Abstract
AIMS
Rac-formoterol is effective as maintenance treatment for both asthma and chronic obstructive pulmonary disease (COPD) and is now used as relief therapy in asthma. Using rac-formoterol for relief and maintenance treatment could involve inhalation of high doses, and whether this is of benefit in COPD is uncertain. Our aim was to determine whether higher doses of inhaled rac-formoterol produce systemic adverse effects that outweigh the limited bronchodilator benefit seen in subjects with COPD.
METHODS
We examined airway and systemic effects of 6, 12, 24 and 48 μg rac-formoterol and placebo on separate days in 20 subjects with symptomatic COPD [forced expiratory volume in 1 s (FEV1) 47% predicted]. FEV1, oxygen saturation, dyspnoea, 6-min walk distance, patient satisfaction and systemic effects were measured and treatment was assessed against placebo and for dose–response effects.
RESULTS
FEV1[area under the time–response curve (AUC)] and satisfaction scores increased with all formoterol doses compared with placebo, as did AUC tremor with the 24- and 48-μg doses and AUC heart rate with the 48-μg dose. A dose–response relationship was seen with FEV1 and tremor, but not with satisfaction scores. There was no difference between placebo and rac-formoterol for other variables.
CONCLUSIONS
Our results show that in patients with COPD rac-formoterol improves FEV1 and patient satisfaction without a corresponding reduction in dyspnoea. Since the systemic effects from a relatively high dose were minimal, its use as relief medication in COPD merits further evaluation.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The long-acting inhaled β2-agonist formoterol has systemic effects when taken in high doses.
It can be used as relief medication in asthma and there is interest in this approach in chronic obstructive pulmonary disease (COPD).
Relief medication can involve high doses, and in subjects with COPD who have limited ability to bronchodilate the adverse effects can outweigh the benefits.
There are concerns with the overall safety of high-dose β2-agonists in subjects with COPD, and this study looks at the balance of beneficial and adverse effects of a range of doses of inhaled formoterol.
WHAT THIS STUDY ADDS
Among subjects with COPD, high-dose inhaled formoterol produced a dose-related increase in forced expiratory volume in 1 s without a corresponding reduction in dyspnoea or increase in walk distance.
Systemic effects were modest, however, and high doses did not appear to reduce patient satisfaction.
Although further safety data are needed, inhaled formoterol may have a role as relief medication in COPD.
Keywords: β2-agonists, COPD, formoterol
Introduction
Bronchodilators are widely used by patients with symptomatic chronic obstructive pulmonary disease (COPD). Short-acting agents are used for symptom relief, but long-acting β2-agonists have additional benefits [1, 2], and management algorithms recommend their introduction if symptoms persist [3].
Rac-formoterol, a selective β2-agonist with a 12-h duration of action at conventional doses, has been shown to improve lung function and reduce symptoms when inhaled twice daily by patients with COPD [1, 4]. Its rapid onset of action within 5 min [5, 6] has led to it being used as relief medication in asthma, and this approach was more effective than rac-terbutaline in terms of improving lung function and prolonging the time to the first severe exacerbation [7].
A similar strategy has been suggested for patients with COPD [8], but the balance of beneficial and adverse effects may be different since additional bronchodilation with higher doses may be minimal in these patients, whereas the potential for adverse effects will increase. The main safety concern with high-dose rac-formoterol relates to cardiovascular adverse effects. This is an important consideration among patients with COPD, who may have coexisting ischaemic heart disease and hypoxaemia, both risk factors for cardiac arrhythmia.
We therefore carried out a randomized, crossover dose–response study to compare efficacy, systemic effects and subject satisfaction following single doses of inhaled rac-formoterol (6, 12, 24 and 48 μg) and placebo in 20 patients with symptomatic COPD.
Methods
Subjects
Patients aged 40–85 years with a clinical diagnosis of COPD were recruited if they had exertional breathlessness, limited reversibility to inhaled salbutamol 200 μg (<200 ml), a forced expiratory volume in 1 s (FEV1) <70% predicted and a FEV1/forced vital capacity (FVC) ratio of <70%. Subjects were excluded if they were current smokers [9] or had an exhaled carbon monoxide level above 10 parts per million on study days (Smokerlyser; Bedfont Scientific, Rochester, UK), had experienced an acute exacerbation of COPD within 4 weeks or a myocardial infarction or unstable angina within 3 months, had an unstable medical condition or were taking β-adrenergic antagonists. Nottingham City Hospital Research Ethics Committee approved the study and written informed consent was obtained from each subject at least 24 h before the first study day.
Measurements
Oxygen saturation was measured using a fingertip pulse oximeter (Minolta Pulsox-7, Tokyo, Japan), continuous heart rate by three-lead ambulatory monitor (DMS 300-7; Numed Cardiac Diagnostics, Sheffield, UK) and blood pressure by electronic sphygmomanometry (HEM-705CP; Omron, Tokyo, Japan). Dyspnoea and tremor were measured using a 100-mm visual analogue scale (VAS) ranging from ‘no breathlessness/tremor’ to ‘worst possible breathlessness/tremor’. Tremor amplitude was also measured for 1 min using an accelerometer fitted to the terminal phalanx of the right middle finger with the forearm supported and the hand outstretched. The waveform was amplified and filtered to remove frequencies above 50 Hz and acceleration measured on 5-s samples using Fourier analysis (Pulse Platform; Bruel and Kjaer, Naerum, Denmark). Spirometry was performed with the patient seated using a dry bellows spirometer (Vitalograph, Buckingham, UK), taking the better of two successive measurements of FEV1 within 100 ml. Plasma potassium concentration was measured by flame photometry (Olympus AU5000, Eastleigh, UK). A 6-min walk test was performed according to American Thoracic Society guidelines with standardized verbal encouragement [10] and with breathlessness measured on a Borg scale before and after exercise. Subjects carried out two familiarization walk tests before treatment randomization [11]. Overall satisfaction with each drug/dose was measured on a 200-mm VAS from ‘completely dissatisfied, side-effects outweigh any benefit’ to ‘completely satisfied, benefit outweighs any side-effects’.
Study design and methods
This was a randomized, double-blind, crossover study performed on 5 days within a 2-week period and with each visit commencing at the same time of day. Subjects were required to withhold short-acting bronchodilators for at least 10 h and theophylline, tiotropium bromide and long-acting β2-agonists for at least 24 h before each study day. Inhaled corticosteroids and nonpulmonary medications were taken as usual unless they were known to affect any of the study outcomes. After an early breakfast with a maximum of one cup of tea or coffee, subjects attended the laboratory at 08.00 h and rested in a comfortable chair for 20 min. After checking exhaled carbon monoxide levels, the ambulatory cardiac monitor was attached and baseline measures of SpO2, heart rate, blood pressure, breathlessness, tremor (VAS and accelerometer) and FEV1 were taken. Blood was then taken for plasma potassium measurement and followed by a 6-min walk test. After a further 10-min rest, subjects inhaled rac-formoterol (6, 12, 24 or 48 μg) or placebo, with the dose and order determined by computer-generated randomized sequence. On each day and under independent supervision, subjects took four inhalations at 1-min intervals from one or more of three identical Turbohalers® containing placebo, rac-formoterol 6 μg or 12 μg; each device was withdrawn between inhalations to ensure blinding. Subjects remained seated for the next 4 h with repeat measures of oxygen saturation, heart rate, blood pressure, breathlessness, tremor and FEV1 in that order at 30, 60, 120, 180 and 240 min post inhalation. Every 60 min, subjects were asked, ‘Have you felt any adverse effects that may relate to inhaling the medication’. At 4 h a further blood sample was taken for plasma potassium assay and the subject was asked to record overall satisfaction with the treatment. The 6-min walk test was then repeated.
We were interested in the balance between beneficial and adverse effects, but with no data available on which to base power calculations, we opted to base the calculations on FEV1. Randomizing 20 subjects provided 80% power to detect a 160-ml change in FEV1[12] and allowed us to assess systemic effects of interest [13].
Analysis
The area under the curve for the variables measured at intervals over the 4 h, the change in mean plasma potassium and 6-min walk distance between baseline and 4 h and ectopic beat rate and satisfaction scores were determined for each subject and treatment. AUC was calculated by computer program and reflects the change over 4 h for each treatment. The differences between placebo and each dose of rac-formoterol were then assessed using repeated measures anova and the dose–response relationship was assessed by statistical significance of the linear contrast with increasing rac-formoterol dose. All analyses were performed with SPSS Version 12 (SPSS Inc., Chicago, IL, USA), and statistical significance accepted as P < 0.05.
Results
All 20 subjects (six women) completed the study (Table 1). Their mean age was 66 years, mean cigarette consumption was 42 pack-years, body mass index 26 kg m−2 and FEV1 1.32 l (47% predicted) with a mean increase of 33 ml (range 0–160 ml) following rac-albuterol 200 μg. Thirteen of the 20 participants used an inhaled corticosteroid and four used tiotropium bromide. Nineteen subjects used inhaled β2-agonists on a daily basis, with 13 taking a long-acting β2-agonist twice daily and six further subjects taking a short-acting β2-agonist four times a day.
Table 1.
Baseline characteristics for the 20 subjects
| Age, years | Sex | BMI | Pack years | FEV1 (l) | FEV1 (% pred) | FEV1/FVC | Reversibility (ml) (<200 ml) | Regular β2-agonists | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | M | 37 | 42 | 0.75 | 25 | 44 | 50 | F, Sb |
| 2 | 75 | F | 35 | 60 | 1.05 | 58 | 62 | 0 | Sb |
| 3 | 54 | F | 28 | 26 | 1.4 | 51 | 54 | 160 | Sm, Sb |
| 4 | 59 | M | 26 | 60 | 1.64 | 48 | 52 | 60 | Sm, Sb |
| 5 | 75 | F | 23 | 41 | 0.88 | 49 | 53 | 150 | Sb |
| 6 | 67 | M | 20 | 10 | 0.5 | 18 | 28 | 20 | Sb |
| 7 | 71 | M | 21 | 69 | 0.74 | 30 | 44 | 60 | Sb |
| 8 | 62 | F | 20 | 40 | 1.22 | 53 | 53 | 0 | F, Sb |
| 9 | 77 | M | 56 | 27 | 0.82 | 31 | 32 | 160 | Sm, Sb |
| 10 | 71 | M | 33 | 0 | 0.95 | 51 | 51 | 150 | Sb |
| 11 | 66 | M | 21 | 25 | 1.4 | 44 | 49 | 0 | Sm, Sb |
| 12 | 64 | M | 28 | 48 | 1.95 | 56 | 65 | 0 | F,T |
| 13 | 63 | F | 19 | 60 | 0.95 | 41 | 44 | 70 | Sm, Sb |
| 14 | 60 | M | 22 | 45 | 2.65 | 69 | 63 | 140 | |
| 15 | 62 | F | 34 | 30 | 1.37 | 59 | 58 | 100 | Sm,Sb |
| 16 | 60 | M | 21 | 56 | 0.75 | 23 | 25 | 50 | F, Sb |
| 17 | 77 | M | 28 | 0 | 1.4 | 55 | 50 | 120 | Sm, T |
| 18 | 75 | M | 22 | 50 | 1.28 | 42 | 49 | 80 | Sm |
| 19 | 52 | M | 29 | 24 | 2.52 | 68 | 53 | 0 | T |
| 20 | 67 | M | 28 | 75 | 2.11 | 63 | 54 | 0 | Sm |
F, Formoterol; Sb, Salbutamol; Sm, Salmeterol; T, Terbutaline; BMI, body mass index, FEV1, forced expiratory volume in 1 s.
Data were available for all time points except for three plasma potassium levels due to blood clotting. There were no significant differences in baseline FEV1 values between study days and no crossover effects.
Response to rac-formoterol (Table 2)
Table 2.
Mean (SD) area under the time–response curve (AUC) for FEV1 and the systemic measurements, made at intervals over 4 h for each rac-formoterol dose
| FEV1 (mm h−1) | SpO2 (ml h−1) | Dyspnoea (% h−1) | HR (mm h−1) | Tremor (bpm h−1) | VAS tremor (m s−1 s−1 h−1) | SBP (mmHg h−1) | DBP | |
|---|---|---|---|---|---|---|---|---|
| Placebo | −104.3 (59) | 0.5 (0.2) | −25 (12) | −8.9 (2.3) | −0.42 (0.1) | −3.2 (2.1) | −21 (5) | −6.7 (3) |
| 6 μg | 225.3† (50) | 1.2 (0.5) | −46.0 (15) | −2.8 (0.8) | −0.26 (0.1) | −4.6 (3.0) | −3.3 (2) | 1.5 (1) |
| 12 μg | 362† (101) | 0.3 (0.2) | −38.2 (19) | −2.5 (0.8) | −0.12 (0.05) | −20.7 (7.0) | −4.1 (2) | 3.1 (2) |
| 24 μg | 510.8† (299) | −1.0 (0.2) | −46.2 (28) | −6.3 (2.3) | 0.57† (0.2) | 0.6 (10) | 8.2 (3) | 3.6 (2) |
| 48 μg | 626.6† (297) | 1.1 (0.4) | −44.3 (19) | 0.6† (3.3) | 0.85† (0.4) | 1.5 (5) | 3.9 (3) | 8.4 (5) |
| P-value | 0.04* | 0.69 | 0.98 | 0.65 | <0.001* | 0.04* | 0.6 | 0.68 |
P = 0.05 for dose–response trend.
P = 0.05 for individual rac-formoterol dose vs. placebo. FEV1, forced expiratory volume in 1 s; HR, heart rate; VAS, visual analogue scale.
FEV1 (Figures 1 and 2)
Figure 1.
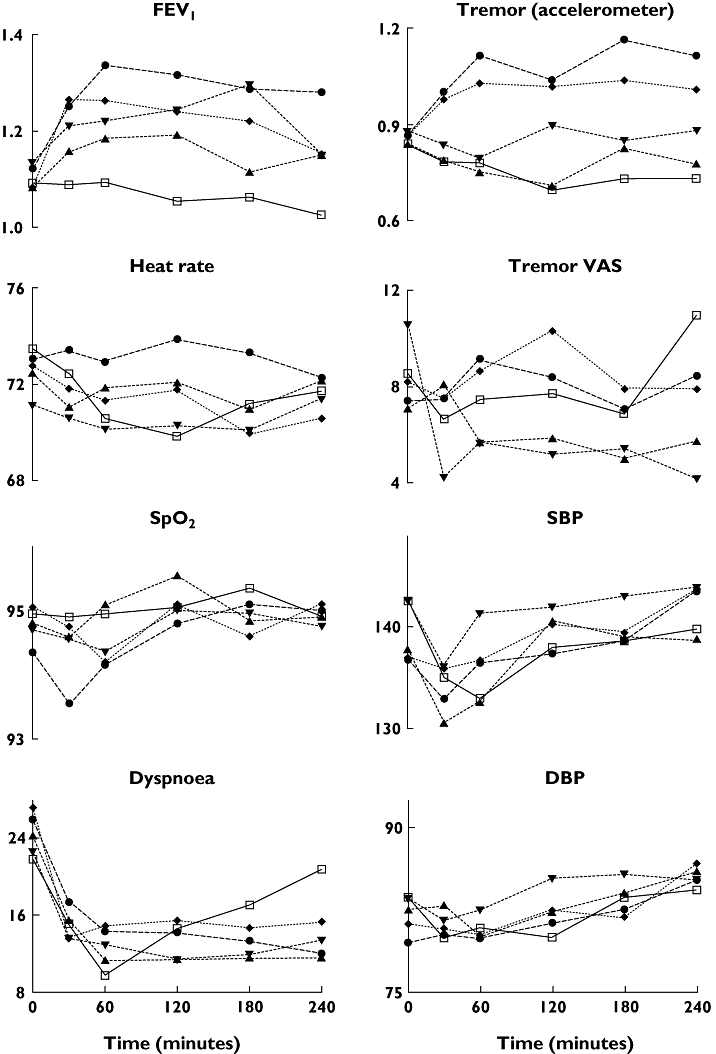
Mean values over 240 min for forced expiratory volume in 1 s (FEV1) (l), heart rate (bpm), SpO2 (%), dyspnoea visual analogue scale (VAS) (mm), tremor acceleration (m s−1 s−1), tremor VAS (mm), systolic (SBP) and diastolic blood pressure (DBP) (mmHg) after rac-formoterol 6 (▴), 12 (▾), 24 (♦) and 48 (▵) μg and placebo (□)
Figure 2.
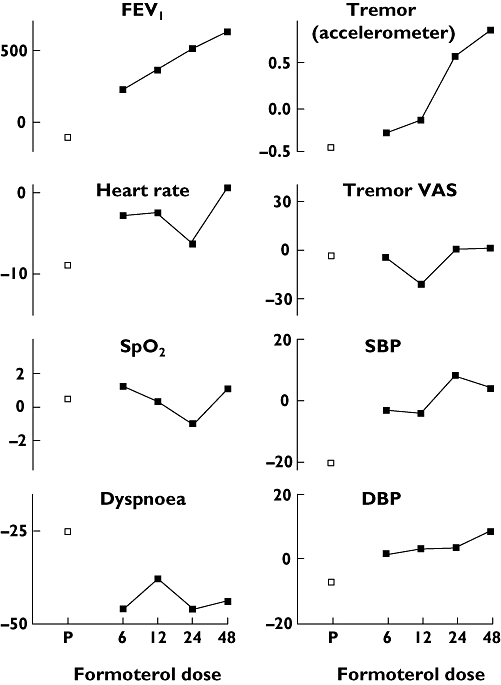
The relationship between log dose rac-formoterol (μg) and mean area under the curve for forced expiratory volume in 1 s (FEV1) (ml h−1), heart rate (bpm h−1), SpO2 (% h−1), dyspnoea visual analogue scale (VAS) (mm h−1), tremor acceleration (m s−1 s−1 h−1), tremor VAS (mm h−1), systolic (SBP) and diastolic blood pressure (DBP) (mmHg h−1) with placebo (P) shown for comparison (□)
FEV1 increased from baseline with all doses of rac-formoterol and the effect was sustained over 4 h. AUC FEV1 was significantly higher after all doses of rac-formoterol compared with placebo and showed a significant dose–response relationship with increasing doses of rac-formoterol. The largest AUC FEV1 (626.6 ml h−1) was seen with the highest dose of rac-formoterol and reflects a mean increase in FEV1 of around 157 ml across the 4-h time period. There was no correlation between the AUC FEV1 and baseline FEV1 (r = 0.7, P = 0.2).
Tremor (Figures 1 and 2)
Tremor measured by accelerometer increased with the two highest rac-formoterol doses compared with placebo and AUC tremor showed a dose–response effect. Tremor measured by VAS was more variable. Although there were no differences between individual doses and placebo, a dose–response relationship was present.
Heart rate (Figures 1 and 2)
Mean heart rate fell after the three lowest doses of rac-formoterol and placebo, but rose following the highest (48 μg) dose of rac-formoterol, and this differed significantly from placebo. There was no difference between placebo and other rac-formoterol doses and no dose–response relationship.
Other end-points (Figures 1–3)
Figure 3.
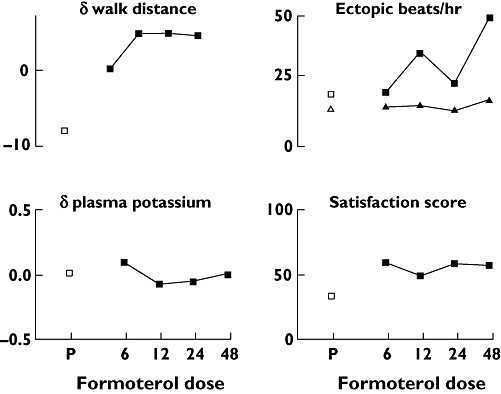
The relationship between log dose rac-formoterol (μg) and change in 6-min walk distance (m), change in plasma potassium concentration (mmol l−1), mean rate of ventricular (▴) and supraventricular (▪) ectopic beats and mean satisfaction scores (mm), with placebo (P) shown for comparison (□)
The following end-points showed no difference between individual doses and placebo and no dose–response relationship: dyspnoea, oxygen saturation, systolic and diastolic blood pressure, ectopic beat rate, walk distance and plasma potassium levels. For example, the maximum change in 6-min walk distance between placebo and any dose of formoterol was around 15 m, which is less than 5% of the mean baseline walk distance of 360 m.
Satisfaction scores (Figure 3)
Each rac-formoterol dose produced higher satisfaction scores than placebo, but with no dose–response relationship.
Adverse events
No serious adverse effects were reported during the study. Two individuals reported headache after placebo and rac-formoterol 6 μg, respectively, and one reported light-headedness 2 h after rac-formoterol 6 μg.
Discussion
In subjects with moderately severe, symptomatic COPD, rac-formoterol produced a dose-dependent increase in FEV1 without a corresponding improvement in dyspnoea or walk distance. Tremor also increased in a dose-dependent manner, whereas heart rate increased significantly only after the highest dose. There was no significant effect on plasma potassium, blood pressure, oxygen saturation or ectopic beat rate. All doses of rac-formoterol produced higher satisfaction scores than placebo, although no dose–response relationship was evident.
Satisfaction with any treatment depends on the balance between beneficial and adverse effects. This is particularly relevant in COPD, where the largely irreversible nature of the disorder will limit the beneficial effects seen with higher drug doses, whereas adverse effects can continue to increase. The increase in FEV1 in this study was small and of similar magnitude to that seen in other studies in subjects with COPD [14]. However, it was not associated with a significant reduction in dyspnoea scores or increase in exercise tolerance. This is consistent with previous studies with rac-formoterol and rac-salmeterol [4, 15] and presumably reflects, in part at least, the relatively small increase in FEV1 seen in patients with COPD.
The adverse systemic effects of β2-agonists are well known, but in this study only tremor and heart rate increased significantly with high doses of rac-formoterol. Moderate doses of rac-formoterol have been shown to increase heart rate, systolic blood pressure and QT interval, and to reduce plasma potassium and diastolic blood pressure in healthy volunteers [16–18]. The effects have been less marked in subjects with asthma [18–23], probably reflecting the development of tolerance [24–26]. Tolerance could also explain the limited systemic effects in our study, since all but one subject were taking β2-agonists regularly.
We have summarized our findings as mean values, and as area under the curve for the measurements that were made on several occasions. Outliers may not be apparent with this approach, but could be important in relation to adverse effects. Checking individual data showed that the data were homogeneous and that there were no worrying outlying results. We limited the study to 4 h so that responses would not be affected by meals, as in previous studies [16]. The greatest pharmacodynamic changes are known to occur within 4 h, so it is unlikely that additional effects after this time period were missed [16].
The dose-related increase in FEV1 and limited systemic effects suggest that rac-formoterol may be suitable for use as rescue medication in COPD. Patients with COPD have an increased incidence of ischaemic heart disease, however, and therefore greater potential for cardiac arrhythmias. In a small study in subjects with COPD and a previous cardiac arrhythmia, rac-formoterol 24 μg caused a greater effect on heart rate, plasma potassium, supraventricular and ventricular premature beats than the 12-μg dose [27]. There was a trend towards increased ectopic beats with higher doses in our study, but a larger long-term study would be needed to assess fully the safety of higher doses in these patients.
Assessing the balance between beneficial and adverse effects is difficult, and asking about overall satisfaction with the drug after each study day was an attempt to address the patients' perception of this balance. Satisfaction scores are difficult to validate, but formoterol produced greater subject satisfaction than placebo in keeping with its effect on FEV1, and a similar approach had face validity in our previous study [28]. This study compared different doses of rac-salbutamol in subjects with COPD and found a bell-shaped response, in that subjects preferred a modest dose of rac-salbutamol (1 mg) to both placebo or the highest dose (4 mg), presumably because the 1-mg dose offered the best balance between beneficial and adverse effects. Satisfaction improved with all doses of rac-formoterol in the current study, however, suggesting that the perceived adverse effects of 48 μg did not exceed the perceived benefit.
Inhaled rac-formoterol up to 48 μg produced a dose-dependent improvement in FEV1 with few systemic effects in subjects with moderate to severe COPD. Satisfaction scores were higher with all doses of rac-formoterol. Further studies are warranted to determine whether rac-formoterol has a useful role as rescue medication for patients with COPD.
Competing Interests: CIW and MPS have received financial support from AstraZeneca to attend meetings. MPS's salary was from a non-conditioned grant from AstraZeneca for a different study. AET and TWH have received honoraria from AstraZeneca for speaking at meetings and TWH received honoraria for being on advisory groups. HJM has no competing interests to declare.
Acknowledgments
We thank the patients who participated, Sarah Pacey in pharmacy for randomization and drug allocation, Professor Sarah Lewis for statistical advice, Dr Heather Wharrad for assistance with the accelerometer, Janet Oborne and Sue Cooper for administrative help and Astra Zeneca for providing placebo inhalers.
REFERENCES
- 1.Dahl R, Greenhorst LAPM, Nowak D, Nonikov V, Byrne AM, Thomson MH, Till D, Della Cioppa G. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:778–84. doi: 10.1164/ajrccm.164.5.2007006. [DOI] [PubMed] [Google Scholar]
- 2.D'Urzo AD, Cristina De Salvo M, Ramirez-Rivera A, Almeida J, Sichletidis L, Rapatz G, Kottakis J. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium. Chest. 2001;119:1347–56. doi: 10.1378/chest.119.5.1347. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Clinical Excellence. Chronic Obstructive Pulmonary Disease. Management of Chronic Obstructive Pulmonary Disease in adults in primary and secondary care. [January 2007];2004 Clinical Guideline 12. Available at http://www.nice.org.uk/CG012NICEguidelines.
- 4.Aalbers R, Ayers J, Backer V, Decramer M, Lier PA, Magyar P, Malolepszy J, Ruffin R, Sybrecht GW. Formoterol in patients with chronic obstructive pulmonary disease: a randomised, controlled, 3-month trial. Eur Respir J. 2002;9:178–85. doi: 10.1183/09031936.02.00240902. [DOI] [PubMed] [Google Scholar]
- 5.Cazzola M, Grella E, Matera MG, Mazzarella G, Marsico SA. Onset of action following formoterol Turbuhaler and salbutamol pMDI in reversible chronic airway obstruction. Pulm Pharmacol Ther. 2002;15:97–102. doi: 10.1006/pupt.2001.0336. [DOI] [PubMed] [Google Scholar]
- 6.Cazzola M, Centanni S, Regorda C, di Marco F, di Perna F, Carlucci P, Boveri B, Santus P. Onset of action of single doses of formoterol administered via Turbuhaler in patients with stable COPD. Pulm Pharmacol Ther. 2001;14:41–5. doi: 10.1006/pupt.2000.0267. [DOI] [PubMed] [Google Scholar]
- 7.Tattersfield AE, Lofdahl C-G, Postma DS, Eivindson A, Schreurs AGM, Rasidakis A, Ekstrom T. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet. 2001;357:257–61. doi: 10.1016/S0140-6736(00)03611-4. [DOI] [PubMed] [Google Scholar]
- 8.Campbell M, Eliraz A, Johansson G, Tornling G, Nihlen U, Bengtsson T, Rabe KF. Formoterol for maintenance and as-needed treatment of chronic obstructive pulmonary disease. Respir Med. 2005;99:1511–20. doi: 10.1016/j.rmed.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Schmekel B, Borgstrom L, Wollmer P. Difference in pulmonary absorption of inhaled terbutaline in healthy smokers and non-smokers. Thorax. 1991;46:225–8. doi: 10.1136/thx.46.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 11.Sciurba F, Criner GJ, Lee SM, Mohsenifar Z, Shade D, Slivka W, Wise RA. Six-minute walk distance in chronic obstructive pulmonary disease. Reproducibility and effect of walking course layout and length. Am J Crit Care Med. 2003;167:1522–7. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 12.Tweeddale PM, Alexander F, McHardy GJ. Short term variability in FEV1 and bronchodilator responsiveness in patients with obstructive ventilatory defects. Thorax. 1987;42:487–90. doi: 10.1136/thx.42.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett JA, Smyth ET, Pavord ID, Wilding PJ, Tattersfield AE. Systemic effects of salbutamol and salmeterol in patients with asthma. Thorax. 1994;49:771–4. doi: 10.1136/thx.49.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cazzola M, Matera M, Santangelo G, Vinciguerra A, Rossi F, D'Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose–response study. Respir Med. 1995;89:357–62. doi: 10.1016/0954-6111(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 15.Boyd G, Morice AH, Poundsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease. Eur Respir J. 1997;10:815–21. [PubMed] [Google Scholar]
- 16.Guhan AR, Cooper S, Oborne J, Lewis S, Bennett J, Tattersfield AE. Systemic effects of formoterol and salmeterol: a dose–response comparison in healthy subjects. Thorax. 2000;55:650–6. doi: 10.1136/thorax.55.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremner P, Woodman K, Burgess C, Crane J, Purdie G, Pearce N, Beasley R. A comparison of the cardiovascular and metabolic effects of formoterol, salbutamol and fenoterol. Eur Respir J. 1993;6:204–10. [PubMed] [Google Scholar]
- 18.Rosenborg J, Bengtsson T, Larsson P, Blomgren A, Persson G, Lotvall J. Relative systemic dose potency and tolerability of inhaled formoterol and salbutamol in healthy subjects and asthmatics. Eur J Clin Pharmacol. 2000;56:363–70. doi: 10.1007/s002280000160. [DOI] [PubMed] [Google Scholar]
- 19.Totterman K, Huhti L, Sutinen E, Backman R, Pietinalho A, Falck M, Larsson P, Selroos O. Tolerability to high doses of formoterol and terbutaline via Turbuhaler for 3 days in stable asthmatic patients. Eur Respir J. 1998;12:573–9. doi: 10.1183/09031936.98.12030573. [DOI] [PubMed] [Google Scholar]
- 20.Burgess C, Ayson M, Rajasingham S, Crane J, Della Cioppa G, Till MD. The extrapulmonary effects of increasing doses of formoterol in patients with asthma. Eur J Clin Pharmacol. 1998;54:141–7. doi: 10.1007/s002280050435. [DOI] [PubMed] [Google Scholar]
- 21.Palmqvist M, Ibsen T, Mellen A, Lotvall J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. Am J Respir Crit Care Med. 1999;160:244–9. doi: 10.1164/ajrccm.160.1.9901063. [DOI] [PubMed] [Google Scholar]
- 22.Maesen FPV, Costongs R, Smeets JJ, Brombacher PJ, Zweers PGMA. The effect of maximal doses of formoterol and salbutamol from a metered dose inhaler on pulse rates, ECG, and serum potassium concentrations. Chest. 1991;99:1367–73. doi: 10.1378/chest.99.6.1367. [DOI] [PubMed] [Google Scholar]
- 23.Malolepszy J, Boszormenyi Nagy G, Selroos O, Larsson P, Brander R. Safety of formoterol Turbuhaler at cumulative dose of 90 μg in patients with acute bronchial obstruction. Eur Respir J. 2001;18:928–34. doi: 10.1183/09031936.01.00251901. [DOI] [PubMed] [Google Scholar]
- 24.Harvey JE, Baldwin CJ, Wood PJ, Alberti KGMM, Tattersfield AE. Airway and metabolic responsiveness to intravenous salbutamol in asthma: effect of regular inhaled salbutamol. Clin Sci. 1981;60:579–85. doi: 10.1042/cs0600579. [DOI] [PubMed] [Google Scholar]
- 25.Lipworth BJ, Struthers AD, McDevitt DG. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high dose inhaled salbutamol in asthmatics. Am Rev Respir Dis. 1989;140:586–92. doi: 10.1164/ajrccm/140.3.586. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor BJ, Aikman SL, Barnes PJ. Tolerance to the nonbronchodilator effects of inhaled β2-agonists in asthma. N Engl J Med. 1992;327:1204–8. doi: 10.1056/NEJM199210223271704. [DOI] [PubMed] [Google Scholar]
- 27.Cazzola M, Imperatore F, Salzillo A, Di Perna F, Calderaro F, Imperatore A, Matera MG. Cardiac effects of formoterol and salmeterol in patients suffering from COPD with preexisting cardiac arrhythmias and hypoxaemia. Chest. 1998;114:411–5. doi: 10.1378/chest.114.2.411. [DOI] [PubMed] [Google Scholar]
- 28.Vathenen AS, Britton JR, Ebden P, Cookson JB, Wharrad HJ, Tattersfield AE. High dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850–5. doi: 10.1164/ajrccm/138.4.850. [DOI] [PubMed] [Google Scholar]


