Abstract
Chronic obstructive pulmonary disease represents a major global health care burden for both primary and secondary care providers and is the most common respiratory condition necessitating hospital admission. Short-acting bronchodilators play a vital role in immediate relief of symptoms, while inhaled long-acting bronchodilators and inhaled corticosteroids are advocated for regular use in individuals with persistent symptoms and exacerbations. Theophylline is a nonspecific phosphodiesterase inhibitor and is usually reserved for patients with ongoing symptoms despite optimum inhaled bronchodilator treatment or when difficulty is encountered with inhaler devices. However, it is often not widely used mainly due to frequency of dose-related adverse effects, numerous drug interactions and narrow therapeutic index. This in turn has lead to the development of more selective phosphodiesterase inhibitors in an attempt to create a drug which patients can use with beneficial effects but without the problems associated with theophylline. Current data do indicate that phosphodiesterase 4 inhibitors confer some benefits in chronic obstructive pulmonary disease when compared to placebo in terms of lung function, quality of life and exacerbations. They are also generally well tolerated. Further studies are required to determine fully their long-term beneficial and adverse effect profiles and ultimately where they might comfortably sit in management algorithms.
Keywords: chronic obstructive pulmonary disease, theophylline, phosphodiesterase 4 inhibitors, management
Introduction
Chronic obstructive pulmonary disease (COPD) is a common cause of morbidity and mortality encompassing large numbers of individuals in both developed and less well developed countries. It places an enormous burden upon patients, families, society and primary and secondary care providers alike. It is predicted that COPD will become the world's third leading cause of mortality and the fifth most common cause of serious morbidity over the next 20 years [1]. Since spirometry is increasingly performed in primary care settings, it is anticipated that earlier diagnosis will become more common, especially if current or previous cigarette smokers are selectively screened.
Smoking cessation is the only intervention demonstrated to slow significantly the inextricable decline in forced expiratory volume in 1 s (FEV1) characteristic of COPD and its encouragement remains at the cornerstone of management [2]. In patients with persistent symptoms, the principle pharmacological strategy comprises the regular use of inhaled long acting bronchodilators such as long acting β2-adrenoceptor agonists and long acting anticholinergics [3]. Inhaled corticosteroids are generally reserved for those with more severe airflow obstruction and recurrent exacerbations [4]. An important disadvantage with all of these drugs is that they are delivered by the inhaled route. Some individuals may dislike such an approach, while the elderly and those with concomitant upper limb locomotor problems may have difficulty in using and co-ordinating hand-held inhaler devices. As a consequence, poor compliance and suboptimal inhaler technique may contribute to inadequate drug delivery to the lungs in patients with obstructive lung disorders [5, 6]. Moreover, it has also been suggested that drug delivery following inhalation may be suboptimal in COPD compared with asthma, mainly due to the different pathophysiological features [7].
Theophylline, an indiscriminate inhibitor of phosphodiesterase, is an oral bronchodilator and anti-inflammatory agent which has been available for many decades to treat obstructive lung disorders [8]. However, its widespread use is often limited due to concerns regarding dose-related adverse effects, numerous drug interactions and narrow therapeutic index. This in turn has lead to the development of a closely related class of drug, phosphodiesterase-4 (PDE4) inhibitors, in an attempt to confer some of the bronchodilator and anti-inflammatory effects of theophylline but exhibit a more favourable adverse effect profile [9]. The current role of PDE4 inhibitors in COPD is yet to be fully defined but this class of drug does represent an exciting and timely development in the management of a common and often disabling condition.
This evidence based review outlines the problems associated with the use of theophylline and the development, pharmacology, clinical effects and potential role of PDE4 inhibitors in COPD. We also highlight the recent studies which have evaluated this class of drug. All authors performed a comprehensive literature search using Medline, Clinical Evidence and Cochrane library. The following keywords were used in the search: chronic obstructive pulmonary disease, PDE4 inhibitor, cilomilast, roflumilast, rolipram, lung function, exacerbations, symptoms, quality of life and adverse effects. We also searched the abstract books from the American Thoracic Society and European Respiratory Society from 2000 to 2006.
Theophylline
Theophylline is one of the oldest drugs for the management of obstructive airways diseases (COPD and asthma) which is still used today. Despite the modest clinical efficacy of theophylline, it is orally active and inexpensive, in turn making it an attractive pharmacotherapeutic option, especially in less developed countries.
Pathophysiology
PDEs (of which at least 11 izoenzymes have been identified) are important enzymes in the hydrolysis of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) to inactive nucleotides [10]. In inflammatory cells, the effects of cAMP are largely inhibitory and it plays an important role in dampening the inflammatory response [11]. Elevated cAMP concentrations are also involved in relaxing smooth muscle in the airway and modulating sensory nerves in the lung.
Theophylline acts as a nonselective PDE inhibitor in a variety of cells throughout the body [8]; indiscriminate PDE inhibition results in an increase in cAMP and cGMP concentrations in organs such as the lungs, kidney, brain, heart, pancreas and liver. It is also thought to have a variety of other effects such as increased interleukin 10 release, enhanced apoptosis, inflammatory mediator inhibition, adenosine receptor antagonism and increased catecholamine release [8]. Studies have also demonstrated that theophylline confers some neutrophil mediated anti-inflammatory effects [12, 13]. Since histone deacetylase, which is able to suppress inflammatory gene expression, is reduced in COPD, it has been suggested that this may be one reason why corticosteroids have limited efficacy [14]. Previous studies have indicated that theophylline can actually increase histone deacetylase concentrations and may as a consequence enhance the responsiveness of corticosteroids [15].
Use of theophylline in COPD
Theophylline is not considered a first line agent in the management of COPD. However, current guidelines suggest that a therapeutic trial should be considered in patients with persistent symptoms and exacerbations despite good compliance with inhaled bronchodilators [16]. Indeed, there are some data which indicate that up to 50% of individuals with more advanced airflow obstruction may derive benefit to some extent from theophylline [17]. Theophylline may also be tried in individuals who have difficulty in using inhaler devices.
Problems encountered with theophylline
Many limitations exist to the more widespread use of theophylline [8]. Since it is a nonselective inhibitor of PDEs, theophylline indiscriminately inhibits izoenzymes in many cell types and organs (Figure 1), in part explaining many of its undesired effects. For example, it often causes dose-related adverse effects such as gastro-intestinal problems, cardiac arrhythmias, headaches, irritability, insomnia and lowering of the seizure threshold. It also interacts with drugs such as fluoroquinolones, macrolides, lithium, rifampicin and anticonvulsants. Moreover, in liver cirrhosis and heart failure there is reduced plasma clearance leading to increased concentrations, while in cigarette smokers, the plasma clearance is increased. This all implies that theophylline doses require to be titrated slowly and plasma monitoring is required.
Figure 1.
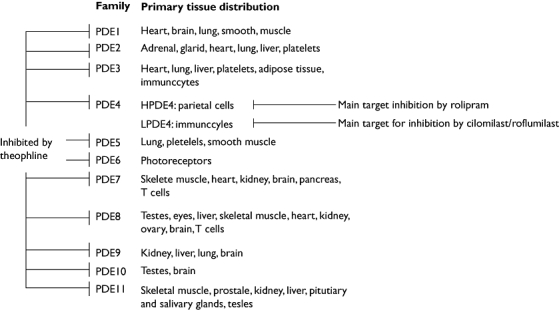
Phosphodiesterase isoenzymes are found in a variety of cells throughout the body. Figure reproduced with permission from Vignola [42], with permission from Elsevier
The isoenzyme PDE4 is expressed in many pro-inflammatory cells found in the airway such as neutrophils, macrophages, eosinophils, mast cells and lymphocytes [11]. As a consequence, selective PDE4 inhibition confers an inhibitory effect upon various inflammatory and immunomodulatory cells. It might therefore be assumed that a theophylline derivative which exhibits more selective PDE isoenzyme inhibition, could result in greater overall benefit in the management of COPD. Moreover, newer theophylline derivatives may not exhibit some of the effects peculiar to theophylline (such as adenosine receptor antagonism which is implicated in unwanted effects) [8] and in turn be of overall greater therapeutic efficacy.
First generation phosphodiesterase inhibitors
Rolipram was one of early selective PDE4 inhibitors and demonstrated some promise in animals [11], although its use resulted in unacceptable levels of nausea and vomiting [18–20]. An important discovery was the fact that PDE4 enzymes exist in both low and high affinity rolipram binding conformations [21]. Inhibition of high affinity rolipram binding sites (expressed in the nervous system) has been hypothesized to be implicated in adverse effects such as nausea and vomiting, while inhibition of low affinity sites may result in immunomodulatory and anti-inflammatory effects [22].
Second generation phosphodiesterase inhibitors
Following the discovery of rolipram, a variety of more selective second generation PDE4 inhibitors have been developed. Roflumilast and cilomilast are the most clinically advanced PDE4 inhibitors currently undergoing clinical evaluation in obstructive lung disorders (Figure 2). Roflumilast is currently manufactured by Nycomed Inc and Cilomilast is manufactured by GlaxoSmithKline.
Figure 2.
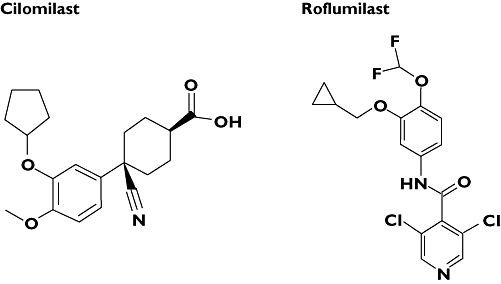
The chemical structures of (a) roflumilast and (b) cilomilast
Unlike rolipram, roflumilast and cilomilast more selectively inhibit low affinity rolipram binding sites in immunomodulatory cells, and have less potency for high affinity rolipram binding sites [20]. A further development was the identification that PDE4 can exist in different isoforms (PDE4A, B, C and D) that are encoded by separate genes. PDE4B is considered to mediate anti-inflammatory effects [23] while PDE4D may be important in unwanted adverse effects, although further work is required to determine the exact functional roles of different PDE4 isoforms across a variety of cellular functions and cell types.
Pharmacology
PDE4 inhibitors exhibit a variety of immunomodulatory and anti-inflammatory effects (Figure 3) [24–27]. Roflumilast is a once daily oral PDE4 inhibitor that is metabolized in the body to an active metabolite, roflumilast N-oxide. Roflumilast and its metabolites are not thought to interact with food or have an altered metabolism depending on whether patients smoke or not [9]. Moreover, no significant interactions with roflumilast or its active metabolite have been identified with warfarin, erythromycin, salbutamol and inhaled budesonide [9].
Figure 3.
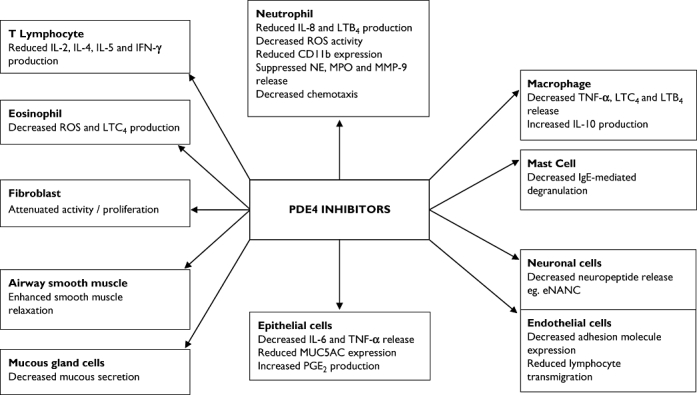
In vivo and in vitro immunomodulatory and anti-inflammatory effects of phosphodiesterase 4 inhibitors. Abbreviations: eNANC, excitatory nonadrenergic noncholinergic; IFN-γ, Interferon gamma; IL, Interleukin; LT, leukotriene; MMP-9, Matrix metalloprotease-9; MPO, Myeloperoxidase; MUC5AC, Mucin 5; subtypes A and C; NE, Neutrophil elastase; ROS, Reactive oxygen species; TNF-α, Tumour necrosis factor alpha. Figure derived with data from Boswell-Smith et al. [39]
Cilomilast is a twice daily administered PDE4 inhibitor. Its metabolism is not significantly affected by cigarette smoking [28], and it has a low potential for interaction with warfarin, digoxin, antacids, prednisolone and salbutamol [29, 30]. The comparative pharmacokinetic profiles of roflumilast, cilomilast and theophylline are shown in Table 1.
Table 1.
Pharmacokinetic profiles of cilomilast, roflumilast and theophylline
| Parameter | Cilomilast | Roflumilast | Theophylline |
|---|---|---|---|
| tmax | 1–2 h | 1.5 h (roflumilast N-oxide 12 h) | Formulation dependant |
| t1/2 | 7 h | 10 h (roflumilast N-oxide 20 h) | 8 h |
| Kinetics | Linear | Linear | Non-linear |
| Oral bioavailability | 96% | 80% | Formulation dependant |
| First pass metabolism | Negligible | Negligible | 90% |
Trials evaluating the use of PDE4 inhibitors
Four fully published randomized placebo controlled trials [31–34] and three studies published in abstract form [35–37] have evaluated the clinical effects of PDE4 inhibitors in patients with COPD (Table 2). The mean FEV1 in all of the studies varied between from 41%−61% predicted and in one study the range of FEV1 was 35%−75% predicted. None of the studies was greater than 1 year duration. From Table 2, it can be seen that when compared with placebo, PDE4 inhibitors do have some benefit upon lung function, exacerbation frequency and quality of life.
Table 2.
Randomized controlled trials evaluating the effects of phosphodiesterase 4 inhibitors vs. placebo in patients with COPD
| Study | n | Duration (weeks) | Mean FEV1 (% predicted) | PDE4 inhibitor | Lung function | Exacerbations | QOL or symptoms |
|---|---|---|---|---|---|---|---|
| Compton [31] | 424 | 6 | 47 | Cilomilast | PDE4+† | NM | PDE4↔ |
| Rennard [34] | 647 | 24 | 47 | Cilomilast | PDE4+† | PDE4+ | PDE4+† |
| Gamble [32] | 59 | 12 | 56 | Cilomilast | PDE4↔ | NM | NM |
| Rabe [33] | 1411 | 24 | 51 | Roflumilast | PDE4+† | PDE4+ | PDE4+† |
| Bredenbroker [35]* | 516 | 26 | 35–75 (range) | Roflumilast | PDE4+ | PDE4+ | NM |
| Calverley [36, 37, 40]* | 1513 | 52 | 41 | Roflumilast | PDE4+ | PDE4+ | PDE4↔ |
| Grootendorst [41]* | 38 | 4 | 61 | Roflumilast | PDE4+ | NM | NM |
Denotes that the study is published in abstract form alone and
denotes primary endpoint. n, number of randomized individuals; FEV1, forced expiratory volume in 1 s; QOL, quality of life; PDE4+ denotes significant improvement with phosphodiesterase 4 inhibitor vs. placebo and PDE4↔ denotes nonsignificant difference with phosphodiesterase 4 inhibitor vs. placebo; NM, information not mentioned or not available from paper or abstract; COPD, chronic obstructive pulmonary disease.
In the largest double-blind, randomized, placebo controlled study, 1411 patients with COPD (mean FEV1 around 51% predicted) were randomized to receive roflumilast 250 μg (n = 576), roflumilast 500 μg (n = 555) or placebo (n = 280) for 24 weeks [33]. For the primary outcome of postbronchodilator FEV1, both doses conferred improvements amounting to 74 ml and 97 ml compared with placebo (P < 0.0001) for the low and high doses, respectively. Similarly, for the other primary outcome of health related quality of life, the differences between both doses of roflumilast and placebo were significant. Although a secondary outcome measure, the mean numbers of exacerbations for each patient were similar at 1.1, 1.0, and 0.8 with placebo, roflumilast 250 μg and roflumilast 500 μg day−1, respectively. A major problem with this study was the fact that only a small proportion of individuals were using regular inhaled treatment. For example, less than 20% were using long acting β2-adrenoceptor agonists, around 20% were using inhaled corticosteroids and less than 40% were using anticholinergics.
In an earlier 6 week study, three dosing regimes for cilomilast (5 mg, 10 mg and 15 mg twice daily) vs. placebo were evaluated in patients with COPD (mean FEV1 47% predicted) [31]. Cilomilast 15 mg twice daily significantly improved the FEV1 compared with placebo (mean 130 ml vs. a reduction of 30 ml, respectively, P < 0.0001). Similar improvements were also observed in terms of forced vital capacity and peak expiratory flow (P = 0.001 and P < 0.0001 for active drug vs. placebo, respectively), although quality of life was not significantly different between groups. In the same study, the postbronchodilator FEV1 improved in the treatment groups, suggesting an additional benefit of cilomilast over that achieved by β2-adrenoceptor agonists may be conferred. It should also be pointed out that in this study of patients with moderately severe airflow obstruction, most individuals were not receiving maximal inhaled treatment.
In a double-blind, placebo-controlled, parallel-group, multicentre study, individuals with COPD were randomized in a 2 : 1 ratio to receive twice daily cilomilast 15 mg or placebo for 24 weeks [34]. The mean change from baseline in FEV1 over 24 weeks in the cilomilast group was an increase of 10 ml compared with a decrease of 30 ml in the placebo group (P = 0.002 for the difference). Taken over 24 weeks, a clinically significant reduction was apparent in the mean total quality of life score in subjects receiving cilomilast compared with placebo (P = 0.001 for the difference). Moreover, a greater proportion receiving cilomilast experienced no exacerbations at 24 weeks (74%) compared with placebo (62%, P = 0.008 for the difference).
Other studies have specifically evaluated the effects of PDE4 inhibitors in terms of inflammatory cell profile. For example, in a parallel-group randomized, placebo-controlled trial lasting 12 weeks [32], CD8+ T lymphocytes and CD68+ monocytes/macrophages (both of which are considered to be involved in the inflammatory process of COPD) significantly fell in bronchial biopsy specimens in patients using cilomilast. However, no significant differences were observed between treatment and placebo groups in terms of sputum neutrophils percentage (which was the primary outcome measure), IL-8 or neutrophil elastase concentrations.
Adverse effects
In contrast to theophylline, PDE4 inhibitors do not require plasma monitoring and pose far less of a problem in terms of interaction with other drugs mainly due to the fact that theophylline is metabolized in the liver through the cytochrome P450 system. However, studies highlighted in this paper do demonstrate that PDE4 inhibitors are associated with a higher incidence of gastrointestinal side-effects, typically nausea, vomiting, diarrhoea and abdominal pain, when compared with placebo. Table 3 summarizes the frequency of adverse effects identified from currently available literature [31–34, 36, 38].
Table 3.
Frequency of adverse effects with phosphodiesterase 4 inhibitors vs. placebo (% of patients)
| Study | Drug | Greater than one adverse event | Nausea | Respiratory disorder | Diarrhoea | Abdominal pain | Headache |
|---|---|---|---|---|---|---|---|
| Compton [31] | Cilomilast | 61 vs. 52 | 12 vs. 1 | 10 vs. 16 | 9 vs. 1 | 8 vs. 3 | 7 vs. 7 |
| Rennard [34] | Cilomilast | 87 vs. 82 | 5 vs. 1 | N/A | 8 vs. 4 | 8 vs. 4 | N/A |
| Gamble [32] | Cilomilast | N/A | 10 vs. 7 | N/A | 21 vs. 14 | N/A | N/A |
| Rabe [33] | Roflumilast | 67 vs. 62 | 3–5 vs. 1 | 23 vs. 23 | 9 vs. 2 | N/A | <1 vs. 2 |
| Calverley [36] | Roflumilast | N/A | 3 vs. N/A | N/A | 6 vs. N/A | N/A | N/A |
| Leichtl [38] | Roflumilast | 49 vs. 49 | 2 vs. N/A | N/A | 1 vs. N/A | N/A | 2 vs. N/A |
N/A, result not available or recorded. Not all studies published as abstracts alone are included due to lack of data surrounding adverse effect profile. Where different doses of active drug were used in the studies, the higher frequency of adverse effects is shown.
In the largest study, evaluating roflumilast 250 μg and 500 μg [33], the numbers of patients withdrawing from the study randomized to placebo, and low and high doses of roflumilast were 32 (11%), 100 (17%) and 124 (22%), respectively. The most common adverse effects, which were not considered related to active treatment, were exacerbations of COPD and nasopharyngitis. Diarrhoea was the most common adverse effect considered to be a result of roflumilast and occurred in no patients using placebo and 13 (2%) and 34 (6%) of those treated with low and high doses of roflumilast, respectively. However, this was considered to be generally mild-to-moderate in severity and occurred more often within the first 4 weeks of treatment. In the same study, nausea occurred in no patients receiving placebo and 6 (1%) and 18 (3%) of those low and high doses of roflumilast, respectively; despite this finding, vomiting was rare. There were no significant changes in terms of electrocardiogram or laboratory findings.
In another study, 424 patients (mean FEV1 47% predicted) were randomized to receive twice daily 5 mg, 10 mg or 15 mg cilomilast [31]. Nausea, which was usually mild to moderate and self-limiting, was the most common adverse effect and occurred in 1 (1%), 1 (1%), 12 (12%) and 12 (11%) of individuals randomized to receive placebo and low, medium and high doses of cilomilast, respectively. Diarrhoea was relatively uncommon and occurred in only 2 (1%), 2 (2%), 4 (4%) and 9 (9%) of those using placebo and low, medium and high doses of cilomilast, respectively. During the study, the most serious adverse event was an exacerbation of COPD. No relevant changes in laboratory parameters or electrocardiograph recordings were observed with any randomised treatment dose.
In a 12 week study of 59 individuals, diarrhoea occurred in four (13%) and six (21%) of patients receiving placebo and cilomilast, respectively, and was reported as being mild to moderate in nature [32]. Two patients receiving placebo reported nausea compared with three treated with cilomilast. No changes in laboratory parameters occurred in either randomized group.
These finding do support the notion that PDE4 inhibitors are associated with some adverse effects which mainly affect the gastro-intestinal system, although direct comparisons with traditional phosphodiesterase inhibitors have not been made. However, they do suggest that this group of drugs are generally safe and tolerated by patients with COPD; whether these findings can be extrapolated into ‘real-life’ and patient long-term tolerability is yet to be discovered.
Conclusions
The studies highlighted in this review do indicate that PDE4 inhibitors confer some benefits in COPD when compared with placebo in terms of lung function, quality of life and exacerbations. They also appear to be generally well tolerated although close pharmacovigilance is required in future long-term studies and real-life settings.
Inhaled bronchodilators form the cornerstone of pharmacological intervention in the management of symptomatic COPD. Non-selective phosphodiesterase inhibitors such as theophylline have usually been confined as a last resort in patients with ongoing symptoms and exacerbations. However, PDE4 inhibitors now represent a specific class of drug which initially appear to confer fewer problems than theophylline in terms of drug interactions, need to monitor plasma levels or adjustment of the dose in individuals with concomitant medical conditions. Indeed, there remains a considerable unmet need in terms of an effective oral bronchodilator in an attempt to avoid the problems encountered with inhaled treatment in COPD and perhaps PDE4 inhibitors might help pave the way in overcoming this problem. The studies highlighted in this paper demonstrate that PDE4 inhibitors appear to confer benefit in improving lung function and health-related outcomes, while the oral route of administration may present a compliance and ease of administration advantage over inhaled medication. However, current data do indicate that there are consistent increases in gastro-intestinal adverse effect profiles for these drugs when compared with placebo.
Further studies are required to establish whether PDE4 inhibitors do in fact have a definite place in the step-wise management of COPD. Ultimately, the prescribing clinician will wish to know whether selective phosphodiesterase inhibitors confer clinical advantages over and above existing management algorithms and if so, under what circumstances they should be prescribed. For example, is this new class of drug effective as monotherapy? Do they confer additive effects to inhaled long acting bronchodilators? Do they confer additive effects to inhaled corticosteroids? Are they more or less effective in individuals with mild, moderate or severe disease? Will they confer overall pharmacoeconomic benefits? Can they alter the natural history of COPD? Moreover, before the long-term safety and tolerability of these drugs are truly established, large numbers of patients with COPD over a prolonged period of time will require to use them not only in the domain of clinical trials, but also in the real world setting. Indeed, no study evaluating the effects of PDE4 inhibitors has had a duration of greater than 1 years or been specifically designed to identify effects upon mortality. The jury must surely be out as to whether PDE4 inhibitors are merely theophylline in disguise.
Competing interests: None declared.
REFERENCES
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava P, Currie GP, Britton J. Smoking cessation. Br Med J. 2006;332:1324–6. doi: 10.1136/bmj.332.7553.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie GP, Rossiter C, Miles SA, Lee DK, Dempsey OJ. Effects of tiotropium and other long acting bronchodilators in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2006;19:112–9. doi: 10.1016/j.pupt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Currie GP, Lipworth BJ. Pharmacological management – inhaled treatment. Br Med J. 2006;332:1439–41. doi: 10.1136/bmj.332.7555.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.Diette GB, Wu AW, Skinner EA, Markson L, Clark RD, McDonald RC, Healy JP, Jr, Huber M, Steinwachs DM. Treatment patterns among adult patients with asthma: factors associated with overuse of inhaled beta-agonists and underuse of inhaled corticosteroids. Arch Intern Med. 1999;159:2697–704. doi: 10.1001/archinte.159.22.2697. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Chronic obstructive pulmonary disease * 12: New treatments for COPD. Thorax. 2003;58:803–8. doi: 10.1136/thorax.58.9.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–8. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- 9.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–75. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 10.Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;108:671–80. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- 11.Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998;157:351–70. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M, Nasuhara Y, Betsuyaku T, Shibuya E, Tanino Y, Tanino M, Takamura K, Nagai K, Hosokawa T, Nishimura M. Effect of low-dose theophylline on airway inflammation in COPD. Respirology. 2004;9:249–54. doi: 10.1111/j.1440-1843.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 13.Culpitt SV, de Matos C, Russell RE, Donnelly LE, Rogers DF, Barnes PJ. Effect of theophylline on induced sputum inflammatory indices and neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1371–6. doi: 10.1164/rccm.2105106. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ. Reduced histone deacetylase in COPD: clinical implications. Chest. 2006;129:151–5. doi: 10.1378/chest.129.1.151. [DOI] [PubMed] [Google Scholar]
- 15.Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med. 2004;200:689–95. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl. 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsten DK, Wegner RE, Jorres RA, Magnussen H. Effects of theophylline withdrawal in severe chronic obstructive pulmonary disease. Chest. 1993;104:1101–7. doi: 10.1378/chest.104.4.1101. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–63. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Renau TE. The potential of phosphodiesterase 4 inhibitors for the treatment of depression: opportunities and challenges. Curr Opin Invest Drugs. 2004;5:34–9. [PubMed] [Google Scholar]
- 20.Torphy TJ, Barnette MS, Underwood DC, Griswold DE, Christensen SB, Murdoch RD, Nieman RB, Compton CH. Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic. Pulm Pharmacol Ther. 1999;12:131–5. doi: 10.1006/pupt.1999.0181. [DOI] [PubMed] [Google Scholar]
- 21.Jacobitz S, McLaughlin MM, Livi GP, Burman M, Torphy TJ. Mapping the functional domains of human recombinant phosphodiesterase 4A: structural requirements for catalytic activity and rolipram binding. Mol Pharmacol. 1996;50:891–9. [PubMed] [Google Scholar]
- 22.Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9:227–36. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- 23.Manning CD, Burman M, Christensen SB, Cieslinski LB, Essayan DM, Grous M, Torphy TJ, Barnette MS. Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4B. Br J Pharmacol. 1999;128:1393–8. doi: 10.1038/sj.bjp.0702911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005;106:269–97. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–79. [PubMed] [Google Scholar]
- 26.Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297:280–90. [PubMed] [Google Scholar]
- 27.Kumar RK, Herbert C, Thomas PS, Wollin L, Beume R, Yang M, Webb DC, Foster PS. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther. 2003;307:349–55. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- 28.Kelly J, Murdoch RD, Clark DJ, Zussman B, Davie C, Howland K. Smoking status has no effect on the clearance of a single dose of Airiflo (SP207499) (15mg), an orally active, novel, second generation PDE4 inhibitor in healthy male volunteers. Am J Respir Crit Care Med. 1999;159:A807. (Abstract). [Google Scholar]
- 29.Zussman BD, Davie CC, Kelly J, Murdoch RD, Clark DJ, Schofield JP, Walls C, Birrell C, Webber D, Quinlan J, Ritchie SY, Carr A. Bioavailability of the oral selective phosphodiesterase 4 inhibitor cilomilast. Pharmacotherapy. 2001;21:653–60. doi: 10.1592/phco.21.7.653.34569. [DOI] [PubMed] [Google Scholar]
- 30.Giembycz MA. Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease. Expert Opin Invest Drugs. 2001;10:1361–79. doi: 10.1517/13543784.10.7.1361. [DOI] [PubMed] [Google Scholar]
- 31.Compton CH, Gubb J, Nieman R, Edelson J, Amit O, Bakst A, Ayers JG, Creemers JP, Schultze-Werninghaus G, Brabilla C, Barnes NC. Cilomilast, a selective phosphodiesterase-4 inhibitor for treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging study. Lancet. 2001;358:265–70. doi: 10.1016/S0140-6736(01)05481-2. [DOI] [PubMed] [Google Scholar]
- 32.Gamble E, Grootendorst DC, Brightling CE, Troy S, Qiu Y, Zhu J, Parker D, Matin D, Majumdar S, Vignola AM, Kroegel C, Morell F, Hansel TT, Rennard SI, Compton C, Amit O, Tat T, Edelson J, Pavord ID, Rabe KF, Barnes NC, Jeffery PK. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:976–82. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- 33.Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 34.Rennard SI, Schachter N, Strek M, Rickard K, Amit O. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;129:56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- 35.Bredenbroker D, Syed J, Leichtl S, Rathgeb F, Wurst W. Roflumilast, a new orally active, selective phosphodiesterase 4 inhibitor, is effective in the treatment of chronic obstructive pulmonary disease [abstract] Eur Respir J. 2002;20(Suppl. 38):374S. [Google Scholar]
- 36.Calverley PM, Sanchez-Toril F, McIvor RA, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of Roflumilast on lung function: a 1-year study in patients with severe to very severe COPD [abstract] Proc Am Thorac Soc. 2006;3:A725. [Google Scholar]
- 37.Fabbri LM, Sanchez-Toril F, McIvor RA, Teichmann P, Bredenbroeker D, Calverley PM. Effect of roflumilast on exacerbations: a 1-year study in patients with severe to very severe COPD [abstract] Proc Am Thorac Soc. 2006;3:A841. [Google Scholar]
- 38.Leichtl S, Syed J, Bredenbroeker D, Rathgeb F, Wurst W. Roflumilast, a new, orally active, selective phosphodiesterase 4 inhibitor, is safe and well tolerated in patients with chronic obstructive pulmonary disease [abstract] Eur Respir J. 2002;20(Suppl. 38):303s. [Google Scholar]
- 39.Boswell-Smith V, Cazzola M, Page CP. Are phosphodiesterase 4 inhibitors just more theophylline? J Allergy Clin Immunol. 2006;117:1237–43. doi: 10.1016/j.jaci.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 40.McIvor RA, Calverley PM, Sanchez-Toril F, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of roflumilast on quality of life: a 1-year study in patients with severe to very severe COPD [abstract] Proc Am Thorac Soc. 2006;3:A850. [Google Scholar]
- 41.Grootendorst DC, Gauw SA, Sterk PJ, Bethke TD, Hospers JJ, Hiemstra PS, Rabe KF. Treatment with PDE4 inhibitor roflumilast reduces sputum neutrophils and eosinophil numbers in patients with COPD [abstract] Proc Am Thorac Soc. 2005;2:A543. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vignola AM. PDE4 inhibitors in COPD – a more selective approach to treatment. Resp Med. 2004;98:495–503. doi: 10.1016/j.rmed.2003.12.012. [DOI] [PubMed] [Google Scholar]


