Abstract
AIMS
To determine whether, for oxybutynin and risperidone, drug exposure is better with less frequent dosing regimens than with regimens that require more frequent dosing.
METHODS
Pharmacokinetic models of oxybutynin (5 mg twice-daily and 10 mg once-daily) and risperidone (2 mg once-daily orally and 25 mg fortnightly intramuscular injection) were developed. Simulations of multiple doses were performed by use of stochastic models of dose-taking compliance and clinic visit attendance.
RESULTS
At therapeutic concentrations and with typical patterns of noncompliance, intramuscular injections of risperidone resulted in a 41% (SD 12%) greater pharmacokinetic coverage than the oral dose, 76% (SD 10%) vs. 35% (SD 7%). No discernable differences were evident between once- and twice-daily formulations of oxybutynin, 29.2% (SD 10%) vs. 29.0% (SD 13%).
CONCLUSIONS
For equivalent doses for each drug, the longer acting preparation of risperidone, but not oxybutynin, is pharmacokinetically more forgiving of noncompliance than the shorter acting counterparts. Further analysis is required to confirm whether these observations are valid clinically.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Patient compliance is better with formulations that require less frequent dosing than with formulations that require more frequent dosing.
Intramuscular risperidone and long-acting oxybutynin are two examples of medicines reformulated for less frequent dosing.
However, it is not clear whether better compliance with less frequent dosing regimens translates to improved therapeutic outcome.
WHAT THIS STUDY ADDS
At equivalent daily doses and typical patterns of compliance, fortnightly intramuscular depot administrations of risperidone provide better pharmacokinetic coverage than once-daily oral dosing.
Once-daily dosing of oxybutynin is no better at maintaining pharmacokinetic exposure than twice-daily dosing at half strength.
The use of simulated compliance data as input to pharmacokinetic models is useful to assess the impact of noncompliance on internal drug exposure.
Keywords: compliance, oxybutynin, pharmacokinetics, risperidone
Introduction
Failure to comply with dosing instructions is a leading cause of therapeutic nonresponse, as pharmacologically active drugs become ineffective if not taken correctly [1]. The majority of patients miss doses, and have errors in timing, and these manifest as erratic pharmacokinetic profiles which are typically associated with reduced and prolonged trough concentrations that may result in opportunities for the pharmacological effect to subside and for symptoms to reappear.
Strategies to promote compliance are an important component of therapeutics, and the development of less frequent dosing regimens is one strategy that has been shown to be effective. There is extensive evidence to support the inverse relationship between dose frequency and compliance [2]. In recent years there has been an increase in the range of medicines, mainly through reformulation, that require less frequent dosing. Examples are diverse, and include once-daily antihypertensives, once-weekly and once-monthly bisphosphonates for the treatment of postmenopausal osteoporosis; long-acting transdermal, implantable and injectable hormonal contraceptives; and injectable antipsychotic agents.
The consequences of missing a dose from a less frequent dosing regimen, however, are likely to be more severe than missing doses from a more frequent dosing regimen. Missing a single dose from a once-daily formulation, for example, may result in a 24-h interruption in therapeutic activity, compared with a 12-h interruption if a single dose is missed from a twice-daily regimen [3, 4]. The ability of drugs to maintain therapeutic activity in spite of noncompliant dosing behaviour is termed ‘forgiveness’, and drugs that are more forgiving are those whose duration of action far exceeds their dosing interval [5].
Thus, a balance between dosing frequency and drug forgiveness is required for maintenance of therapeutic activity in the presence of noncompliance.
The aim of the present analysis was to evaluate the impact of noncompliance from a pharmacokinetic perspective, by assessing the pharmacokinetic profiles associated with perfect and typical compliance, of two formulations – one immediate release and one extended release – of oxybutynin and risperidone. The extended-release formulation of oxybutynin (Lyrinel XLTM) makes use of osmotic pressure to deliver the drug at a controlled rate over 24 h to facilitate once-daily dosing. This avoids the peaks in plasma concentration that are observed with the immediate-release formulation of oxybutynin [6] and results in a reduction in the incidence and severity of anticholinergic adverse effects [7]. The intramuscular injectable formulation of risperidone (Risperdal ConstaTM) allows for fortnightly dosing and reduces the daily fluctuations in plasma concentrations [8, 9]. Using previously published data to populate models of pharmacokinetics and compliance, simulations were performed to discern whether changes in drug formulation result in more, or less, forgiving products.
Methods
Pharmacokinetics
Pharmacokinetic models were based on published, mean parameter estimates. Data were not available to develop population pharmacokinetic models, and hence the pharmacokinetic simulations were confined to deterministic analyses of expected population means.
Comparability among products was maintained by selecting equivalent total daily doses. For oxybutynin, 10 mg once-daily extended-release formulation was compared with 5 mg twice-daily immediate-release formulation. For risperidone, 25 mg once-fortnightly intramuscular injection was compared with 2 mg once-daily oral dosing. Pharmacokinetic studies suggest that these doses are equivalent in terms of total daily body exposure [6, 7, 9, 10].
A one-compartment model that characterized the total mean plasma concentration profile of risperidone plus the active metabolite, 9-hydroxyrisperidone, was selected for the analysis. It was taken from a study in which participants were administered a 1-mg oral dose of risperidone (Table 1) [11]. Linear kinetics was assumed in order to simulate the administration of 2-mg doses [12]. There are no published pharmacokinetic models of the long-acting injectable formulation of risperidone, and so a noncompartmental model that comprised linear interpolations between mean plasma concentration time points of the active moiety was employed. Data were obtained from a study that evaluated plasma concentrations following a 25-mg intramuscular dose of risperidone [13]. The equations relating to the linear interpolation analysis for the long-acting injectable formulation of risperidone are not presented.
Table 1.
Parameter estimates used for the pharmacokinetics models of oxybutynin and risperidone (sum of active moieties, risperidone plus 9-hydroxyrisperidone)
| Drug/metabolite | Pharmacokinetic parameters |
|---|---|
| Risperidone | |
| Risperidone active moiety (2 mg oral) | V/F = 47.7 l; kel = 0.1 h−1; ka = 6.02 h−1 |
| Risperidone injectable (12.5 mg) | Non-compartmental analysis with linear interpolations between data points |
| Oxybutynin | |
| R-oxybutynin (10 mg) | V/F = 1.89 l; kel = 0.23 h−1; ka = 0.05 h−1 |
| R-oxybutynin (5 mg) | V/F = 1.17 l; ka = 0.99 h−1; α = 0.82 h−1; β = 0.044 h−1 |
| R-desethyloxybutynin (5 mg) | km = 1.40 h−1; α = 0.90 h−1; β = 0.20 h−1 |
| R-desethyloxybutynin (10 mg) | km = 0.07 h−1; kel = 0.21 h−1 |
V/F, apparent volume of distribution normalized by the bioavailable fraction; kel, elimination rate constant; ka, absorption rate constant; α and β are the rate constants for the two-compartment model; km, the rate constant for metabolite formation.
Pharmacokinetic data relating to the immediate- and extended-release preparations of oxybutynin were taken from a study that measured the plasma concentration profiles of active moieties of oxybutynin; namely R-oxybutynin and R-desethyloxybutynin [14]. A two-compartment model was used for the immediate-release formulation, and a one-compartment model for the extended-release preparation (Table 1). A first order rate constant was introduced to account for metabolite formation. The sum of the mean concentrations of both compounds was calculated to represent the concentration of active moieties. Linear kinetics were assumed, on the basis of supporting evidence from a pharmacokinetic study in patients with overactive bladder [15].
Compliance
Various models have been proposed that may be used to simulate typical noncompliant dosing patterns that are characterized by dose omissions, timing variations and drug holidays [16–19]. Patient-level data on compliance with the drugs selected in the current analysis were not available. Instead, a generic two-state Markov model that assigned different probabilities of a dose being taken or not, conditional on whether the preceding dose was taken, was employed to simulate noncompliance [17, 18]. This enabled dose omissions and drug holidays to be modelled, but not variations in timing. Data on mean (and SD) dose-taking compliance were taken from a systematic review of 76 studies that measured compliance by use of electronic monitoring devices [2]. The mean dose-taking compliance (±SD) for once-daily preparations was 79% (±14%) compared with 69% (±15%) for twice-daily preparations.
The interindividual variation in compliance with oral formulations was modelled by assuming that probability P1 of a dose being taken given the preceding dose was taken, and P2 of a dose being taken given that the preceding dose was not taken, followed independent β distributions. The mean and variance of P1 and P2 were estimated from simulations of compliance, which resulted in the dose-taking compliance and SD reported above. Parameters of the β distributions were derived using the methods of moments, such that for once-daily dosing, P1∼β(5.6, 1.4), and P2∼β(6.7, 2.9); and for twice-daily dosing, P1∼β(6.0, 1.8), and P2∼β(2.6, 2.6).
For the long-acting injectable formulation of risperidone, compliance is expected to be determined by fortnightly attendance, by the patient, to an outpatient clinic for dose administration. Typical attendance by psychiatric patients was taken from a study which investigated the frequency and rescheduling of 1620 appointments to seven mental health clinic psychiatrists [20]. For the 8.8% of visits that were missed, it was reported that 73.3% were rescheduled within 2 weeks. The probability of attending a clinic visit at the scheduled time was assumed to follow a Bernoulli distribution. For patient i, the probability of clinic attendance was represented by Pi∼Be(0.912). The timing of rescheduled visits in noncompliant patients was determined by sampling from an exponential distribution that yielded a 2-week probability of 73.3%. The parameter of the distribution was calculated as λ = −1/14 × ln[(100 − 73.3)/100], giving Ti∼Exp(0.0943).
In each case, compliance was measured as the proportion of doses taken over a period of up to 1 year. In some simulations, persistence with therapy was <1 year. In such cases, compliance was measured as the proportion of doses taken until the point of discontinuation.
Simulations
Pharmacokinetic simulations of multiple doses with full and typical compliance patterns – for both formulations of oxybutynin and risperidone – were performed by assuming that the principle of superpositioning for linear pharmacokinetics applied. Pharmacokinetic coverage was calculated as the proportion of time above any given concentration of drug for the period of analysis (up to 1 year). As the relationship between concentration and pharmacodynamic response is unknown, coverage was computed for a range of concentrations up to the maximum steady-state concentration, Cmaxss. The analyses were repeated by 1000 Monte Carlo simulations to capture the interindividual differences in compliance patterns (not pharmacokinetic variability).
The results are presented as plots of differences in percentage pharmacokinetic coverage between long-acting and short-acting preparations. In addition, the probability of each formulation resulting in better coverage was calculated from the results of the simulations. Sensitivity analyses were performed to assess the impact of varying P1, P2 and λ on pharmacokinetic coverage at threshold concentrations of 10 μg l−1 and 15 μg l−1 active moieties of oxybutynin and risperidone, respectively.
All simulations were performed with Microsoft Excel 2002 (Microsoft Corporation, Redmond, WA, USA).
Results
The results of the compliance simulations are presented in Figure 1. These are in the form of a frequency histogram of interdose intervals for twice-daily, once-daily and fortnightly regimens. As expected, the modes of the interdosing intervals are, respectively, 12 h, 1 day and 14 days. Occasional interdose intervals of ≥3 days (representing ‘drug holidays’) are observed for both oral dosing regimens. For fortnightly intramuscular administrations, approximately 12% of interdosing intervals exceeded the prescribed 14 days.
Figure 1.
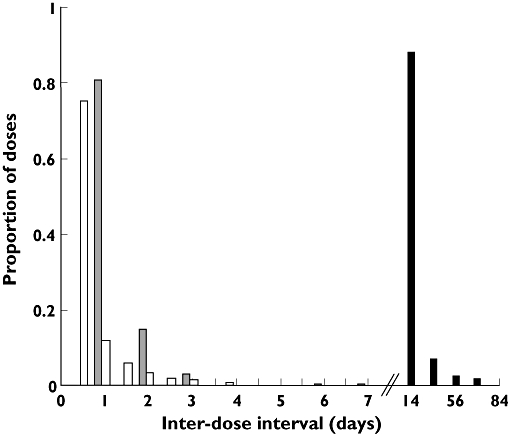
Frequency histogram of interdosing intervals generated from simulated compliance profiles for once- and twice-daily oral dosing regimens, and fortnightly clinic visits. Once daily, (▵); Twice daily, (□); Fortnightly, (▪)
Figures 2 and 3 illustrate how the differences in pharmacokinetic coverage, between long- and short-acting preparations, vary according to threshold concentration. With perfect compliance, coverage at higher concentrations (closer to Cmaxss) is greater in each case with more frequent dosing preparations. This reflects the differences in fluctuations in plasma concentration, as higher peaks are associated with more frequent dosing of shorter-acting formulations. At lower concentrations, pharmacokinetic coverage is greater with the longer-acting formulations of oxybutynin (Figure 2) and risperidone (Figure 3).
Figure 2.
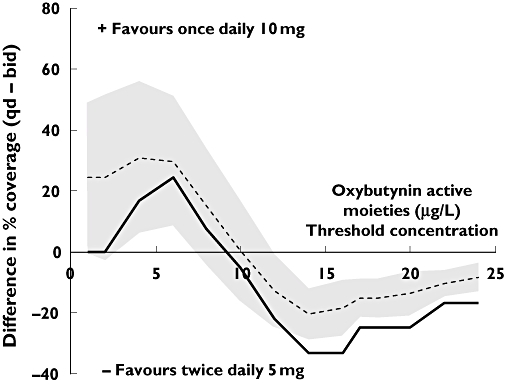
Mean differences in percentage pharmacokinetic coverage between 10 mg once-daily (q.d.) extended-release oxybutynin, and 5 mg twice-daily (b.i.d.) immediate-release oxybutynin. The solid line represents differences with perfect compliance; broken line represents typical compliance patterns (shaded area is the standard deviation around the mean)
Figure 3.
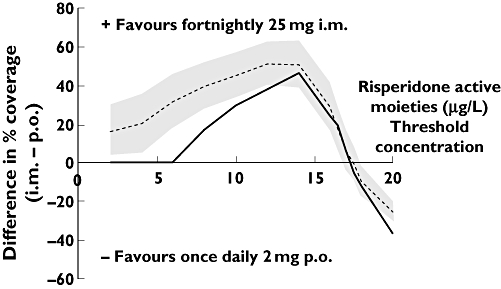
Mean differences in percentage pharmacokinetic coverage between 25 mg fortnightly injectable (i.m.) risperidone, and 2 mg once-daily oral (p.o.) risperidone. The solid line represents differences with perfect compliance; broken line represents typical compliance patterns (shaded area is the standard deviation around the mean)
An appreciable reduction in coverage is evident for all formulations when noncompliance is factored into the analysis. For risperidone, the difference in coverage between short- and long-acting preparations is greatest at concentrations nearer to the average steady-state concentration, Cavss. However, only at lower concentrations were any discernable advantages evident for oxybutynin 10 mg once daily.
When compliance is perfect, and at a concentration of 15 μg l−1 risperidone active moieties (representing Cavss), the pharmacokinetic coverage with 25 mg fortnightly intramuscular injection is 87%, compared with 45% for 2 mg daily dosing of oral risperidone. However, with typical compliance patterns, coverage decreases to 76% (SD 10%) and 35% (SD 7%), respectively, although maintaining a similar difference of 41% (SD 12%) (Figure 3). The probability that 25-mg fortnightly injections offer greatest coverage is 1.0 (i.e. certainty). This considers only variability in compliance, however, and excludes any pharmacokinetic variability.
With perfect compliance, and at a concentration of 10 μg l−1 oxybutynin active moieties (representing Cavss), the pharmacokinetic coverage with 10 mg daily is 45%, compared with 50% for 5 mg twice daily. When typical compliance patterns are introduced, coverage decreases to 29.2% (SD 10%) and 29.0% (SD 13%), respectively; a net difference of 0.2% (SD 17%) (Figure 2). The resulting probability of the longer-acting preparation offering greatest pharmacokinetic coverage at this concentration is 0.51.
Figures 4 and 5 present the results of the bivariate sensitivity analyses for oxybutynin and risperidone, respectively. As different combinations of P1 and P2 (and P1 and λ) can result in the same mean values of compliance, the purpose of the sensitivity analyses was to assess whether pharmacokinetic coverage is influenced sufficiently by the choice of parameter estimates so as to affect the interpretation of the analysis. The figures suggest that for oxybutynin, pharmacokinetic coverage is similar for once- and twice-daily formulations, regardless of the specification of the compliance model. For risperidone, better coverage is achieved with the fortnightly intramuscular injection across the evaluable range of P1 and λ.
Figure 4.
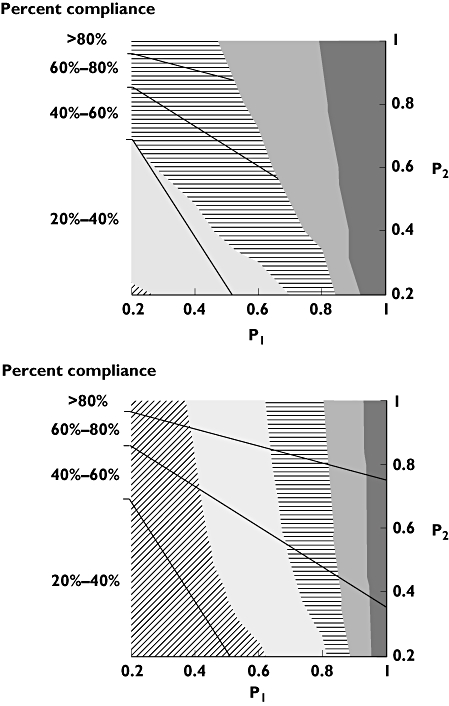
Two-way sensitivity analyses, illustrating the impact of varying P1 and P2 on the pharmacokinetic coverage of 5 mg twice-daily (upper panel) and oxybutynin 10 mg once-daily oxybutynin (lower panel). Plots are segmented to areas of equal compliance, and the shaded contours highlight different coverage for a given combination of probabilities at a threshold concentration of 10 μg l−1 of active moieties of oxybutynin. 40%–50% (▵); 30%–40% (○); 20%–30% (•); 10%–20% (□); 0%–10% (▪)
Figure 5.
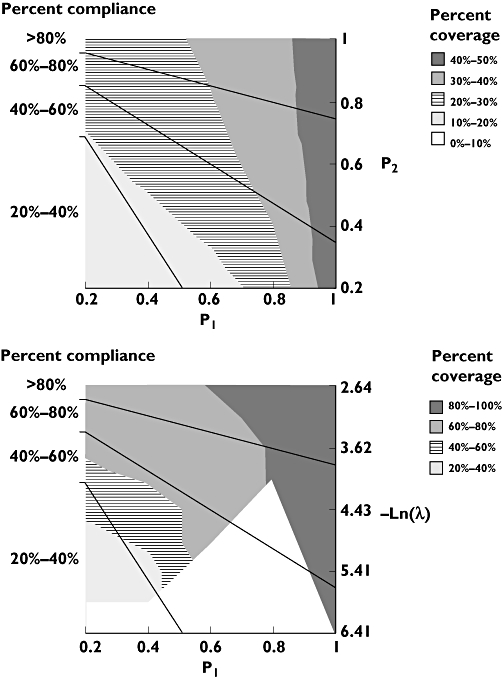
Two-way sensitivity analyses, illustrating the impact of varying P1 and λ on the pharmacokinetic coverage of 2 mg once-daily risperidone (upper panel) and once-fortnightly 25 mg i.m. risperidone (lower panel). Plots are segmented to areas of equal compliance, and the shaded contours highlight different coverage for a given combination of probabilities at a threshold concentration of 15 μg l−1 of active moieties of risperidone. The unshaded area in the lower panel represents indeterminable combinations of P1 and λ
Discussion
The results of the present analysis suggest that in the presence of noncompliance, and at concentrations close to Cavss, the longer-acting formulation of risperidone, but not oxybutynin, may offer a pharmacokinetic advantage over their shorter-acting counterpart. For risperidone, improved compliance with the less frequent dosing regimen might compensate for the greater interdose intervals that exist when doses are missed.
The figures presenting the results of the analysis summarize the difference in coverage for each drug/formulation for concentrations up to Cmaxss. Besides providing an indication of relative pharmacokinetic forgiveness [21] (i.e. maintenance of coverage for a given plasma concentration), they illustrate the avoidance, by the longer-acting drug formulations, of the higher concentration peaks that are evident for the regimens that require more frequent dosing. Indeed, it would appear that for oxybutynin, avoidance of peak concentrations (that are associated with side-effects), and not improvements in forgiveness, is the principal advantage of the longer-acting formulation.
There are certain caveats to the study, however, and care should be exercised to infer improvements in pharmacodynamic coverage and hence clinical advantages from the pharmacokinetic modelling presented here.
The estimates for compliance with oral therapy were taken from a review that included a range of medications and diseases [2], as no relevant studies for risperidone or oxybutynin were identified. The mean compliance from four studies in psychiatric patients in the review was reported as 78% [2]. Compared with other publications using electronic monitoring devices, this is a high estimate of compliance in patients taking oral antipsychotic agents. Diaz et al.[22] have reported mean compliance rates to be 63% for the first month of therapy, and ranged from 56% to 45% over the next five. In another study in patients with schizophrenia, which evaluated compliance as a dichotomous variable and using a threshold of 80%, noncompliance as measured by electronic monitoring devices was reported to be 52% [23]. A systematic review of 161 articles that have assessed compliance with oral antipsychotic agents [24] did not present direct evidence on the proportion of doses taken.
Pharmacokinetic advantages, in terms of coverage and forgiveness, may not necessarily translate into corresponding pharmacodynamic advantages. First, despite the fact that the doses selected for evaluation were comparable in terms of total daily exposure dose, clinical evidence suggests that only in the case of oxybutynin is therapeutic equivalence observed [25]. For oral risperidone, evidence from clinical trials [26] and from pharmacoepidemiological studies [27] suggests that doses >2 mg day−1 are required to achieve therapeutic responses that are considered comparable to those observed with 25-mg fortnightly injections.
Second, it was not possible to develop pharmacokinetic–pharmacodynamic (PK–PD) models of drug response in this instance because of the nature of the outcome measures in the diseases for which these drugs are prescribed. Decreases in the number and frequency of urinary incontinent episodes and changes in psychiatric rating scales do not lend themselves well to PK–PD modelling. Previously published models for oxybutynin related dose (as opposed to concentration) to clinical response [28]. Models of the pharmacodynamic actions of risperidone are limited to analyses of intermediate end-points, such as changes in electroencephalographic parameters [13] and dopamine D2 receptor occupancy [29–31]. Extrapolating from the pharmacokinetic observations in the present analysis would also require that the potencies of parent drugs and metabolites are assumed to be equivalent. This may be justified in the light of supporting evidence that demonstrates pharmacodynamic equivalence between risperidone and 9-hydroxyrisperidone [32] and between R-oxybutynin and R-desethyloxybutynin [33].
The same difficulties associated with PK–PD modelling extend to identifying the therapeutic concentration range for both oxybutynin and risperidone. For this reason, the discussion has been limited to assessing pharmacokinetic coverage with reference to Cavss and Cmaxss. For both formulations of risperidone, Cavss for the active moieties was approximately 15 μg l−1, corresponding to what is considered to be pharmacokinetically defined therapeutic levels [34]. For the active moieties of oxybutynin, Cavss ranged between 9 μg l−1 (extended-release preparation) and 12 μg l−1 (immediate-release preparation), comparable to those required for symptom relief in patients with overactive bladder [15].
The modelling could have been improved significantly if a population pharmacokinetic approach had been adopted in order to capture pharmacokinetic variability both within and among individuals, in addition to the variability in compliance patterns already accounted for. By using mean concentration data, variability is reduced to that resulting from dose-taking behaviour and may result in a biased estimation of the true pharmacokinetic impact of noncompliance.
Despite these weaknesses, it is reasonable to assume that pharmacokinetic coverage is likely to be a conservative estimate of pharmacodynamic coverage, as drugs that act via hypothetical effect compartments, which is what may be expected for risperidone and oxybutynin, are more forgiving of noncompliance than drugs that act directly [35].
The use of modelling to predict the effect of poor compliance on plasma concentrations has been presented before. Rubio et al.[36] predicted the plasma concentrations of diltiazem by use of individual pharmacokinetic parameter estimates in conjunction with compliance history measured by medication event monitoring systems. Vrijens et al.[37] used compliance data, acquired from electronic monitoring devices, to successfully project the pharmacokinetic profiles of lopinavir prescribed for human immunodeficiency virus (HIV) patients. They further analysed the impact on pharmacokinetic exposure of noncompliance with once- and twice-daily regimens, using methods similar to those described in the present analysis [4]. Few studies have made use of pharmacodynamic models to predict the impact of noncompliance. Levy et al.[38] and Blesius et al.[39] used a pharmacokinetic model linked to an indirect response model to assess the implications of noncompliance with warfarin therapy. Hughes et al.[16] used population pharmacodynamic simulations to predict the influence of noncompliance and premature discontinuation on the efficacy of atorvastatin, and Vrijens et al.[40] demonstrated that compliance with antiretroviral therapy, serving as an input to a pharmacokinetic model, allowed pharmacodynamic activity (plasma viral load HIV RNA) to be predicted successfully.
The model used to simulate variable compliance patterns with oral formulations considered that patients are increasingly likely to be noncompliant, as successive doses are skipped. It ignored timing variations and double-dosing, as supportive data from accurate electronic monitoring devices were not available for more sophisticated modelling. As a consequence, although the total number of doses ingested is captured adequately, the introduction of timing errors may further reduce pharmacokinetic coverage. Other analyses have employed multivariate normal distributions for timing errors [17].
Conclusions
Claims that less frequent dosing regimens are superior are often unfounded [3]. Indeed, a systematic review of the evidence has proved inconclusive as to whether compliance benefits offered by less frequent dosing regimens translate to improved health outcomes [41]. Of the 36 evaluable studies identified, no differences in efficacy were observed in 22 studies; less frequent dosing was better in seven but inferior in seven other studies [41].
The present analysis simulated the impact of noncompliance on the pharmacokinetics of different formulations of risperidone and oxybutynin. The results suggest that pharmacokinetic coverage is reduced substantially for both drugs once typical compliance patterns are introduced. The degree of reduction in coverage is dependent on both concentration and drug formulation. Within therapeutically meaningful concentrations of risperidone, the injectable preparation provides superior pharmacokinetic coverage to oral dosing in the presence of noncompliance. The pharmacokinetic advantage gained by the longer-acting formulation of oxybutynin is the reduction in peak plasma concentration, and not increased forgiveness of noncompliance. Further studies are required to confirm whether these findings are valid clinically.
Competing interests
This study was completed with the assistance of a research grant from Janssen Pharmaceutica N.V., Belgium.
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 3.Hughes D. Less is more: medicines that require less frequent administration improve adherence, but are they better? Pharmacoeconomics. 2006;24:211–3. doi: 10.2165/00019053-200624030-00001. [DOI] [PubMed] [Google Scholar]
- 4.Comte L, Vrijens B, Tousset E, Gerard P, Urquhart J. Estimation of the comparative therapeutic superiority of QD and BID dosing regimens, based on integrated analysis of dosing history data and pharmacokinetics. J Pharmacokinet Pharmacodyn. 2007;34:549–58. doi: 10.1007/s10928-007-9058-0. [DOI] [PubMed] [Google Scholar]
- 5.Urquhart J. Pharmacodynamics of variable patient compliance: implications for pharmaceutical value. Adv Drug Deliv Rev. 1998;33:207–19. doi: 10.1016/s0169-409x(98)00029-5. [DOI] [PubMed] [Google Scholar]
- 6.Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharmacol. 1999;39:289–96. [PubMed] [Google Scholar]
- 7.Sathyan G, Chancellor MB, Gupta SK. Effect of OROS controlled-release delivery on the pharmacokinetics and pharmacodynamics of oxybutynin chloride. Br J Clin Pharmacol. 2001;52:409–17. doi: 10.1046/j.0306-5251.2001.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane JM, Eerdekens M, Lindenmayer JP, Keith SJ, Lesem M, Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160:1125–32. doi: 10.1176/appi.ajp.160.6.1125. [DOI] [PubMed] [Google Scholar]
- 9.Mannaert E, Vermeulen A, Remmerie B, Bouhours P, Levron JC. Pharmacokinetic profile of long-acting injectable risperidone at steady-state: comparison with oral administration. Encephale. 2005;31(5 Pt 1):609–15. doi: 10.1016/s0013-7006(05)82420-0. [DOI] [PubMed] [Google Scholar]
- 10.Huang ML, Rasmussen M, Woestenborghs R, Delor I, Van Peer A, Lowenthal R. Steady-state bioavailability in chronic schizophrenic patients comparing once daily oral administration of risperidone with intramuscular injections of a risperidone depot microspheres formulation given every two weeks. RIS-INT-32. Janssen Research Foundation, Clinical Research Report, February 2000. N137257.
- 11.Lee DY, Lee KU, Kwon JS, Jang IJ, Cho MJ, Shin SG, Woo JI. Pharmacokinetic–pharmacodynamic modelling of risperidone effects on electroencephalography in healthy volunteers. Psychopharmacology. 1999;144:272–8. doi: 10.1007/s002130051003. [DOI] [PubMed] [Google Scholar]
- 12.Heykants J, Huang ML, Mannens G, Meuldermans W, Snoeck E, Van Beijsterveldt L, Van Peer A, Woestenborghs R. The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatry. 1994;55(Suppl.):13–7. [PubMed] [Google Scholar]
- 13.Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res. 2004;70:91–100. doi: 10.1016/j.schres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Pitsiu M, Sathyan G, Gupta S, Verotta D. A semiparametric deconvolution model to establish in vivo–in vitro correlation applied to OROS oxybutynin. J Pharm Sci. 2001;90:702–12. doi: 10.1002/jps.1026. [DOI] [PubMed] [Google Scholar]
- 15.Preik M, Albrecht D, O'Connell M, Hampel C, Anderson R. Effect of controlled-release delivery on the pharmacokinetics of oxybutynin at different dosages: severity-dependent treatment of the overactive bladder. BJU Int. 2004;94:821–7. doi: 10.1111/j.1464-410X.2004.05040.x. [DOI] [PubMed] [Google Scholar]
- 16.Hughes DA, Walley T. Predicting ‘real world’ effectiveness by integrating adherence with pharmacodynamic modeling. Clin Pharmacol Ther. 2003;74:1–8. doi: 10.1016/S0009-9236(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 17.Girard P, Blaschke TF, Kastrissios H, Sheiner LB. A Markov mixed effect regression model for drug compliance. Stat Med. 1998;17:2313–33. doi: 10.1002/(sici)1097-0258(19981030)17:20<2313::aid-sim935>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Wong D, Modi R, Ramanathan M. Assessment of Markov-dependent stochastic models for drug administration compliance. Clin Pharmacokinet. 2003;42:193–204. doi: 10.2165/00003088-200342020-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kenna LA, Labbé L, Barrett JS, Pfister M. Modeling and simulation of adherence: approaches and applications in therapeutics. AAPS J. 2005;7:E390–407. doi: 10.1208/aapsj070240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparr LF, Moffitt MC, Ward MF. Missed psychiatric appointments: who returns and who stays away. Am J Psychiatry. 1993;150:801–5. doi: 10.1176/ajp.150.5.801. [DOI] [PubMed] [Google Scholar]
- 21.Boissel J-P, Nony P. Using pharmacokinetic– pharmacodynamic relationships to predict the effect of poor compliance. Clin Pharmacokinet. 2002;41:1–6. doi: 10.2165/00003088-200241010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Diaz E, Levine HB, Sullivan MC, Sernyak MJ, Hawkins KA, Cramer JA, Woods SW. Use of the Medication Event Monitoring System to estimate medication compliance in patients with schizophrenia. J Psychiatry Neurosci. 2001;26:325–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B. The use of electronic monitoring (MEMS) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr Res. 2007;90:229–37. doi: 10.1016/j.schres.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Velligan DI, Lam YW, Glahn DC, Barrett JA, Maples NJ, Ereshefsky L, Miller AL. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32:724–42. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol. 1999;161:1809–12. [PubMed] [Google Scholar]
- 26.Chue P, Eerdekens M, Augustyns I, Lachaux B, Molcan P, Eriksson L, Pretorius H, David AS. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005;15:111–7. doi: 10.1016/j.euroneuro.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Williams R. Optimal dosing with risperidone: updated recommendations. J Clin Psychiatry. 2001;62:282–9. doi: 10.4088/jcp.v62n0411. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SK, Sathyan G, Lindemulder EA, Ho PL, Sheiner LB, Aarons L. Quantitative characterization of therapeutic index: application of mixed-effects modeling to evaluate oxybutynin dose–efficacy and dose–side effect relationships. Clin Pharmacol Ther. 1999;65:672–84. doi: 10.1016/S0009-9236(99)90089-9. [DOI] [PubMed] [Google Scholar]
- 29.Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry. 2002;7:317–21. doi: 10.1038/sj.mp.4001009. [DOI] [PubMed] [Google Scholar]
- 30.Takano A, Suhara T, Ikoma Y, Yasuno F, Maeda J, Ichimiya T, Sudo Y, Inoue M, Okubo Y. Estimation of the time-course of dopamine D2 receptor occupancy in living human brain from plasma pharmacokinetics of antipsychotics. Int J Neuropsychopharmacol. 2004;7:19–26. doi: 10.1017/S1461145703003912. [DOI] [PubMed] [Google Scholar]
- 31.Gefvert O, Eriksson B, Persson P, Helldin L, Björner A, Mannaert E, Remmerie B, Eerdekens M, Nyberg S. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta) in patients with schizophrenia. Int J Neuropsychopharmacol. 2005;8:27–36. doi: 10.1017/S1461145704004924. [DOI] [PubMed] [Google Scholar]
- 32.Megens AA, Awouters FH, Schotte A, Meert TF, Dugovic C, Niemegeers CJ, Leysen JE. Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology (Berl) 1994;114:9–23. doi: 10.1007/BF02245439. [DOI] [PubMed] [Google Scholar]
- 33.Waldeck K, Larsson B, Andersson KE. Comparison of oxybutynin and its active metabolite, N-desethyl-oxybutynin, in the human detrusor and parotid gland. J Urol. 1997;157:1093–7. [PubMed] [Google Scholar]
- 34.Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(Suppl. 16):18–23. [PubMed] [Google Scholar]
- 35.Nony P, Cucherat M, Boissel J-P. Revisiting the effect compartment through timing errors in drug administration. Trends Pharmacol Sci. 1998;19:49–54. doi: 10.1016/s0165-6147(97)01159-0. [DOI] [PubMed] [Google Scholar]
- 36.Rubio A, Cox C, Weintraub M. Prediction of diltiazem plasma concentration curves from limited measurements using compliance data. Clin Pharmacokinet. 1992;22:238–46. doi: 10.2165/00003088-199222030-00006. [DOI] [PubMed] [Google Scholar]
- 37.Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J Clin Pharmacol. 2005;45:461–7. doi: 10.1177/0091270004274433. [DOI] [PubMed] [Google Scholar]
- 38.Levy G, Zamacona MK, Jusko WJ. Developing compliance instructions for labelling. Clin Pharmacol Ther. 2000;68:586–91. doi: 10.1067/mcp.2000.110976. [DOI] [PubMed] [Google Scholar]
- 39.Blesius A, Chabaud S, Cucherat M, Mismetti P, Boissel JP, Nony P. Compliance-guided therapy: a new insight into the potential role of clinical pharmacologists. Clin Pharmacokinet. 2006;45:95–104. doi: 10.2165/00003088-200645010-00007. [DOI] [PubMed] [Google Scholar]
- 40.Vrijens B, Goetghebeur E, de Klerk E, Rode R, Mayer S, Urquhart J. Modelling the association between adherence and viral load in HIV-infected patients. Stat Med. 2005;24:2719–31. doi: 10.1002/sim.2130. [DOI] [PubMed] [Google Scholar]
- 41.Richter A, Anton SE, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25:2307–35. doi: 10.1016/s0149-2918(03)80222-9. [DOI] [PubMed] [Google Scholar]


