Abstract
AIMS
Relative to nonsmokers, the bioavailability of inhaled human insulin (Exubera®; EXU) is markedly increased in chronic smokers. The pharmacokinetics of EXU following passive cigarette smoke exposure is unknown.
METHODS
In an open-label, crossover study, healthy nonsmoking volunteers received two treatments in randomized sequence separated by a 2-week wash-out: (i) EXU 3 mg with no passive smoke exposure and (ii) EXU 3 mg after passive smoke exposure (atmospheric nicotine levels 75–125 μg m−3) for 2 h. Blood samples were obtained at prespecified times up to 6 h after EXU administration.
RESULTS
Twenty-seven subjects completed both study periods. Mean plasma insulin AUC0−360 decreased by 17% [ratio 83%, 95% confidence interval (CI) 68.8, 99.5] and mean Cmax by 29% (ratio 71%, 95% CI 59.8, 83.1) after passive cigarette smoke exposure. The median (range) tmax was 60 min (20–120 min) and 75 min (20–360 min) in the EXU with no exposure and EXU passive exposure groups, respectively. EXU was well tolerated.
CONCLUSIONS
Unlike active chronic smoking, acute passive cigarette smoke exposure modestly decreases EXU bioavailability and thus should not increase hypoglycaemia risk. These results are consistent with those from published literature involving technetium-labelled diethylenetriamine penta-acetic acid and suggest that passive cigarette smoke exposure causes an acute decrease in lung permeability vs. active smoking, which causes an increase in permeability.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Active cigarette smoking is associated with increased permeability of the pulmonary alveolar epithelium, resulting in faster absorption of inhaled drugs such as Exubera® (EXU). Absorption of EXU is increased approximately twice to four times as much in chronic smokers compared with nonsmokers.
The rate of clearance of radioaerosols such as technetium-labelled diethylenetriamine penta-acetic acid is decreased in response to passive smoke exposure.
WHAT THIS STUDY ADDS
Passive smoke exposure causes a decrease in lung permeability, an effect opposite to that of active smoking.
Acute passive smoke exposure results in a decrease in EXU bioavailability and does not create a risk of hypoglycaemia.
These results are consistent with previous studies of radioaerosol lung clearance.
Keywords: inhaled human insulin, passive smoking, pharmacokinetics
Introduction
Cigarette smoking remains prevalent in many countries. Passive smoking is defined as involuntary exposure to a diluted combination of cigarette sidestream smoke (gas and particle phases evolved from the smouldering end of a cigarette) and mainstream smoke (mixture inhaled by the smoker and exhaled after lung filtration) [1]. Sidestream smoke is believed to be the primary source of cigarette smoke exposure of nonsmokers [2]. Cigarette smoke constituents can affect the pharmacokinetic and pharmacodynamic properties of many drugs through alterations in drug absorption, distribution, metabolism, excretion and effectiveness [3, 4].
Active cigarette smoking is also associated with increased permeability of the pulmonary alveolar epithelium to solutes. This has been measured by the rate of clearance from lung to blood of inhaled diethylenetriamine penta-acetic acid (DTPA) labelled with technetium-99m(99mTc). Following aerosol inhalation, DTPA is deposited primarily in the alveoli and diffuses across the interepithelial pores of epithelia with no apparent active transport mechanism [5]. The rate of clearance of 99mTc-DTPA from the lung may therefore be used as a measure of the integrity of the alveolar capillary barrier [6]. Active smoking increases the clearance of 99mTc-DTPA [7–9], indicating increased alveolar permeability. Increased alveolar permeability also results in faster absorption of inhaled drugs such as terbutaline [10].
In contrast, acute passive smoking modestly decreases pulmonary permeability of DTPA, as shown in a study of 1-h exposure to sidestream smoke in 20 nonsmoking volunteers [11]. It is not clear whether long-term and high-level exposure to smoke in nonsmokers causes permeability changes similar to those seen in smokers.
Inhalable forms of human insulin are being developed to facilitate the uptake of insulin therapy and improve diabetes outcomes [12, 13]. Inhaled human insulin (Exubera®; EXU) is a novel, non-invasive, pulmonary dry-powder human insulin and delivery system developed by Pfizer Inc. in collaboration with Nektar Therapeutics, which has been approved in both the USA and European Union for the treatment of type 1 and type 2 diabetes in adults [13]. Long-term pulmonary tolerability has been demonstrated with EXU in combination with oral agent regimens in patients with type 2 diabetes [14]. In particular, changes in pulmonary function compared with comparator groups were small, nonprogressive, and reversed upon discontinuation of therapy. Importantly, rates of lung function change were indistinguishable between EXU and comparator between 6 months and 2 years of therapy [14].
The effects of cigarette smoking on EXU pharmacokinetics have been previously studied in healthy subjects [15, 16]. In comparison with nonsmokers, smokers had a significantly greater Cmax (three- to fivefold increase) and AUC (two- to threefold increase) following EXU administration [15, 16]. Smoking cessation diminished both the insulin exposure and pharmacodynamic response [15, 16], but resumption of smoking for only 2–3 days almost completely returned EXU exposure and pharmacodynamic response to precessation levels [16].
The pharmacokinetics of EXU in subjects who are passively exposed to cigarette smoke on an acute basis, such as that which might occur under typical social conditions, has not been studied previously. Considering the increase in EXU exposure that is observed in chronic smokers, additional pharmacokinetic data that explores the impact of passive cigarette smoke may be useful to help guide the use of EXU in patients passively exposed to cigarette smoke. The objective of the current study was to assess the effect of acute passive cigarette smoke exposure on the single-dose pharmacokinetics of EXU in healthy, nonsmoking subjects.
Materials and methods
This was an open-label, randomized, two-centre study. The study protocol was approved by the Institutional Review Board of each participating centre, and all subjects provided written informed consent. The study was conducted in accordance with the ethical principles originating from the Declaration of Helsinki. Financial support was provided by Pfizer Central Research and Development (the sponsor).
Participants
Inclusion criteria were: (i) healthy male or female nonsmoking volunteers aged 18–55 years; (ii) body mass index 18–35 kg m−2; (iii) total body weight >50 kg (110 lb); (iv) negative screening and predose semiquantitative urinary cotinine test results (NicAlertTM); (v) quantitative cotinine levels at screening <5 ng ml−1 (28.4 nmol l−1); (vi) written informed consent. Exclusion criteria included a history of moderate or severe asthma or chronic obstructive pulmonary disease; pulmonary function tests in the following ranges: forced expiratory volume in 1 s (FEV1) <80% of predicted value, carbon monoxide diffusion capacity (DLco) >120% or <70% predicted value, FEV1/forced vital capacity (FVC) <0.70; major organ system disease; psychiatric, neurological or allergic disease; history of use of any tobacco- or nicotine-containing products within 6 months of screening or a positive urine cotinine test; any history of chronic smoking defined as ≥15 cigarettes per day for a period of ≥6 months; known drug misuse (positive urine screen) or history of alcohol dependence in the 6 months preceding enrolment; use of prescription or nonprescription drugs, vitamins and dietary supplements within 14 days prior to the first dose of study medication (with the exception of paracetamol, hormone replacement therapy and hormonal contraceptives); and pregnant, lactating or women of childbearing age not using an acceptable method of contraception. It was deemed reasonable to assume that results gained in nonsmoking members of the general population would be applicable to persons with diabetes, although it is acknowledged that the average rate of decline of lung function as measured by FEV1 is greater in comparison with that of healthy nonsmokers [17].
Study design
The study was crossover in design and randomized with respect to passive smoke exposure order. In one study period EXU was administered with no passive cigarette smoke exposure, and in the other study period EXU was administered after 2 h of passive smoke exposure. Numbers were assigned to subjects sequentially as they were screened for the trial. A randomization schedule was provided by the sponsor, and subjects received the trial treatments in accordance with this schedule.
Each subject received a training dose [one inhalation of the 3-mg dose (EXU is available in 1- and 3-mg dose blister packs)] 15 min prior to receiving breakfast on days −3 and −1 of both study periods. On study day 1 of each study period, following an 8-h overnight fast, subjects received a single EXU dose (3 mg) following either acute exposure to passive cigarette smoke or no exposure in randomized crossover fashion. In the passive smoke exposure plus EXU treatment arm, subjects were required to remain seated in the study room in the presence of cigarette smoke for approximately 2 h. During this time subjects were permitted to read, converse and watch television. Subjects were then taken to a separate room to administer the EXU dose. There was a wash-out interval of at least 2 weeks between study periods.
Blood glucose levels were measured at approximately 5–10-min intervals up to 6 h after dosing. Infusion of a dextrose solution was to be initiated when postdose glucose concentration dropped by ≥5 mg dl−1 (0.3 mmol l−1) from baseline glucose concentration, and was to be infused as necessary until 6 h postdose. The infusion rate was to be adjusted on a subject-by-subject basis so that blood glucose levels were maintained at or below the baseline blood glucose concentration and at a level sufficient to prevent hypoglycaemia. This modified euglycaemic clamp was implemented solely to prevent hypoglycaemia and, as such, glucose infusion rates were not included in the study database.
Atmospheric nicotine levels
Sidestream smoke was generated using a commercially available smoking machine (Model CSM2072i; CH Technologies Inc., Westwood, NJ, USA) and full-flavour cigarettes with a goal of achieving an atmospheric nicotine concentration between 75 and 125 μg m−3. This level was selected as it is expected to reproduce levels of nicotine in various social settings of high involuntary smoke exposure where subjects treated with EXU may visit [2]. As real-time measurement of nicotine concentrations was not possible, the level of passive smoke was titrated to the amount of total suspended particulate matter that was measured using a real-time monitoring probe (Casella Microdust Pro; Casella USA, Nashua, NH, USA). Once the relationship between suspended particulate level and atmospheric nicotine concentration had been established in the smoke exposure room, two pilot studies were conducted prior to any subjects being administered study drug. The pilot studies were conducted to verify that the passive smoke exposure was resulting in changes in urinary cotinine levels. During each of the pilot studies, four to eight healthy volunteers were exposed to passive smoke exposure for approximately 2 h. Urinary cotinine and plasma carboxyhaemoglobin measurements were collected at various time intervals. No EXU was administered during these pilot studies. Subjects who participated in the pilot studies were not eligible for participation in the main study.
During the 2-h passive smoke exposure sessions, air was continuously drawn in parallel, through three resin-filled glass filters (XAD-4) set up in parallel at approximately 1 l min−1. At the conclusion of each smoke exposure session, quantification of nicotine in the filter was determined (Arista Laboratories, Richmond, VA, USA). The mean and standard deviation of the three reported concentrations normalized by volume (m3) of air sampled was reported for each 2-h smoke exposure session.
Insulin pharmacokinetics
To standardize the conditions for pharmacokinetic sampling, all subjects were required to refrain from lying down, eating (with the exception of the training session, when breakfast was consumed) and drinking anything other than water during the first 6 h after dosing.
Whole-blood samples were collected and ethylenediamine tetraaceticacid plasma samples were harvested for pharmacokinetic analysis of total insulin and C-peptide at the following time points in both study periods: day 1 at 30, 20 and 10 min prior to dosing; and 0 (just prior to insulin dosing), 10, 20, 30, 40, 50, 60, 75, 90, 120, 180, 240 and 360 min after insulin dosing. Blood samples were centrifuged for approximately 10 min at 4°C and the plasma was stored at −20°C within 1 h of collection until analysis at a central laboratory.
Plasma total insulin concentrations were determined by radioimmunoassay using a Linco Research Inc. (catalogue no. HI-11K; Billerica, MA, USA) ultrasensitive human insulin kit. 125I-labelled insulin antigen was incubated with insulin antiserum and the radioactivity of the samples was measured using a gamma counter to determine the level of insulin. Insulin concentrations were determined by back-calculating the results against a standard curve containing known insulin concentrations. The limit of quantification (LOQ) for the assay was 2.02 μU ml−1; the interassay precision [overall percent coefficient of variation (CV%)] and the bias were <12% and 9%, respectively, in the calibration range of 2.02–100 μU ml−1.
Plasma C-peptide concentrations were determined by radioimmunoassay using a Diagnostic Products Corporation (catalogue no. KPED2; Los Angeles, CA, USA) double antibody C-peptide kit. 125I-labelled C-peptide antigen was incubated with C-peptide antiserum and the radioactivity of the samples was measured using a gamma counter to determine the level of C-peptide. C-peptide concentrations were determined by back-calculating the results against a standard curve containing known C-peptide concentrations. The LOQ for the assay was 0.095 ng ml−1; the interassay precision and bias were <7% and 12%, respectively, in the calibration range of 0.095–9.02 ng ml−1. For each subject and on each treatment day, insulin concentrations at each time point were corrected for endogenous insulin to yield exogenous insulin concentration by assuming a constant ratio between endogenous insulin and C-peptide concentrations [18]. The following equation was used:
where Insulinobs = observed plasma insulin concentration, f = mean of the ratios of plasma insulin/C-peptide concentrations at −30, −20, −10 and 0 min, and C-peptideobs = observed plasma C-peptide concentration.
This method of correcting for endogenous insulin can give negative values in certain circumstances. These negative values were set to zero. Insulinexog was summarized to give the maximum observed insulin concentration (Cmax), the first time to maximum insulin concentration (tmax), the area under the insulin concentration–time curve to time t = 120, 240 and 360 min postdose (AUC0–t), and the apparent terminal half-life (t1/2), as data permitted.
Safety
Safety was assessed by reporting of adverse events. Spirometry measurements (FEV1 and DLco) were obtained at screening and follow-up (3–5 days after period 2 dosing).
Statistical methods
A sample size of 24 completers was planned to ensure at least 95% power to detect a 30% change in the EXU–AUC0−360 for the acute passive cigarette smoke exposure followed by EXU treatment relative to EXU alone. A change in AUC0−360 of ≥30% was thought to represent a clinically relevant effect on EXU exposure related to prior passive cigarette smoke exposure.
Log-transformed AUC0−360 and Cmax were analysed by means of a factorial analysis of variance (anova) using the SAS® MIXED procedure and the Restricted Maximum Likelihood (REML) estimation (SAS Inc., Cary, NC, USA). Treatment (A: EXU alone; B: passive cigarette smoke exposure followed by EXU), sequence and period were specified as the fixed effects, with a random effect for subject nested within sequence. The point estimates of treatment differences (treatment B − treatment A) and the 95% confidence interval (CI) for the true differences were obtained using the appropriate linear contrast. The antilog transformation was applied to the estimated difference and the CI to obtain a ratio (treatment B/treatment A) and CI for the true ratio. Treatment A was considered the reference in the comparisons.
Results
Subject disposition and baseline characteristics
Fifty subjects were enrolled in this study; 14 were pilot subjects receiving passive smoke exposure only. Of the remaining 36 volunteers who did receive study drug, three subjects withdrew or were terminated from the study during the training phase prior to contributing any pharmacokinetic data, and one subject who completed all pharmacokinetic assessments was found to have a protocol violation (elevated urine cotinine at baseline) and was therefore not included in the statistical analyses. In total, 32 volunteers contributed data from one or both treatment periods (EXU alone and/or EXU +passive smoke exposure). Twenty-six subjects completed both treatment periods, four completed only the EXU +passive smoke exposure treatment and two completed the EXU in the absence of smoke exposure treatment. Of the nine subjects who did not complete the study, two were no longer willing to participate, one was found not to meet the entry criteria, and six were discontinued because they had completed only one of two treatment arms when the sponsor terminated the study at one centre due to low enrollment. Baseline demographic characteristics for the 32 subjects included in the statistical analysis are presented in Table 1.
Table 1.
Baseline demographic characteristics
| Total (n = 32) | |
|---|---|
| Age (years) | |
| Mean (SD) | 35.9 (11.0) |
| Range | 18–56 |
| M/F | 17/15 |
| Race | |
| White | 9 |
| Black | 5 |
| Hispanic | 18 |
| Body mass index (kg m−2) | |
| Mean (SD) | 29.0 (4.3) |
| Range | 18.9–35.2 |
Atmospheric nicotine levels
Mean (SD) atmospheric nicotine concentrations in the passive smoke exposure room ranged from 60 (0.3) μg m−3 to 135 (1.9) μg m−3 (Table 2). Urine cotinine concentrations approximating values reported for nonsmokers who are passively exposed to tobacco smoke [5–30 ng ml−1 (28–170 nmol l−1)][19] were achieved (Table 2).
Table 2.
Descriptive statistics [mean ± SD] for atmospheric nicotine and urine cotinine sampling in subjects receiving Exubera (EXU) 3 mg after 2 h of passive cigarette smoke exposure
| Atmospheric nicotine | Number subjects exposed (n) | Atmospheric nicotine concentration (μg m−3) |
|---|---|---|
| 2 | 97.96 ± 10.11 | |
| 2 | 128.80 ± 4.00 | |
| 3 | 135.19 ± 1.88 | |
| 7 | 59.66 ± 0.33 | |
| 3 | 70.71 ± 0.56 | |
| 6 | 108.16 ± 2.73 | |
| 7 | 111.49 ± 0.99 |
| Urine cotinine treatment sequence | Time post smoke exposure (h) | Urine cotinine (ng ml−1) |
|---|---|---|
| AB (n = 15) | Pre-exposure | 0.2 ± 0.6 |
| 3 | 3.5 ± 1.7 | |
| 6 | 3.8 ± 1.9 | |
| 12 | 4.4 ± 2.4 | |
| 24 | 4.4 ± 1.4 | |
| BA (n = 15) | Pre-exposure | 0.4 ± 1.1 |
| 3 | 3.4 ± 1.9 | |
| 6 | 3.8 ± 1.8 | |
| 12 | 3.8 ± 1.2 | |
| 24 | 4.1 ± 2.0 |
A, Exubera alone; B, Exubera + passive cigarette smoke exposure.
Insulin pharmacokinetics
The bioavailability of EXU was less when administered following 2 h of passive cigarette smoke exposure than without (Table 3, Figure 1). The effects of passive smoke exposure on insulin pharmacokinetic parameters after adjustment for endogenous insulin using mean C-peptide correction are shown in Table 3. Mean (SD) C-peptide corrected plasma insulin AUC0−360 values were 7280 (3100) min μU−1 ml−1 and 5610 (2200) min μU−1 ml−1 in the EXU alone and passive smoke exposure followed by EXU groups, respectively. Corresponding mean (SD) Cmax values were 48.7 (28.8) μU−1 ml−1 and 30.1 (9.9) μU−1 ml−1, respectively. Passive smoke exposure decreased mean plasma insulin AUC0−360 by 17% (ratio 83%, 95% CI 68.8, 99.5) and plasma insulin Cmax by 29% (ratio 71%, 95% CI 59.8, 83.1) relative to the control condition.
Table 3.
Mean (SD) C-peptide corrected insulin pharmacokinetic parameters following Exubera (EXU) 3 mg in the presence and absence of passive smoke exposure
| Arithmetic mean values | EXU alone (n = 28) | Passive smoke + EXU (n = 30) |
|---|---|---|
| AUC0-360 (min μU−1 ml−1) | 7280 (3100) | 5610 (2200) |
| AUC0−240 | 6240 (2680) | 4600 (1750) |
| AUC0−120 | 3790 (1670) | 2590 (1010) |
| Cmax (μU ml−1) | 48.7 (28.8) | 30.1 (9.9) |
| Tmax (min)* | 60 (20–120) | 75 (20–360) |
Median (range).
Figure 1.
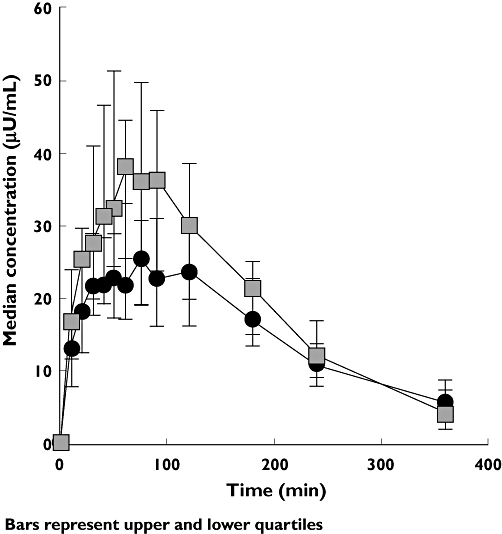
Median C-peptide-corrected insulin concentration–time profiles in the presence and absence of passive smoke exposure. EXU, inhaled human insulin (Exubera). Passive smoke + EXU 3 mg, (▵); EXU 3 mg alone, (▪)
The median (range) tmax was 60 min (20–120 min) and 75 min (20–360 min) in the EXU alone and with passive smoke exposure groups, respectively. The majority of subjects received some glucose via the variable rate glucose infusion during one or both treatment periods. Review of total immunoreactive insulin, C-peptide and glucose concentrations did not reveal any systematic trends suggesting that the variable glucose infusion had an adverse impact on pharmacokinetic assessments for either treatment condition.
Safety
The safety results reflect data from enrolled subjects who received at least one dose of EXU. There were no discontinuations due to adverse events. The number of adverse events for each treatment was similar and the majority of adverse events were mild in intensity and not considered treatment related. Seven of 29 subjects receiving EXU 3 mg in the absence of passive smoke exposure reported a total of 11 adverse events, and eight of 31 subjects receiving EXU 3 mg in the presence of passive smoke exposure reported a total of 12 adverse events. The most frequently reported adverse events in both treatment arms were headache (two subjects in each group), nausea (one in each group), and chest pain (one without and two with smoke exposure). In the two subjects reporting chest pain in the smoke inhalation group, the investigator noted that the events were reported after passive smoke exposure along with tightness or discomfort on inhalation. In the investigator's opinion, these events were respiratory related. One subject receiving EXU in the absence of passive smoke exposure experienced one mild hypoglycaemic event. Mild vomiting, somnolence, or increased cough were each reported by one person in each group. Of these, a single episode of cough was considered to be treatment related.
Discussion
Lung physiology plays a critical role in the absorption, delivery and systemic exposure of inhaled drugs such as EXU. Thus, any factors altering the permeability of the lung may alter drug absorption. When EXU was inhaled after passive exposure to cigarette smoke for 2 h, the overall amount of EXU absorbed and peak EXU concentration after inhalation were modestly reduced by 17% and 29%, respectively, and median tmax was increased by 15 min. These changes in EXU pharmacokinetics indicate a reduction in both the rate and extent of insulin absorption from the lung after acute passive cigarette smoke exposure.
The observations of the present study are in contrast to the markedly greater rate and extent of EXU absorption that has been observed in previous studies in healthy volunteers who were active smokers compared with nonsmokers. In these studies, the rate and extent of EXU absorption were greater, as indicated by a faster time to Cmax and larger (approximately three- to fivefold) AUC in smokers than in nonsmokers [15, 16].
These differences in EXU pharmacokinetics observed between passive and active smoking may be explained by changes in alveolar permeability. Several studies have demonstrated that inhaled 99mTc-DTPA can be used to assess alveolar epithelial injury and that active smoking increases lung permeability, which could in turn result in increased drug exposure [7–9, 11]. It has been shown that when healthy volunteers are passively exposed to sidestream cigarette smoke, 99mTc-DTPA clearance is reduced as a result of decreased lung permeability [11]. The exact mechanisms for this are not fully understood, although it has been suggested that this could be the result of changes to pulmonary microvascular blood flow and/or surfactant due to an active phase irritant response [11].
The duration of passive smoke exposure in this study of 2 h is considered to be a realistic amount of time that an individual would be exposed to high levels of acute passive smoke (atmospheric nicotine concentration of 75–125 μg m−3) in a social setting such as dining at a restaurant, and is within the range of other investigations in this area of research [11, 20–23]. For example, typical atmospheric nicotine levels in a residence with one or more smokers would average 2–10 μg m−3 with high of around 14 μg m−3, and in smoking sections of bars and aircraft would average 50–75 μg m−3[2]. The range specified in this study was selected in order to cover all levels of exposure (including the highest) that might reasonably be expected during the course of a person's typical daily activities.
A limitation of this study is that assessments of bronchial hyper-reactivity following passive smoke exposure were not conducted. We cannot therefore exclude the possibility that acute exposure to passive smoke temporarily increased the bronchial tone, contributing to the observed decrease in insulin bioavailability following passive cigarette smoke exposure. Furthermore, subjects in this study were exposed to acute passive smoke. Chronic exposure is known to carry a risk of bronchial hyper-reactivity [24] and may affect the pharmacokinetics of EXU differently from acute exposure.
As with all methods of correcting for endogenous insulin production in healthy fasting volunteers, the C-peptide correction methodology also has assumptions that should be noted. More specifically, the C-peptide correction methodology assumes that the rate of clearance of insulin and C-peptide is similar, when in fact it is recognized that the clearance rates are different [18]. It is therefore acknowledged that estimates of exogenous insulin concentration derived using this correction methodology are approximations and that the accuracy of this methodology improves as the endogenous insulin contribution gets smaller relative to the measured insulin concentration. To this point, the observed blood glucose concentrations did not appear grossly different [91.2 ± 11.4 mg dl−1 (EXU alone) and 89.8 ± 9.5 mg dl−1 (EXU + passive smoke exposure)], suggesting that endogenous insulin production was similar during both treatments and that the resulting pharmacokinetic parameter estimates and study results are reliable.
During this study, EXU therapy was well tolerated. The majority of adverse events were mild in intensity and there did not appear to be a difference in frequency or intensity of adverse events reported after administration of EXU 3 mg in the absence or presence of passive cigarette smoke exposure.
In conclusion, while cessation of smoking and avoidance of passive smoking is consistent with general health recommendations in patients with diabetes, this study offers some understanding that the exposure of EXU can be influenced by external factors such as passive smoking. This effect is opposite to that seen with active chronic smoking, but consistent with results from published studies of radioaerosol lung clearance. Unlike active chronic smoking, acute passive inhalation of cigarette smoke appears to decrease lung permeability modestly for reasons not fully understood at this time. Despite these observed modest decreases in bioavailability of EXU, acute passive smoke inhalation is unlikely to compromise glycaemic control to any clinically relevant extent, and presents no risk of hypoglycaemia in patients with diabetes. Patients should, however, be made aware of a potential small reduction in insulin absorption that may result from involuntary tobacco smoke exposure, but there is no need on the basis of current data to recommend adjustments in dosage of EXU under these circumstances.
Acknowledgments
Editorial support was provided by J. Grice of PAREXEL and was funded by Pfizer Inc.
REFERENCES
- 1.Nelson E. The miseries of passive smoking. Hum Exp Toxicol. 2001;20:61–83. doi: 10.1191/096032701670538508. [DOI] [PubMed] [Google Scholar]
- 2.Office of Environmental Health Hazard Assessment. Health Effects of Exposure to Environmental Tobacco Smoke. [March 2006]; Final Report September 1997. Available at http://www.oehha.org/air/environmental_tobacco/finalets.html.
- 3.Miller LG. Cigarettes and drug therapy: pharmacokinetic and pharmacodynamic considerations. Clin Pharm. 1990;9:125–35. [PubMed] [Google Scholar]
- 4.Zevin S, Benowitz NL. Drug interactions with tobacco smoking: an update. Clin Pharmacokinet. 1999;36:425–38. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 5.Groth S, Mortensen J, Lange P, Vest S, Rossing N, Swift D. Effect of change in particle number on pulmonary clearance of aerosolized 99mTc-DTPA. J Appl Physiol. 1989;66:2750–5. doi: 10.1152/jappl.1989.66.6.2750. [DOI] [PubMed] [Google Scholar]
- 6.Effros RM, Mason GR. Measurements of pulmonary epithelial permeability in vivo. Am Rev Respir Dis. 1983;27:S59–65. [PubMed] [Google Scholar]
- 7.Jones JG, Lawler P, Crawley JC, Minty BD, Hulands G, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1:66–8. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 8.Nolop KB, Maxwell DL, Fleming JS, Braude S, Hughes JMB, Royston D. A comparison of 99mTc-DTPA and 113mIn-DTPA aerosol clearances in humans. Effects of smoking, hyperinflation, and in vitro oxidation. Am Rev Respir Dis. 1987;136:1112–6. doi: 10.1164/ajrccm/136.5.1112. [DOI] [PubMed] [Google Scholar]
- 9.Mason GR, Uszler JM, Effros RM, Reid BA. Rapidly reversible alterations of pulmonary epithelial permeability induced by smoking. Chest. 1983;83:7–11. doi: 10.1378/chest.83.1.6. [DOI] [PubMed] [Google Scholar]
- 10.Schmekel B, Borgstrom L, Wollmer P. Difference in pulmonary absorption of inhaled terbutaline in healthy smokers and non-smokers. Thorax. 1992;46:225–8. doi: 10.1136/thx.46.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates DH, Havill K, Thompson MM, Rittano AB, Chu J, Glanville AR. Sidestream smoke inhalation decreases respiratory clearance of 99mTc-DTPA acutely. Aust NZ J Med. 1996;26:513–8. doi: 10.1111/j.1445-5994.1996.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 12.Barnett AH. Exubera inhaled insulin: a review. Int J Clin Pract. 2004;58:394–401. doi: 10.1111/j.1368-5031.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 13.Patton JS, Bukar JG, Eldon MA. Clinical pharmacokinetics and pharmacodynamics of inhaled insulin. Clin Pharmaokinet. 2004;43:781–801. doi: 10.2165/00003088-200443120-00002. [DOI] [PubMed] [Google Scholar]
- 14.Barnett AH, Lange P, Dreyer M, Serdarevic-Pehar M Exubera® Phase 3 Study Group. Long-term tolerability of inhaled human insulin (Exubera®) in patients with poorly controlled type 2 diabetes. Int J Clin Pract. 2007;61:1614–25. doi: 10.1111/j.1742-1241.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 15.Sha S, Becker RHA, Willavise SA, Schumacher DA, Lee JD, Carroll RS, Fryburg DA. The effect of smoking cessation on the absorption of inhaled insulin (Exubera®) Diabetes. 2002;51(Suppl. 2):538. (Abstract). [Google Scholar]
- 16.Becker RHA, Sha S, Frick AD, Fountaine RJ. The effect of smoking cessation and subsequent resumption on absorption of inhaled insulin. Diabetes Care. 2006;29:277–82. doi: 10.2337/diacare.29.02.06.dc05-1913. [DOI] [PubMed] [Google Scholar]
- 17.Davis WA, Knuiman M, Kendall P, Grange V, Davis TME. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27:752–7. doi: 10.2337/diacare.27.3.752. [DOI] [PubMed] [Google Scholar]
- 18.Owens DR. Human insulin. Intermediate-acting insulin preparations ‘initial ratio’ method. In: Owens DR, editor. Clinical Pharmacological Study in Normal Man. MTD Press Limited; 1986. p. 138. [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. Deposition and absorption of tobacco smoke constituents. In: Donald R, editor. Surgeon General Report: Health Consequences Involuntary Smoking. Washinton, DC: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health; 1986. pp. 177–224. [Google Scholar]
- 20.Wiedemann HP, Mahler DA, Loke J, Virgulto JA, Snyder P, Matthay RA. Acute effects of passive smoking on lung function and airway reactivity in asthmatic subjects. Chest. 1986;89:180–5. doi: 10.1378/chest.89.2.180. [DOI] [PubMed] [Google Scholar]
- 21.Mahmud A, Feely J. Effects of passive smoking on blood pressure and aortic pressure waveform in healthy young adults – influence of gender. Br J Clin Pharmacol. 2003;57:37–43. doi: 10.1046/j.1365-2125.2003.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausberg M, Mark A, Winniford MD, Brown RE, Somers VK. Sympathetic and vascular effects of short-term passive smoke exposure in healthy nonsmokers. Circulation. 1997;96:282–7. [PubMed] [Google Scholar]
- 23.Kato M, Roberts-Thomson P, Phillips BG, Narkiewicz K, Haynes WG, Pesek CA, Somers VK. The effects of short-term passive smoke exposure on endothelium-dependent and independent vasodilation. J Hypertens. 1999;17:1395–401. doi: 10.1097/00004872-199917100-00006. [DOI] [PubMed] [Google Scholar]
- 24.Leuenberger P, Schwartz J, Ackermann-Liebrich U, Blaser K, Bolognini G, Bongard JP, Brandli O, Braun P, Bron C, Brutsche M. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study) Am J Respir Crit Care Med. 1994;150:1222–8. doi: 10.1164/ajrccm.150.5.7952544. [DOI] [PubMed] [Google Scholar]


