1996) observed evidence of a mecamylamine-resistant cholinergic receptor, stimulation of which increased the opening of a non-selective cation channel, an effect that was not blocked by atropine, mecamylamine or tubocurarine. In addition Segerberg and Stretton (1993) reported that pilocarpine, a muscarinic agonist, produced a concentration-dependent depolarization in A. suum muscle that was not antagonized by a muscarinic antagonist (N-methyl-scopolamine) nor the nicotinic antagonist, tubocurarine. The lack of effect of oxtremorine and atropine in previous A. suum studies, together with the modest effect of atropine in our studies, suggests that a GAR-3-like receptor is not involved in mediating the effects of MFI.
Acknowledgments
The project was supported by Grant Number R 01 AI 047194 from the national Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceves J, Erlij D, Martinez-Maranon R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br J Pharmacol. 1970;38:602–607. doi: 10.1111/j.1476-5381.1970.tb10601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J Physiol. 2006;570:45–51. doi: 10.1113/jphysiol.2005.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo JI, Saz HJ. Effects of cholinergic agents on the metabolism of choline in muscle from Ascaris suum. J Parasitol. 1992;78:387–392. [PubMed] [Google Scholar]
- Aubry ML, Cowell P, Davey MJ, Shevde S. Aspects of the pharmacology of a new anthelmintic: pyrantel. Br J Pharmacol. 1970;38:332–344. doi: 10.1111/j.1476-5381.1970.tb08521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading AF, Caldwell PC. The resting membrane potential of the somatic muscle cells of Ascaris lumbricoides. J. Physiol. 1971;217:605–624. doi: 10.1113/jphysiol.1971.sp009588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim GD, Milligan G, Brown DA. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alpha o in rat sympathetic neurones. J Physiol. 1994;477:415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun L, Holden-Dye L, Walker RJ. The Pharmacology of cholinoceptors on the somatic muscle cells of the parasitic nematode Ascaris suum. J.Exp.Biol. 1991;158:509–530. doi: 10.1242/jeb.158.1.509. [DOI] [PubMed] [Google Scholar]
- Dale VM, Martin RJ. Oxantel-activated single channel currents in the muscle membrane of Ascaris suum. Parasitology. 1995;110(Pt 4):437–448. doi: 10.1017/s0031182000064775. [DOI] [PubMed] [Google Scholar]
- Evans AM, Martin RJ. Activation and cooperative multi-ion block of single channel currents of Ascaris muscle by the tetrahydropyrimidine anthelmintic, morantel. Br J Pharmacol. 1996;118:1127–1140. doi: 10.1111/j.1476-5381.1996.tb15515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JM, Chang DJ, Kim US, Lee YS, Park YS, Kaang BK, Cho NJ. Cloning and functional characterization of a Caenorhabditis elegans muscarinic acetylcholine receptor. Receptors Channels. 1999;6:415–424. [PubMed] [Google Scholar]
- Lee YS, Park YS, Nam S, Suh SJ, Lee J, Kaang BK, Cho NJ. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 2000;75:1800–1809. doi: 10.1046/j.1471-4159.2000.0751800.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, LeBoeuf B, Garcia LR. G alpha(q)-coupled muscarinic acetylcholine receptors enhance nicotinic acetylcholine receptor signaling in Caenorhabditis elegans mating behavior. J Neurosci. 2007;27:1411–1421. doi: 10.1523/JNEUROSCI.4320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982;77:255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. doi: 10.1017/S0031182007000029. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP, Wolstenholme AJ. Mode of action of the macrocyclic lactones. In: Vercruysse J, Rew RS, editors. Macrocyclic Lactones in Antiparasitic Therapy. Wallingford: CABI; 2002. pp. 125–140. [Google Scholar]
- Martin RJ, Thorn P, Gration KA, Harrow ID. Voltage-activated currents in somatic muscle of the nematode parasite Ascaris suum. J Exp Biol. 1992;173:75–90. doi: 10.1242/jeb.173.1.75. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Valkanov MA. Effects of acetylcholine on a slow voltage-activated non-selective cation current mediated by non-nicotinic receptors on isolated Ascaris muscle bags. Exp Physiol. 1996;81:909–925. doi: 10.1113/expphysiol.1996.sp003992. [DOI] [PubMed] [Google Scholar]
- Melliti K, Meza U, Adams BA. RGS2 blocks slow muscarinic inhibition of N- type Ca(2+) channels reconstituted in a human cell line. J Physiol. 2001;532:337–347. doi: 10.1111/j.1469-7793.2001.0337f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry NR, Priestley T. Pharmacological differences between two muscarinic responses of the rat superior cervical ganglion in vitro. Br J Pharmacol. 1987;92:817–826. doi: 10.1111/j.1476-5381.1987.tb11386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington AJ, Martin RJ. A patch-clamp study of acetylcholine-activated ion channels in Ascaris suum muscle. J Exp Biol. 1990;154:201–221. doi: 10.1242/jeb.154.1.201. [DOI] [PubMed] [Google Scholar]
- Purcell J, Robertson AP, Thompson DP, Martin RJ. The time-course of the response to the FMRFamide-related peptide PF4 in Ascaris suum muscle cells indicates direct gating of a chloride ion-channel. Parasitology. 2002;124:649–656. doi: 10.1017/s0031182002001695. [DOI] [PubMed] [Google Scholar]
- Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006;20:2606–2608. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Roberts KE, Newberry NR. A pharmacological study of the responses induced by muscarinic agonists on the isolated superior cervical ganglion of the guinea-pig. Eur J Pharmacol. 1990;186:257–265. doi: 10.1016/0014-2999(90)90441-8. [DOI] [PubMed] [Google Scholar]
- Robertson AP, Clark CL, Burns TA, Thompson DP, Geary TG, Trailovic SM, Martin RJ. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J Pharmacol Exp Ther. 2002;302:853–860. doi: 10.1124/jpet.102.034272. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Martin RJ. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br J Pharmacol. 1993;108:170–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Pennington AJ, Evans AM, Martin RJ. The action of pyrantel as an agonist and an open channel blocker at acetylcholine receptors in isolated Ascaris suum muscle vesicles. Eur J Pharmacol. 1994;271:273–282. doi: 10.1016/0014-2999(94)90784-6. [DOI] [PubMed] [Google Scholar]
- Segerberg MA, Stretton AO. Actions of cholinergic drugs in the nematode Ascaris suum. Complex pharmacology of muscle and motorneurons. J Gen Physiol. 1993;101:271–296. doi: 10.1085/jgp.101.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger KA, Avery L. The GAR-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics. 2004;167:633–643. doi: 10.1534/genetics.103.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sugawara R, Ohinata H, Takai A. Two types of non-selective cation channel opened by muscarinic stimulation with carbachol in bovine ciliary muscle cells. J Physiol. 2004;559:899–922. doi: 10.1113/jphysiol.2004.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trailovic SM, Robertson AP, Clark CL, Martin RJ. Levamisole receptor phosphorylation: effects of kinase antagonists on membrane potential responses in Ascaris suum suggest that CaM kinase and tyrosine kinase regulate sensitivity to levamisole. J Exp Biol. 2002;205:3979–3988. doi: 10.1242/jeb.205.24.3979. [DOI] [PubMed] [Google Scholar]
- Verma S, Robertson AP, Martin RJ. The nematode neuropeptide, AF2 (KHEYLRF-NH(2)), increases voltage-activated calcium currents in Ascaris suum muscle. Br J Pharmacol. 2007;151:888–899. doi: 10.1038/sj.bjp.0707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblat DP, Byerly L, Russel RL. Ionic Mechanisms of electrical activity in the somatic muscle cell of the nematode Ascaris lumbricoides. J Comp Physiol. 1976;111:93–113. [Google Scholar]
- Zholos AV, Tsytsyura YD, Gordienko DV, Tsvilovskyy VV, Bolton TB. Phospholipase C, but not InsP3 or DAG, -dependent activation of the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br J Pharmacol. 2004;141:23–36. doi: 10.1038/sj.bjp.0705584. [DOI] [PMC free article] [PubMed] [Google Scholar]


