Abstract
AIMS
Dietary supplements (DS) promoted to enhance athletic performance often contain herbal sympathomimetics such as Citrus aurantium (synephrine) and caffeine. We aimed to characterize the pharmacology of a performance-enhancing DS in the setting of exercise.
METHODS
Ten healthy adults (three women) aged 20–31 years participated in a three-arm, double-blind, placebo-controlled, crossover study. Subjects ingested one dose of DS (Ripped Fuel Extreme Cut® with 21 mg synephrine and 304 mg caffeine by analysis) under resting conditions and 1 h prior to moderately intense exercise (30 min on cycle ergometer at 75–80% HRmax), with a placebo (PLC)/exercise control. Plasma synephrine and caffeine concentrations were measured over 12 h, and vital signs, serum electrolytes, oxygen consumption and perceived exercise exertion were monitored.
RESULTS
No significant adverse events occurred. Synephrine and caffeine pharmacokinetics were unaffected by exercise. Post-exercise diastolic blood pressure was higher after DS (peak mean 71.7 ± 8.7 mmHg) than PLC (63.0 ± 4.9 mmHg) (p = 0.007). There were no substantial treatment-related differences in post-exercise HR, systolic blood pressure, or temperature. Postprandial plasma glucose increased to 121.0 ± 31.6 mg dl−1 with DS and exercise vs. 103.7 ± 25.5 mg dl−1 with PLC and exercise (P = 0.004). No treatment differences in exercise-related oxygen consumption, serum lactate, or insulin were observed. Exercise was rated less difficult with DS than PLC (P = 0.001).
CONCLUSIONS
Blood pressure and plasma glucose increased post-exercise with DS use, which could be detrimental in some people. Exercise was perceived as less strenuous after DS, presumably due to the stimulant effects of caffeine.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Performance-enhancing dietary supplements have not been clinically tested for safety or efficacy.
In clinical trials performed under resting conditions, performance-enhancing supplements raise blood pressure and affect glucose homeostasis.
The effect of exercise on the pharmacokinetics and pharmacodynamics of stimulant herbals is unknown.
WHAT THIS STUDY ADDS
Supplement-induced effects on blood pressure and glucose levels are not ameliorated by exercise.
Exercise does not affect the kinetics of stimulant ingredients, caffeine and synephrine.
Performance-enhancing supplement use modestly improves exercise tolerance.
Keywords: caffeine, dietary supplement, ergogenic, exercise, pharmacology, synephrine
Introduction
Substances that improve strength or stamina during strenuous exercise are described as ergogenic. Many pharmaceuticals and illicit substances with proven or alleged ergogenic actions have been used by athletes to enhance performance. These agents include anabolic steroids, hormones and sympathomimetic stimulants. Proposed mechanisms of ergogenic actions for sympathomimetics include increased cardiac output, promotion of energy substrate utilization, and enhanced psychological motivation through central nervous system (CNS) stimulation. Sympathomimetic ergogenic agents include not only amphetamine-related drugs, but also natural products marketed as dietary supplements. Many dietary supplements marketed for athletic performance enhancement formerly contained ephedra, until ephedra was banned by the Food and Drug Administration in 2004 because of reports of adverse cardiovascular events and lack of evidence demonstrating efficacy for performance enhancement [1–4]. Reformulated ergogenic supplements have combinations of herbal caffeine derivatives (guarana, green tea) and extracts of Citrus aurantium, a natural source of several biogenic amines, including p-synephrine. Despite widespread availability of these ergogenic dietary supplements, there are no published reports demonstrating efficacy or tolerability of these alleged performance-enhancing products.
Previous exercise studies have examined the ergogenic effects of pharmaceutical ephedrine and caffeine alone and in combination [5–8]. Studies of healthy individuals have shown that ephedrine decreases the run time in 5-km and 10-km distance events, and increases time to exhaustion after cycling at maximal effort in a controlled laboratory setting [5, 6, 8]. The mean decrease in 10-km run time observed between ephedrine and placebo groups was 48 s. Increased motivation mediated through heightened CNS stimulation may be the ergogenic effect in long duration exercises. Favourable changes in skeletal muscle metabolism may be the ergogenic effect in short-duration tests, lasting 10–20 min.
Caffeine ingestion prior to exercise improves endurance and performance in trained athletes [9, 10], although the mechanism is not clear. It has been suggested that caffeine promotes lipolysis and fatty acid oxidation by skeletal muscle, which has a glycogen-sparing effect. However, in recent studies of trained athletes, caffeine did not affect glucose kinetics or free fatty acid concentrations, but increased lactate levels [11, 12]. Caffeine appears to enhance the ergogenic effect of ephedrine in short-duration exercise tests lasting 10–20 min, but the evidence is equivocal for activities of longer duration [6, 7].
Sympathomimetic drug use during strenuous exercise may increase the risk of heat-related illnesses, by increasing metabolic heat production, particularly in ambient conditions of high temperature and humidity. Sympathomimetics increase sweating, but evaporative cooling may be compromised by peripheral vasoconstriction produced by sympathomimetic drugs, and core body temperature may rise rapidly. A study of caffeine and ephedrine effects on temperature regulation showed no change in rectal temperature during moderate exercise in a hot, dry laboratory environment [13], but these results may not be generalizable to actual training conditions.
Human studies on the pharmacology of ergogenic dietary supplements containing the botanical sympathomimetics guarana (caffeine) and C. aurantium (synephrine) in the setting of exercise have not been published. Because these products are widely promoted to athletes, clinical investigations are needed to evaluate safety and efficacy. Our aims were to (i) determine the effects of exercise on the pharmacokinetics of synephrine and caffeine; and (ii) evaluate differences in haemodynamic and metabolic responses to exercise after a single dose of a performance-enhancing dietary supplement vs. placebo.
Methods
Clinical study design
We conducted a three-arm, randomized, placebo-controlled, crossover study involving 10 healthy men and women aged 18–45 years who exercised at least three times weekly including cycling. Volunteers gave written informed consent prior to study participation. The Committee on Human Research at the University of California, San Francisco approved the study protocol. Subject eligibility was determined by history and physical examination and screening laboratory tests, including urine pregnancy testing for women. Exclusion criteria included significant medical conditions, pregnancy or lactation, obesity [body mass index (BMI) >30], prescription or illicit drug use, smoking, and heavy caffeine use (three or more cups of coffee or equivalent per day). Subjects were instructed to abstain from caffeine, over-the-counter and herbal products for 24 h before the study.
Subjects underwent a screening examination and a practice visit in the General Clinical Research Center (GCRC) to become familiar with the exercise routine and to determine their peak exercise capacity as described below. Subjects were admitted to the GCRC on three separate occasions on the evening prior to the study and fasted after midnight, but water was allowed. At 07.00 h breakfast was given, which was identical during all three visits. A urine sample was analysed for drugs of abuse. Subjects were administered study medications at 09.00 h, 1 h prior to exercise. During two of three study visits, subjects received a single oral dose of a synephrine and caffeine-containing supplement (Ripped Fuel Extreme Cut®; Twin Laboratories Corp., American Fork, UT, USA) and were randomized to exercise or rest. On the third visit, placebo was administered followed by exercise. Placebo and supplements were packaged in identical gelatin capsules for blinding. Treatment order was determined by an online randomization program (http://www.randomization.com). Subjects rested in their hospital room after dosing. If randomized to an exercise arm, subjects completed a 30-min stationary cycling routine 1 h after dosing, as detailed below. Pre- and post-exercise levels of plasma lactate, glucose and insulin were measured. Blood concentrations of synephrine and caffeine were serially measured over 12 h. Subjective responses of perceived exertional effort were recorded. On the third study visit, subjects received the same dose of dietary supplement and all measurements were performed under resting conditions. A minimum 1-week wash-out period occurred between study visits.
Exercise procedures
Subjects participated in a screening graded exercise test to exhaustion on an electromagnetically braked cycle ergometer to determine maximal oxygen consumption (VO2max) and maximal work load. A 3-min warm-up at 50 W and 60 rpm was done, and then the work load was increased 25 W every 2 min until the point of volitional exhaustion. The test was terminated when the subject was unable to sustain a constant pedal cadence of 60 rpm or when he/she indicated a desire to stop. Heart rate (HR) was recorded continuously during the test. Breath-by-breath measurements of O2 and CO2 were performed throughout the exercise and during a 10-min recovery period with a SensoMedics metabolic cart (Vmax Spectra; Sensomedics, Yorba Linda, CA, USA). Maximal HR and VO2max were calculated as the average values of the last 2 min of the test. The ambient room temperature was controlled at 21°C for the practice routine and study visits.
During exercise visits subjects were weighed in a hospital gown, then suited up in exercise clothes and began the cycling routine at 10.00 h. A 3-min warm up at 50 W and 60 rpm was performed, and then the constant-load cycle ride was done for 30 min at an intensity of 75–80% HRmax determined from the screening visit. Subjects were required to remain seated and pedal continuously at 60 rpm during the exercise test. After completing the test, subjects were asked to towel-off, change into a hospital gown, and were weighed again to determine if significant weight change due to fluid loss had occurred during exercise. Every 5 min, subjects were asked to rate their perceived exercise exertion using the 15-grade Borg scale [14]. The Borg scale rates perceived exertion from a low score of 6 indicating the lightest exertion to a high score of 20 meaning the hardest exertion.
Measurements
Blood samples (7 ml) were collected from a venous catheter at baseline, 30, 60, 90 min, and 2, 3, 4, 6, 8 and 12 h after dosing. Blood was centrifuged and separated plasma was stored at −20°C for later analysis. HR and blood pressure were recorded with an automatic sphyngomanometer, and body temperature was measured via skin and ear thermocouples for 1 h prior to dosing, and before each blood sample collection. Heart rate and rhythm were continuously monitored during exercise sessions, but blood pressure cuff readings could not be done while subjects were on the cycle ergometer because of exertional flexion of upper extremity muscles.
Analysis
Plasma concentrations of synephrine and caffeine were measured over 12 h, and pharmacokinetic parameters were estimated by noncompartmental methods with use of WinNonlin (Version 3.1; Pharsight Corp., Mountain View, CA, USA). Area under the plasma concentration–time curve (AUC) was calculated using the linear/log trapezoidal rule for the 12-h postdosing period and extrapolated to infinity (0–∞).
A novel liquid chromatography-tandem mass spectrometry (LC/MS-MS) method developed in our laboratory and previously described [16] was used to measure levels of synephrine and caffeine in plasma samples and in dietary supplements. Standard curves were linear from 0.1 to 25 ng ml−1 for synephrine and from 10 to 5000 ng ml−1 for caffeine in plasma.
Statistics
Results are expressed as means ± standard deviations (SD) in the text and tables, and, for clarity, as means ± standard errors (SEM) in the figures. Comparisons of changes from baseline were made between treatments by multivariate analysis using anova, paired t-tests and Wilcoxon signed-ranks tests for non-normal data. Data were analysed using SAS (Version 8.2; SAS Institute, Cary, NC, USA). Statistical significance was defined as a two-sided α<0.05.
Results
Subjects
Twelve subjects were enrolled, and seven men and three women completed the study. One woman was dropped because of an unrelated sports injury, and one man was dropped because of scheduling conflicts. The average age of participants was 24.3 years (range 20–31 years), with a mean total body weight of 71.9 ± 12.2 kg and BMI of 23.6 ± 2.1 kg m−2. Self-reported race/ethnicity included seven Whites and three Asian/Pacific Islanders. VO2max measurements ranged from 25.6 to 54.2 ml kg−1 min−1 (mean 40.8 ml kg−1 min−1). No significant adverse events requiring medical intervention occurred during the study. One subject complained of moderate headache (5 out of 10) with dietary supplement ingestion under resting conditions.
Pharmacology
Analysis of the dietary supplements by LC-MS/MS revealed that each two-capsule dose of Ripped Fuel Extreme Cut® contained 21.0 mg synephrine (from C. aurantium) and 303.8 mg caffeine (from green tea leaf, guarana seed, and caffeine). Other listed constituents of the product that were not confirmed by analysis include niacin (40 mg), biotin (150 μg), pantothenic acid (12 mg), ginger root extract, cocoa seed extract, cayenne fruit, quercetin, wasabi extract, naringin complex, white willow bark extract, l-tyrosine, catuaba bark, and citrus bioflavanoids.
Mean values for synephrine and caffeine pharmacokinetic parameters under resting and exercise conditions are shown in Table 1. No significant treatment-related differences in kinetic parameters were observed, but there was a trend towards faster absorption of synephrine under exercise conditions, with mean Tmax of 108.0 ± 32.3 min during exercise vs. 126 ± 41.9 min at rest.
Table 1.
Pharmacokinetic values for synephrine and caffeine under resting and exercise conditions
| Parameter | Synephrine | Caffeine | ||
|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | |
| Cmax | 1.75 ± 0.79 ng ml−1 | 1.86 ± 0.72 ng ml−1 | 5.9 ± 1.9 μg ml−1 | 5.9 ± 1.4 μg ml−1 |
| Tmax (min) | 126 ± 41 | 108 ± 32 | 144 ± 51 | 135 ± 32 |
| AUC(0–∞) | 427.9 ± 172.6 ng min−1 ml−1 | 466.4 ± 222.1 ng min−1 ml−1 | 3534 ± 1956 µg min−1 ml−1 | 3723 ± 1681 μg min−1 ml−1 |
| CL/F | 60.0 ± 32.5 l min−1 | 55.8 ± 27.5 l min−1 | 102.6 ± 36.4 ml min−1 | 94.3 ± 34.5 ml min−1 |
| T1/2 (h) | 2.56 ± 0.80 | 2.64 ± 1.11 | 5.96 ± 1.87 | 6.38 ± 1.92 |
Values are means ± standard deviations, except for Tmax for which medians are shown (n = 10). Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC(0–∞), area under the plasma concentration vs. time curve extrapolated to infinity; CL/F, clearance divided by bioavailability; V/F, apparent volume of distribution divided by bioavailability; T1/2, elimination half-life. All comparisons of exercise vs. resting values were nonsignificant (P-value >0.05 by paired Student's t-tests).
Clinical effects
Blood pressure
Diastolic blood pressure (DBP) was higher with dietary supplement vs. placebo, and exercise did not alter this treatment effect (Figure 1a). Peak DBP at 3 h (90 min post exercise) was 71.7 ± 8.7 mmHg with supplement vs. 63.0 ± 4.9 mmHg with placebo (P = 0.007). However, exercise appeared to diminish slightly the effect of the dietary supplement on systolic blood pressure (SBP) (Figure 1b), with a peak SBP post exercise of 121.3 ± 12.8 mmHg with supplement vs. 111.6 ± 11.0 mmHg with placebo, although the difference was not statistically significant (P = 0.077).
Figure 1.
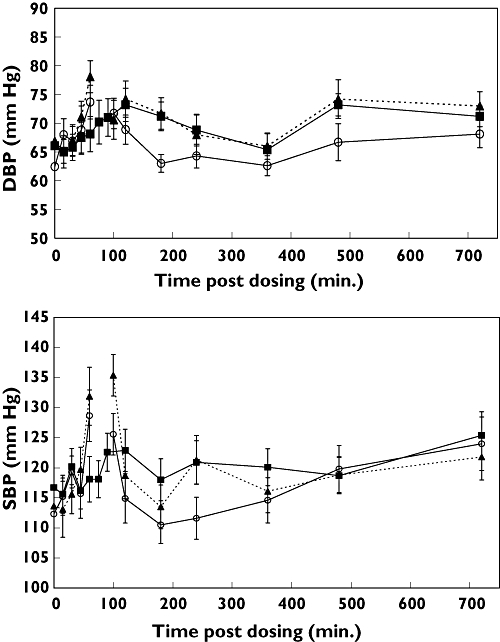
Diastolic (a) and systolic (b) blood pressures with dietary supplement and exercise (▴); dietary supplement and rest (▪); and placebo and exercise (○). Data are means ± SEM (n = 10). Blood pressure was not recorded during exercise, which was performed from 60 to 90 min (indicated by break in dotted line). P < 0.05 for treatment vs. placebo diastolic blood pressure (DBP) at 120, 180 and 480 min
Heart rate
There was no dietary supplement-related effect on mean HR during or post exercise. However, episodes of tachycardia were observed during exercise in three subjects, twice with dietary supplement treatment and once with placebo. Heart rates as high as 188 bpm were recorded on these occasions, prompting a reduction in cycling work load to decrease HR into target ranges. No cardiac dysrhythmias were observed and subjects remained asymptomatic during these brief periods.
Body temperature/fluid loss
Mean skin temperature was significantly elevated after exercise (33.3 ± 1.4°C) compared with resting conditions (29.3 ± 4.8°C, P = 0.04) (Figure 2), but tympanic membrane temperature was not altered. There was no difference between exercise-induced temperature rise with supplement vs. placebo, suggesting that the performance-enhancing supplement did not exacerbate the thermic effects of strenuous physical activity. Mean body weight decrease after exercise averaged 0.37 ± 0.13 kg with dietary supplement and 0.47 ± 0.62 kg with placebo, which was not statistically significant (P = 0.59).
Figure 2.
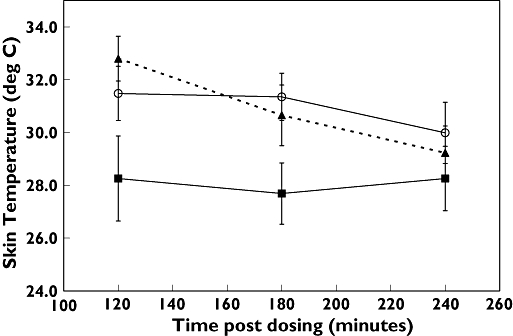
Skin temperature with dietary supplement and exercise (▴); dietary supplement and rest (▪); and placebo and exercise (○). Data are means ± SEM (n = 10). Exercise was performed from 60 to 90 min. P = 0.025 for dietary supplement (DS)/exercise vs. DS/rest at 120 min; differences were nonsignificant at other time points
Metabolic data
Dietary supplement treatment significantly increased mean postprandial plasma glucose (Figure 3). Peak plasma glucose 1 h after subjects ate a midday meal (90 min post exercise) was 121.0 ± 31.6 mg dl−1 with dietary supplement vs. 103.7 ± 25.5 mg dl−1 with placebo (P = 0.004). This hyperglycaemic action of the dietary supplement was unaffected by exercise. Serum insulin concentrations were not significantly affected by the dietary supplement. Blood lactate increased in response to exercise, with a peak increase of 2.08 ± 0.86 mEq l−1 at 120 min (30 min after exercise) vs. 1.13 ± 0.38 mEq l−1 at rest (P = 0.013). Ingestion of dietary supplement did not affect lactate levels in response to exercise. There was no significant treatment-related difference in oxygen consumption, VO2, during exercise.
Figure 3.
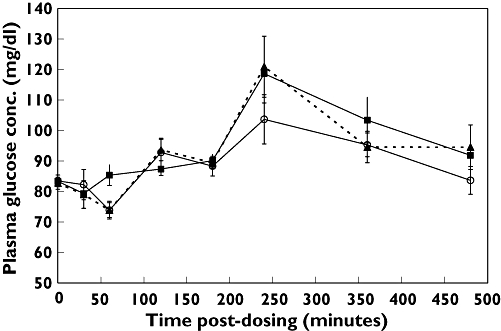
Plasma glucose concentrations with dietary supplement (DS) and exercise (▴); dietary supplement and rest (▪); and placebo and exercise (○). Data are means ± SEM (n = 10). P < 0.05 for DS/exercise vs. placebo/exercise at 120 and 240 min. Exercise was performed during 60–90-min period. Midday meal was served at 180 min
Subjective effects
Mean Borg scores for perceived exertion during exercise (Figure 4) showed that supplement use ameliorated the increase in exertional effort reported over time with placebo (P = 0.001). Subjects were asked at the end of each blinded session about their overall impression of the difficulty of the exercise. Dietary supplement sessions were rated easier than placebo sessions 83% of the time.
Figure 4.
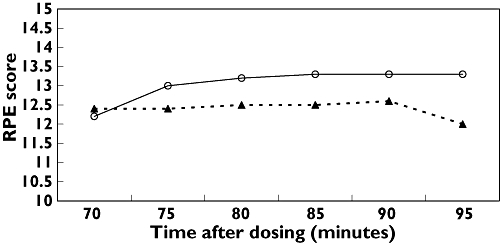
Ratings of perceived exercise exertion (RPE) during exercise session from 60 to 90 min for dietary supplement and exercise (▴); and placebo and exercise (○). Data are means ± SEM (n = 10). P = 0.001 for supplement vs. placebo at all time points
Discussion
This study has provided new data on the pharmacology of a dietary supplement marketed for athletic performance enhancement that contains caffeine and C. aurantium, and the interactive effects of exercise and dietary supplement use on haemodynamic and metabolic parameters in healthy volunteers who exercise regularly. We found that short-duration, moderate-intensity exercise does not affect the pharmacokinetics of synephrine or caffeine, or significantly change pharmacodynamic responses to the dietary supplement. Effects of the stimulant-containing supplement on blood pressure and plasma glucose were not ameliorated or enhanced by exercise. The dietary supplement modestly improved subjective exercise tolerance.
Peak plasma levels of synephrine after a single oral 21-mg dose averaged <2 ng ml−1, similar to what was observed with our previous human study on C. aurantium[15]. Peak caffeine levels averaged approximately 6 μg ml−1, which is within the range of reported concentrations associated with improved exercise endurance effects of caffeine [16–18].
The kinetics of caffeine and synephrine were not significantly affected by exercise. Results of prior studies on exercise effects on drug kinetics have been variable [19]. Intense exercise reduces splanchnic and hepatic blood flow [20] and delays gastric emptying [21], which is expected to decrease the rate of gastrointestinal absorption, but may also reduce first-pass drug metabolism. Prolonged exercise also alters renal blood flow [17, 22], potentially decreasing clearance of drugs that are renally eliminated. It is possible that the exercise duration was not long enough to change significantly the absorption, distribution and elimination of caffeine and synephrine. However, a prior study involving 1.5 h of exercise on a cycle ergometer has similarly shown no effect of exercise on the pharmacokinetics of caffeine [23]. We conclude that moderate-intensity exercise does not alter the pharmacokinetics of caffeine and synephrine, two primary active constituents of performance-enhancing dietary supplements.
DBP and SBP were increased, but HR was unchanged by the dietary supplement. These results are consistent with our previous findings that synephrine and caffeine raise resting blood pressure; however, we also previously observed transient increases in resting HR [15]. Although strenuous exercise acutely increases blood pressure, it also induces vasodilation in peripheral tissues, particularly skeletal muscle, thereby lowering systemic vascular resistance and decreasing resting blood pressure. In this study, however, the magnitude and duration of vasopressor action of the dietary supplement persisted in the face of exercise, apparently counteracting the vasodilatory effects of exercise. Maintenance of a regular exercise routine is known to lower blood pressure in both normal and hypertensive subjects [24], but it is not known what effect chronic supplement use during regular exercise would have on resting blood pressure.
Exercise significantly raised mean skin temperature with both supplement and placebo, but did not affect tympanic membrane temperature, suggesting that intrinsic responses to heat generation of skin flushing and evaporative cooling were effective, and core body temperature was unperturbed. These findings indicate that at least during short-duration moderate-intensity exercise at 21°C, the dietary supplement did not inhibit skin flushing by peripheral vasoconstriction or impair sweating through diuresis and fluid loss. However, supplement effects on thermoregulation could be different with longer duration exercise or in hot, humid ambient conditions.
Metabolic effects of the supplement included increased postprandial blood glucose concentration that was unaffected by exercise. Previous studies on caffeine effects on glucose regulation are highly variable, with a few reports showing no effect of caffeine on glucose in the setting of exercise [17, 18]. Several recent studies have indicated that acute caffeine use impairs glucose tolerance, but chronic coffee ingestion decreases the risk of developing Type 2 diabetes [25–27]. We have previously shown that dietary supplements containing ephedra and guarana significantly raise postprandial glucose [28]. We postulated that exercise would counteract this hyperglycaemic effect of the supplement by promoting glucose uptake in skeletal muscle. Our findings that postprandial plasma glucose concentrations were, on average, 20% higher with supplement than placebo indicate that hyperglycaemic effects of the dietary supplement are not ameliorated by moderate-intensity exercise. Although further investigation is needed to determine the mechanism of effect on glucose disposition, these preliminary findings indicate that glucose control could be aggravated by use of performance-enhancing dietary supplements in patients with insulin resistance or Type 2 diabetes.
The primary aim of this study was to assess the pharmacology of a performance-enhancing dietary supplement during moderate-intensity exercise. Assessment of the supplement's effect on exercise performance and characterization of the mechanism of ergogenic action were beyond the scope of the study. However, a positive effect of the dietary supplement on subjective ratings of exercise exertion was observed. The ingredient(s) responsible for the ergogenic effect is not known, but caffeine is presumed to be the primary active constituent. Previous studies on the ergogenic effects of caffeine have produced conflicting results. Acute ingestion of caffeine has been shown to prolong time to exhaustion in endurance exercises lasting ≥30 min [5–7, 9, 16, 17], but improved performance in short-term, high-intensity physical activity has not been demonstrated [8, 29]. More rigorous examination of the effects of performance-enhancing dietary supplements in various subject populations and under different exercise settings is needed before definitive conclusions can be drawn about the efficacy of these products.
These findings are derived from a small study of one performance-enhancing dietary supplement used by healthy individuals who exercise regularly. These results may not apply to other supplement brands, and cannot be generalized to predict results in other people, such as those who are sedentary, overweight, or have pre-existing conditions such as hypertension. Such individuals could have exaggerated cardiovascular responses to the supplement, and different effects on exercise tolerance. Caution should be used in advising patients about use of these products to enhance exercise performance until further studies have been performed.
Acknowledgments
This work was supported by Public Health Service grants K23AT00069-04 (National Center for Complementary and Alternative Medicine), DA12393, and a General Clinical Research Center Award (M01RR00083-41) from the National Institutes of Health. The authors are grateful to Kathy Mulligan, PhD for her guidance and expertise in conducting the exercise and metabolic studies. We thank Gina Lowry for subject recruitment and assistance with the clinical study; Faith Allen for protocol and data management; Dr Peter Bacchetti for statistical analysis; and the nurses and staff of the SFGH GCRC for care of the research subjects. We acknowledge Pharsight Corporation (Mountain View, CA, USA) for donation of a WinNonlin®3.1 license.
REFERENCES
- 1.Perrotta DM, Coody G, Culmo C. Adverse events associated with ephedrine-containing products – Texas, Dec. 1993 to Sept. 1995. JAMA. 1996;276:1711–2. [PubMed] [Google Scholar]
- 2.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000;343:1833–8. doi: 10.1056/NEJM200012213432502. [DOI] [PubMed] [Google Scholar]
- 3.Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagne J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance. JAMA. 2003;289:1537–45. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- 4.FDA Press Office. FDA announces plans to prohibit sales of dietary supplements containing ephedra. [28 February 2008]; Available at http://www.hhs.gov/news/press/2003pres/20031230.html.
- 5.Bell DG, Jacobs I. Combined caffeine and ephedrine ingestion improves run times of Canadian Forces Warrior Test. Aviat Space Environ Med. 1999;70:325–9. [PubMed] [Google Scholar]
- 6.Bell DG, Jacobs I, Zamecnik J. Effects of caffeine, ephedrine, and their combination on time to exhaustion during high-intensity exercise. Eur J Appl Physiol Occup Physiol. 1998;77:427–33. doi: 10.1007/s004210050355. [DOI] [PubMed] [Google Scholar]
- 7.Bell DG, McLellan TM, Sabiston CM. Effect of ingesting caffeine and ephedrine on 10-km run performance. Med Sci Sports Exerc. 2002;34:344–9. doi: 10.1097/00005768-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Bell DG, Jacobs I, Ellerington K. Effect of caffeine and ephedrine ingestion on anaerobic exercise performance. Med Sci Sports Exerc. 2001;33:1399–403. doi: 10.1097/00005768-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Graham TE, Spriet LL. Performance and metabolic responses to a high caffeine dose during prolonged exercise. J Appl Physiol. 1991;71:2292–9. doi: 10.1152/jappl.1991.71.6.2292. [DOI] [PubMed] [Google Scholar]
- 10.Erikson MSA, Schwarzkopf RJ, McKenszie RD. Effects of caffeine, fructose and glucose ingestion on muscle glycogen utilization during exercise. Med Sci Sports Exerc. 1987;19:579–83. [PubMed] [Google Scholar]
- 11.Laurent D, Schneider KE, Prusaczyk WK, Franklin D, Vogel SM, Krssak M, Petersen KF, Goforth HW, Shulan GI. Effects of caffeine on muscle glycogen utilization and the neuroendocrine axis during exercise. J Clin Endocrinol Metab. 2000;85:2170–5. doi: 10.1210/jcem.85.6.6655. [DOI] [PubMed] [Google Scholar]
- 12.Roy BD, Bosman MJ, Tarnopolsky MA. An acute oral dose of caffeine does not alter glucose kinetics during prolonged dynamic exercise in trained endurance athletes. Eur J Appl Physiol. 2001;85:280–6. doi: 10.1007/s004210100456. [DOI] [PubMed] [Google Scholar]
- 13.Bell DG, Jacobs I, McLellan TM, Miyazaki M, Sabiston CM. Thermal regulation in the heat during exercise after caffeine and ephedrine ingestion. Aviat Space Environ Med. 1999;70:583–8. [PubMed] [Google Scholar]
- 14.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 15.Haller CA, Benowitz NL, Jacob P., III Hemodynamic effects of ephedra-free weight loss supplements in humans. Am J Med. 2005;118:998–1003. doi: 10.1016/j.amjmed.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Greer F, Friars D, Graham TE. Comparison of caffeine and theophylline ingestion: exercise metabolism and endurance. J Appl Physiol. 2000;89:1837–44. doi: 10.1152/jappl.2000.89.5.1837. [DOI] [PubMed] [Google Scholar]
- 17.Graham TE, Hibbert E, Sathasivam P. Metabolic and exercise endurance effects of coffee and caffeine ingestion. J Appl Physiol. 1998;85:883–9. doi: 10.1152/jappl.1998.85.3.883. [DOI] [PubMed] [Google Scholar]
- 18.Van Soeren MHV, Graham TE. Effect of caffeine on metabolism, exercise endurance, and catecholamine responses after withdrawal. J Appl Physiol. 1998;85:1493–501. doi: 10.1152/jappl.1998.85.4.1493. [DOI] [PubMed] [Google Scholar]
- 19.Van Baak MA. Influence of exercise on the pharmacokinetics of drugs. Clin Pharmacokinet. 1990;19:32–43. doi: 10.2165/00003088-199019010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Van Nieuwenhoven MA, Brummer RJM, Brouns F. Gastrointestinal function during exercise: comparison of water, sports drink, and sports drink with caffeine. J Appl Physiol. 2000;89:1079–85. doi: 10.1152/jappl.2000.89.3.1079. [DOI] [PubMed] [Google Scholar]
- 21.Brouns F. Gastric emptying as a regulatory factor in fluid uptake. Int J Sports Med. 1998;19(Suppl. 2):S125–8. doi: 10.1055/s-2007-971976. [DOI] [PubMed] [Google Scholar]
- 22.Pederson KE, Madsen J, Kjaer K, Klitgaard NA, Hvidt S. Effects of physical activity and immobilization on plasma digoxin concentration and renal digoxin clearance. Clin Pharmacol Ther. 1983;34:303–8. doi: 10.1038/clpt.1983.172. [DOI] [PubMed] [Google Scholar]
- 23.McLean C, Graham TE. Effects of exercise and thermal stress on caffeine pharmacokinetics in men and eumenorrheic women. J Appl Physiol. 2002;93:1471–8. doi: 10.1152/japplphysiol.00762.2000. [DOI] [PubMed] [Google Scholar]
- 24.Jennings GL, Deakin G, Korner P, Meredith I, Kingwell B, Nelson L. What is the dose–response relationship between exercise training and blood pressure. Ann Med. 1991;23:313–8. doi: 10.3109/07853899109148066. [DOI] [PubMed] [Google Scholar]
- 25.Van Dam RM, Hu FB. Coffee consumption and risk of Type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 26.Battram DS, Arthur R, Weekes A, Graham TE. The glucose intolerance induced by caffeinated coffee ingestion is less pronounced than that due to alkaloid caffeine in men. J Nutr. 2006;136:126–80. doi: 10.1093/jn/136.5.1276. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg JA, Boozer CN, Geliebter A. Coffee, diabetes, and weight control. Am J Clin Nutr. 2006;84:682–93. doi: 10.1093/ajcn/84.4.682. [DOI] [PubMed] [Google Scholar]
- 28.Haller CA, Jacob P, III, Benowitz NL. Short-term metabolic and hemodynamic effects of ephedra and guarana combinations. Clin Pharmacol Ther. 2005;77:560–71. doi: 10.1016/j.clpt.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Greer F, McLean C, Graham T. Caffeine, performance, and metabolism during repeated Wingate exercise tests. J Appl Physiol. 1998;85:1502–8. doi: 10.1152/jappl.1998.85.4.1502. [DOI] [PubMed] [Google Scholar]


