Abstract
High blood pressure (BP) and monocyte activation are associated with atherogenic processes. Especially, CD16 expressing monocytes are shown to be activated in many inflammatory conditions but their characteristics in hypertension is unknown. We compared CD16++, CD16+ and CD16− monocyte populations and their cellular adhesion molecule (CAM), chemokine receptor, and activation marker expression in response to a moderate 20-min treadmill exercise bout at 65–70% VO2peak in 44 participants with elevated (EBP) or normal BP (NBP). Blood was drawn before, immediately after, and 10min after exercise. Phenotyping of monocytes and detection of surface markers were done by flow cytometry. Monocyte subset by exercise [pre, post, 10-min post] repeated measures ANOVA and group [EBP vs. NBP] by exercise repeated measures of ANCOVA with age, BMI, and fitness as covariates were employed. Circulating numbers of all the three monocyte subsets increased after exercise (p< 0.001), with the largest % increase for CD16+CD14++. Percents of CD16++CD14+ and CD16+CD14++ increased, whereas % CD16−CD14++ decreased (p< 0.001). Also, pre to post exercise changes in CD62L, CD11b, CXCR2, and HLA-DR expression were different among the monocyte subsets (p’s< 0.001). BP status did not significantly affect monocyte subset trafficking, although post-exercise changes in CD62L and CXCR2 levels were greater in EBP individuals (p< 0.05). We conclude that exercise leads to a different mobilization among monocyte subsets based on CD16 expression. Individuals with high BP showed greater responses to a physical challenge in some monocyte chemokine receptors and selectins, but its clinical implications need further examination.
Keywords: CD16, cell adhesion molecule, chemokine receptor, exercise, hypertension, monocytes
Introduction
High blood pressure (BP) is a major risk factor for developing coronary heart disease, stroke, heart failure, and atherosclerosis (Black, 2003; Chobanian et al., 2003). Atherosclerosis is an inflammatory disease of the vessel wall where endothelial inflammation and activation of immune cells play an important role in its pathogenesis (Ross, 1999). Hypertension poses high mechanical shear stress on the endothelium and may initiate endothelial injury, leading to adherence and infiltrations of monocytes that result in the development of plaques (Kher and Marsh, 2004). Upregulation of cellular adhesion molecules (CAMs) and chemokine receptors on monocytes is critical in recruitment, adherence, and infiltration of monocytes to the endothelium. Monocyte chemoattractant protein (MCP-1) recruits monocytes into the developing atheroma (Deo et al., 2004; De Lemos et al., 2003), and carotid artery intima-media thickness, a widely accepted index for assessing atherosclerosis, is associated with reactive oxygen species synthesis by monocytes in hypertensive patients (Watanabe et al., 2006). Thus, high BP and monocyte activation appear to contribute to the atherogenic processes in individuals with hypertension. However, the literature on CAM expression on monocytes in hypertension is limited (Mills et al., 2003; Zapolska-Downar et al., 2006).
The majority of blood monocytes strongly express CD14 on their surface, but a small subset also expresses CD16. These cells are shown to exhibit dendritic cell (DC)-like characteristics indicated by surface markers (Ancuta et al., 2000; Grage-Griebenow et al., 2001), to become DCs when cultured and stimulated in vitro, to express higher levels of CD1a, CD11a, and CD11c (Sanchez-Torres et al., 2001), to be more mature, to be more potent antigen presenting cells, to produce less cytokines, and to show less phagocytic activity compared to CD16− monocytes (CD16−CD14+) (Ziegler-Heitbrock, 1996). However, the findings are mixed on their maturity and cytokine production (Clanchy et al., 2006; Szaflarska et al., 2004). CD16+CD14+ monocytes are also shown to express less CCR2 (MCP-1 receptor) but higher levels of CCR5 (receptor for various chemokines including macrophage inflammatory protein-1 alpha; MIP-1α, MIP-1β, RANTES) than CD16−CD14+ monocytes, suggesting that CD16+ monocytes may exhibit different trafficking patterns from CD16− monocytes (Weber et al., 2000).
Numbers of CD16+ monocytes also increase in circulation in several inflammatory and pathological conditions such as AIDS (Ellery and Crowe, 2005; Pulliam et al., 2004), renal disease (Brauner et al., 1998; Ulrich et al., 2006), sepsis (Tsujimoto et al., 2006), and in response to exercise (Steppich et al., 2000). However, little is known about their migratory responses or CAM or chemokine receptor expression in response to acute stressors in individuals with high blood pressure. Thus, we investigated CD16+ vs. CD16− monocyte responses and their CAM (CD62L, CD11b) and chemokine receptor (CCR5, CXCR2) expression in response to a moderate exercise challenge in individuals with elevated BP (EBP) vs. normal BP (NBP). Examining monocyte demargination in response to stressors in individuals with high BP helps to investigate monocyte trafficking responses under the sympathetic nervous system (SNS), endocrine, and cardiovascular system activation.
Methods
Subjects
All subjects gave informed consent to the protocol, which was approved by the University of California, San Diego Institutional Review Board. Forty-four healthy men and women (age of 20–55 years) with both normal and high blood pressure were recruited from the local community via various advertisements (e.g., newspaper, flyers, brochures, and websites) and word of mouth referrals for a larger study of sympathetic nervous system regulation of cellular adhesion of immune cells1. Participants were categorized as having “normal” (screening Systolic BP (SBP) ≤ 129 and/or Diastolic BP (DBP) ≤ 84 mmHg) or “elevated” (screening SBP≥ 130 and/or DBP≥ 85 mmHg) BP2. Screening BP was measured using a Dinamap Compact BP® monitor (Critikon, Tempa, FL) and defined as the average of six seated blood pressure measures taken over two separate days. The study was conducted at the UCSD General Clinical Research Center, UCSD Clinical Trials Center, and the UCSD Pulmonary Function Laboratory. To confirm eligibility, all subjects underwent a history and physical examination by a licensed physician, blood tests for liver, metabolic, lipid, and thyroid panels were obtained, and a normal resting electrocardiogram (ECG) was confirmed before the protocol began. Individuals who had resting BP > 180/110 at screening, a history of heart disease, liver or renal disease, diabetes, psychosis, severe asthma, pregnancy, morbid obesity (>150% of the ideal body weight based on Metropolitan life tables), or ongoing inflammatory diseases (i.e., rheumatoid arthritis, multiple sclerosis, lupus) were excluded. For six subjects who were taking anti-hypertension medications they were carefully tapered off followed by a three-week wash-out period upon study enrollment after approval had been obtained from each patient’s physician. BP was monitored and recorded on a daily basis throughout the duration of the study for these participants to ensure safety.
Procedure
Exercise tests were performed by a certified cardiopulmonary technician under the supervision of an investigator and a physician. Subjects underwent a test on a treadmill using the standard Bruce protocol where the speed and grade of the treadmill increased gradually from 1.7mph and 10% every three minutes until exhaustion. Treadmill exercise has its benefits of familiarity for the motion (walking) and ease regardless of the individual’s fitness level as compared to other type of exercise (e.g., bicycle exercise requires lower body strength and may be unfamiliar to some individuals). Subject’s expired gas was analyzed by Sensormedics metabolic cart equipped with Vmax software (version 6–2A), and ECG was recorded using Marquette CardioSoft V.3 (GE medical systems, Milwaukee, WI). Oxyhemoglobin saturation (SpO2) was monitored using pulse oximetry_(Ohmeda, Datex, Louisville, CO), and perceived effort during the exercise was recorded using Borg’s 6–20 scale ratings of perceived exertion (RPE; Borg, 1970).
Subjects returned to the laboratory for a 20-min steady state exercise approximately one week after the peak exercise test. Subjects were instructed to refrain from caffeine, vigorous exercise, alcohol, and smoking for 24 hours prior to the testing day. Upon arrival, subjects were supine, and a 19-gauge catheter was inserted into the antecubital vein followed by rest in order to establish the baseline values; resting ECG, BP, and blood samples were acquired. Following a 2–5min warm-up period (speed gradually increased until the target was reached), 20-min treadmill exercise was performed at 65–70% of . Throughout the exercise task ECG, heart rate (HR), BP, respiratory exchange ratio (R), and metabolic equivalent (MET), Oxygen saturation (SpO2) levels, and RPE were monitored. Subjects were informed to notify the investigator immediately of warning signs of excess exertion (e.g., faintness, chest pain, shortness of breath, dizziness, and muscle cramps). Following the 20-min treadmill test, subjects continued to walk slowly for 2 minutes as a cool down period. Blood samples were acquired before, after, and 10-min after the exercise in a supine position.
Flow cytometry analyses
Antibody staining
Blood was drawn into a tube with EDTA and maintained at room temperature (23°C). Complete blood count (CBC) analysis was performed using a Cell-Dyn 4000 CBC counter (Abbott Diagnostics, Santa Clara, CA). Within 3 hours of collection, whole blood (100 µl) cells were incubated with various monoclonal antibodies (20 µl) for 15-min in the dark. Monoclonal antibodies against monocyte phenotype (CD14 and CD16), adhesion (CD62L and CD11b), chemokine receptors (CCR5, and CXCR2), and activation (HLA-DR, CD69) markers were conjugated to 4-color fluorchromes, fluoresceine isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC) (BD-PharMingen, San Diego, CA). Erythrocytes were removed using 2 ml of FACS Brand Lysing Solution (Becton-Dickinson, San Jose, CA); following 10-min incubation and centrifugation (5 min at × 300g), supernatant was aspirated, and cells were washed and resuspended in PBS with 5% formaldehyde.
Acquisition
A flow cytometer (FACSCalibur, Becton-Dickinson, San Jose, CA) equipped with 15mW argon (488m) and red diode (635nm) lasers and with CellQuest software (version 3.2, 1998), was used to acquire data. Isotype controls were used to determine non-specific antibody binding, and compensation of the signal between the photomultiplier tubes (PMTs) was confirmed using a compensation control. The voltage, gain, and compensation parameters were set based on monocytes identified in a forward and side scatter plot. Events of 4,000 – 5,000 gated monocytes on forward and side scatter plots were analyzed per tube. For the estimation of antibodies bound (against the molecules of interest) per cell was obtained using Quantibrite PE beads (Becton-Dickinson), as they provide the known reference values of the number of PE molecules bound. The Quantibrite PE beads were run at the same instrument settings as the cells.
Analysis
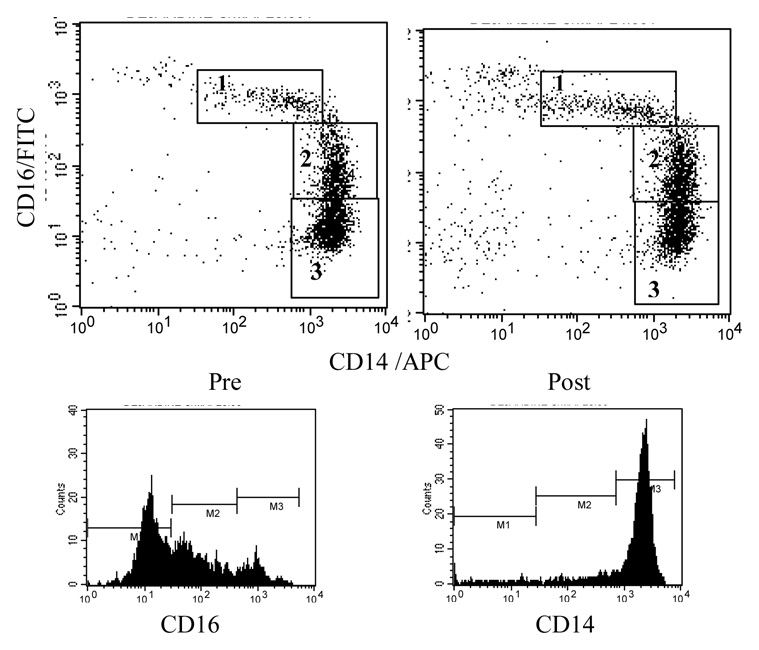
First of all, peripheral blood monocytes were identified using a forward and side scatter plot, and a monocyte gate was placed. Then, the monocyte gate was applied to the CD14/APC and CD16/FITC plots to identify three monocyte subsets based on the levels of CD16 expression: CD16++CD14+, CD16+CD14++, and CD16−CD14++ cells (Figure 1). Categorization of blood monocyte subsets based on CD16 and CD14 expression levels varies across the studies in the literature. We divided CD16 expressing monocytes further into CD16+ and CD16++ monocytes (Ancuta et al., 2000, Ulrich et al., 2006). Based on our previous observations, one may more readily be mobilized than the other. Cell subsets were expressed as percentages (%) of the gated population (i.e., total monocytes). Absolute numbers of the three monocyte subsets were calculated using the % data and a total monocyte count from the CBC differential data and adjusted for blood volume change post exercise (see Dill and Costill, 1974, Bosch et al., 2005). Also, expression levels of cell adhesion molecules (CD62L, CD11b, CD11a), chemokine receptors (CCR5 and CXCR2), and activation markers (CD69, and HLA-DR) were analyzed for each monocyte subsets. The Quantibrite PE beads provide a reliable estimation of antigen expression levels on the cell by converting fluorescent intensity of the FL2 (PE) axis into the number of PE molecules bound/cell (for CD11b, CD62L, and CCR5). Of note, the number of PE molecules bound per antibody is variable depending on the antibody. For the molecules of interest that were not stained with PE-conjugated antibodies (HLA-DR, CD69, and CXCR2), geo-mean fluorescent intensity (MFI) was calculated. Analyses of the flow cytometry data were performed using FlowJo analysis software (Tree Star, Ashland, Oregon).
Figure 1.
Scatter plots indicating monocyte subset designation based on CD16 (Y axis) and CD14 (X axis) expression levels pre and post exercise. Based on CD16 and CD14 expression levels three monocyte subsets were identified: 1. CD16++CD14+, 2. CD16+CD14++, and 3. CD16-CD14++. Lower plots are histograms of CD16 and CD14 fluorescence to show the expression patterns and distribution for their significance in monocyte subset gating.
Statistical analysis
Two stages of statistical analyses were performed (SPSS Statistical Software 14.0). First, 3 exercise [time: pre, post, and 10-min recovery]-by-3 monocyte subsets [CD16++CD14+, CD16+CD14++, and CD16−CD14++] repeated measures ANOVA was performed to examine monocyte subset differences in their response to exercise regardless of the BP groups. Secondly, in order to examine the responses of each monocyte subset to and recovery from exercise in individuals according to BP status, interactions were analyzed using two-way, 3 exercise [pre, post, and 10-min recovery]-by-2 group [EBP and NBP] repeated measures analysis of covariance (ANCOVA) for each monocyte subset separately. Age, fitness, and BMI (that were previously shown to be different between the two BP groups) were used as covariates in order to control for the possible effects of them on monocyte responses to exercise. Post-hoc tests with Bonferroni adjustment for multiple comparisons were done where appropriate, and simple main effects of Group or Exercise were examined. Polynomial expansion was employed under the apriori assumption of quadratic function through the time point of assessments, pre, post, and 10-min post (recovery) exercise, for most dependent variables. F and P values are presented based on linear or quadratic function as appropriate. Chi-square analyses were done for non-parametric analyses for the demographic data that were compared between the EBP and NBP groups, and log transformation was performed to approximate normal distribution as necessary. Statistical significance was determined at an alpha level of 0.05.
Results
Demographics
The demographic data for subjects are presented in Table 1 according to the BP groups. Age, BMI, and resting BPs were different between the two groups; on average, EBP individuals were 9 years older and heavier (by a BMI difference of 5) and, by definition, showed significantly higher systolic and diastolic BP at rest compared to that of the NBP group. However, resting HR and gender and ethnicity distribution were not different between the two groups. The overall ethnic composition was as following: 66% Caucasian American, 14% African American, 9% Asian American, 5% Hispanic American, and 7% other. There were two current smokers each in the NBP and EBP group. Among the EBP individuals, six subjects who were tapered off their antihypertensive medication(s) showed similar BP levels on average as the others (142/85 compared to 140/86 mm Hg).
Table 1.
Demographic characteristics in participants with elevated and normal BP
| Demographic Variables | Elevated BP | Normal BP | t/χ2 |
|---|---|---|---|
| (N=15) | (N=29) | ||
| Age (years) | 45 (7.95) | 36 (9.19) | −3.47** |
| Gender (male/female)^ | 9/6 | 15/14 | 0.27 |
| Systolic blood pressure (mmHg) at rest | 140.72 (9.69) | 114.60 (9.36) | −8.68*** |
| Diastolic blood pressure (mmHg) at rest | 85.45 (7.42) | 72.12 (9.77) | −4.63*** |
| Heart Rate (bpm) at rest | 73.02 (7.50) | 72.94 (8.78) | 0.03 |
| Body mass index (kg/m2) | 29.07 (4.10) | 24.41 (4.26) | −3.48* |
Values are presented as mean (SD).
indicate p< 0.05, p< 0.01, and p< 0.001, respectively.
denotes non-parametric χ2 test.
The t and p values are based on the results of Levene’s test for equality of variances, and equal variance was shown for all variables.
Metabolic responses during exercise in EBP vs. NBP groups
Table 2 shows the metabolic measurements of the BP groups during the peak exercise and 20-min steady state exercise. Although absolute VO2peak expressed in liters did not differ between the two groups, cardiovascular fitness (VO2peak in ml/kg/min) adjusted for body mass was significantly higher in the NBP group as compared to the EBP group. Consequently, absolute 65–70% VO2peak values for EBP individuals during a steady state exercise challenge were lower than those of NBP individuals. During the 20-min steady state exercise, EBP individuals exhibited significantly higher SBP and DBP, but lower metabolic equivalents (METs) than the NBP individuals, indicating a lower absolute work load. However, respiratory exchange ratio and perception of exertion assessed by Borg’s RPE during 20-min exercise were not different between the two groups, indicating that the relative difficulty of the exercise challenge was similar for the two groups regardless of their BP or fitness levels, as intended. The average rating of perceived exertion during the 20-min exercise challenge was 13 (“somewhat hard”).
Table 2.
Metabolic measurements during peak exercise and 20-min steady state exercise at 65–70% in participants with elevated and normal BP
| Metabolic Variables | Elevated BP | Normal BP | t |
|---|---|---|---|
| (N=15) | (N=29) | ||
| (ml/kg/min) | 30.53 (9.64) | 37.78 (9.98) | 2.31* |
| (L) | 2.63 (0.96) | 2.65 (1.13) | 0.05 |
| systolic blood pressure (mmHg) during 20-min exercise | 181.43 (17.65) | 149.38 (23) | −4.53*** |
| diastolic blood pressure (mmHg) during 20-min exercise | 86.86 (10.63) | 70.46 (10.90) | −4.58*** |
| heart rate (bpm) during 20-min exercise | 142.36 (16.93) | 154.28 (24.68) | 1.60 |
| respiratory exchange ratio (R) during 20-min exercise | 0.93 (0.06) | 0.96 (0.06) | 1.71 |
| Metabolic equivalent (MET) during 20-min exercise | 6.11 (1.70) | 7.46 (2.10) | 2.11* |
| ratings of perceived exertion (RPE) during 20-min exercise | 13.1 (2.00) | 13.4 (2.37) | 0.42 |
Values are presented as mean (SD).
indicate p< 0.05, p< 0.01, and p< 0.001, respectively.
The t and p values are based on the results of Levene’s test for equality of variances, and equal variance was shown for all variables.
Differences in monocyte subset responses to a moderate exercise challenge
Three subpopulations of peripheral blood monocytes were identified based on the expression levels of CD16 and CD14: CD16++(“bright”)CD14+(“dim”), CD16+(“dim”)CD14++(“bright”), and CD16−(“negative”)CD14++(“bright”) monocytes (Figure 1) with average % of total monocytes as 11%, 17%, and 64%, respectively.
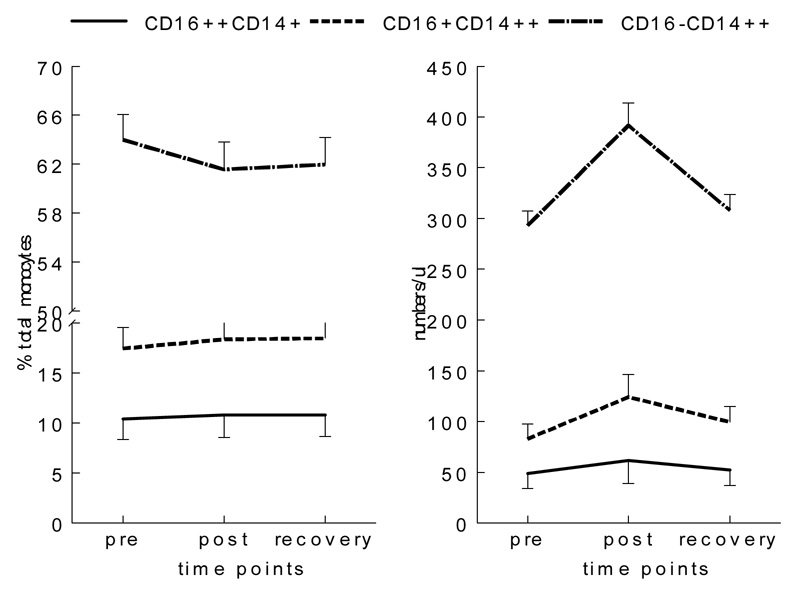
Exercise-by-monocyte subset interaction was significant for % monocyte change [F(1,129)= 6.00, p< 0.01], indicating different responses to exercise among the three subsets of monocytes: % CD16++ and % CD16+ (of total monocytes) slightly increased, but % CD16− monocytes decreased (Figure 1 & Figure 2). Post hoc analyses further revealed that these changes in % subsets were significantly different from each other. When absolute changes in cell numbers were compared, the exercise-by-subset interaction was also significant [F(1,129)= 14.28, p< 0.001], and the numbers of all three subsets of monocytes increased in circulation after exercise (overall exercise-induced monocytosis) and returned near the baseline values after a 10-min rest. These absolute number increases in cells were largest for the CD16− followed by CD16+ and CD16++ monocytes mainly because CD16− monocytes are the largest population of the three. However, % increase in cell numbers pre to post exercise was the largest, again, in CD16+ (by 49%) followed by that of CD16− (by 34%) and CD16++ (by 24%).
Figure 2.
Percent and number changes of monocyte subsets before and after exercise. % of CD16++CD14+ and CD16+CD14+ increased, but % CD16−CD14+ decreased post exercise. The absolute numbers of all three monocyte subsets increased with the highest % number increase for CD16+CD14+ monocytes.
Surface molecule expression on monocyte subsets in response to exercise
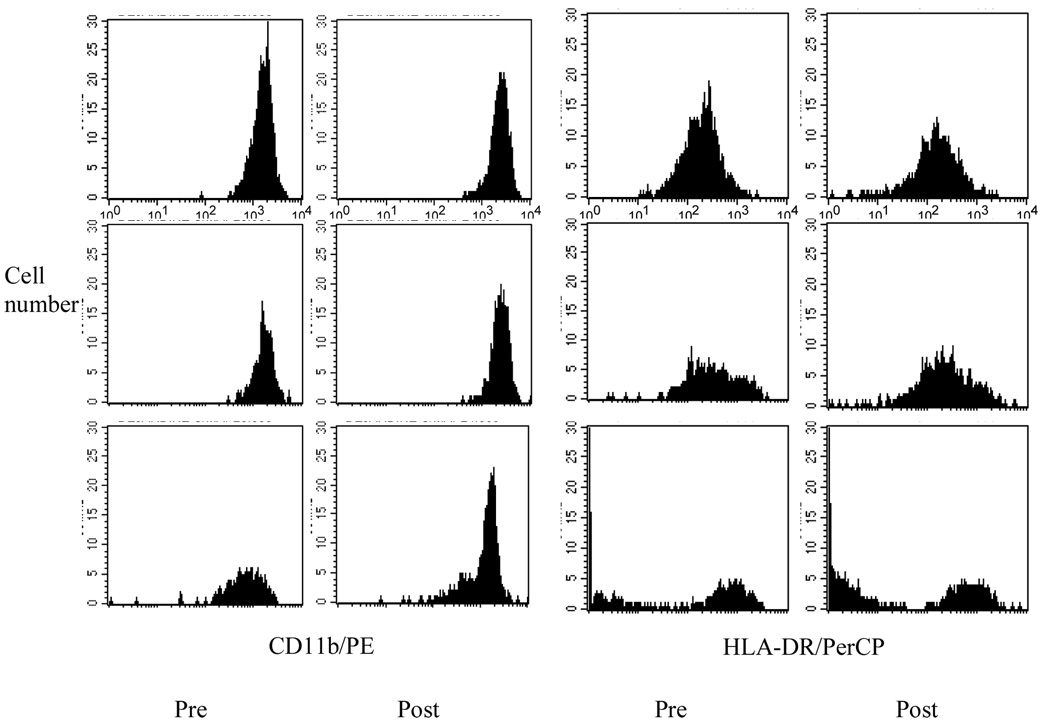
Table 3 presents CAMs, chemokine receptor, and activation marker expression pre, post, and 10min post exercise on CD16++, CD16+, and CD16− monocyte populations. The exercise-by-subset interaction was significant for CD62L such that its average per cell expression levels (quantified by using standard PE beads) decreased after exercise and remained lower than the baseline values on CD16+ and CD16−, but its levels on CD16++ increased [F(1,126)= 61.79, p< 0.001]. CD62L expression was significantly different among the monocyte subsets at all three time points with the highest level on CD16− followed by CD16+ monocytes. Meanwhile, the expression levels of CD11b increased after exercise and continued to increase after a 10-min rest on CD16+ and CD16− but only slightly increased on CD16++ in response to exercise (Figure 3). CD11b expression on CD16+ and CD16− monocytes was significantly higher than that on CD16++ at all three time points [F(1,126)= 8.37, p< 0.001]. As for the chemokine receptors, CXCR2 expression levels (assessed by MFI) in response to exercise also decreased significantly with the largest % decline shown on CD16− (25%) and remained low at recovery [F(1,126)= 13.08, p< 0.001]. At all time points, the levels of CXCR2 expression were the highest on CD16− followed by CD16+ monocytes. As a marker of activation, the level of HLA-DR decreased after exercise with the largest % decrease shown on CD16+ (22%) followed by CD16++ (12%) and was the highest for CD16++ monocytes at all time points (figure 3). For CAM, chemokine receptors, and activation marker expression the sample size decreased to 43, as those surface marker data were not available for one subject with normal BP. It should be also noted that the expression levels of some surface markers were based on small cell numbers, especially for CD16 expressing monocytes because of their presence in lower percentages among total blood monocytes.
Table 3.
Expression of adhesion molecules and chemokine receptors on monocyte subsets in response to exercise
| Molecules | roles | CD16++CD14+ | CD16+CD14++ | CD16−CD14++ |
|---|---|---|---|---|
| 1CD62L*** | L-selectin; mediates initial rolling on the endothelium | 84 (89), | 4561 (2559), | 9367 (4602), |
| 444 (392), | 3471 (4285), | 6941 (5907), | ||
| 437 (413) | 3550 (2269) | 6782 (4036) | ||
| 1CD11b*** | α chain of β-Integrin; mediates firm adhesion on the endothelium | 11620 (4894), | 20808 (11124), | 19710 (10215), |
| 12687 (5187), | 23639 (13820), | 22685 (13527), | ||
| 13603 (6060) | 28003 (14819) | 26903 (13622) | ||
| 2CCR5 | C-C chemokine receptor 5; mediates chemotaxis to chemokines such as MIP-1α | 84 (90), | 154 (117), | 173 (120), |
| 74 (80), | 133 (95), | 154 (100), | ||
| 85 (103) | 150 (125) | 170 (126) | ||
| 2CXCR2*** | CX-C chemokine receptor 2; mediates chemotaxis to chemokines such as IL-8 | 12 (7), | 44 (25), | 64 (29), |
| 10 (7), | 37 (37), | 49 (24), | ||
| 10 (6) | 36 (22) | 48 (23) | ||
| 2HLA-DR*** | MHC class II human leukocyte antigen-DR; mediates antigen presentation to T cells | 812 (349), | 407 (249), | 167 (90), |
| 716 (341), | 319 (186), | 155 (91), | ||
| 755 (357) | 380 (231) | 165 (99) |
Values are presented as mean (SD).
indicates exercise-by-monocyte subset interactions at p≤ 0.001.
PE molecules bound per cell or
mean fluorescence intensity (MFI) for each molecule pre, immediately post, and 10-min post exercise
Figure 3.
Representative histograms of CD11b and HLA-DR expression on three monocyte subsets (CD16++CD14+; top panel, CD16+CD14++; middle panel, CD16−CD14+; bottom panel) pre and post exercise. Expression levels of CD11a and HLA-DR increased and decreased on monocyte subsets pre to post exercise with the largest changes shown for CD16+CD14++. The X axis represents fluorescence FL2 (for CD11b/PE) and FL3 (for HLA-DR/PerCP) based on a log scale (100 to 104). The Y axis represents cell numbers (0–30).
Monocyte subsets and surface molecule expression in response to exercise in the EBP vs. NBP groups
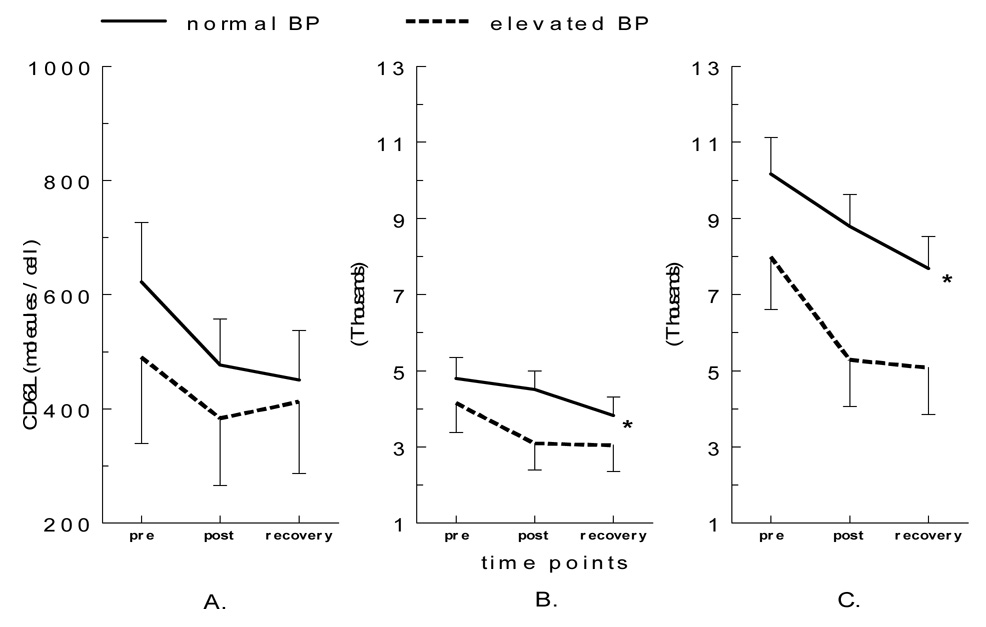
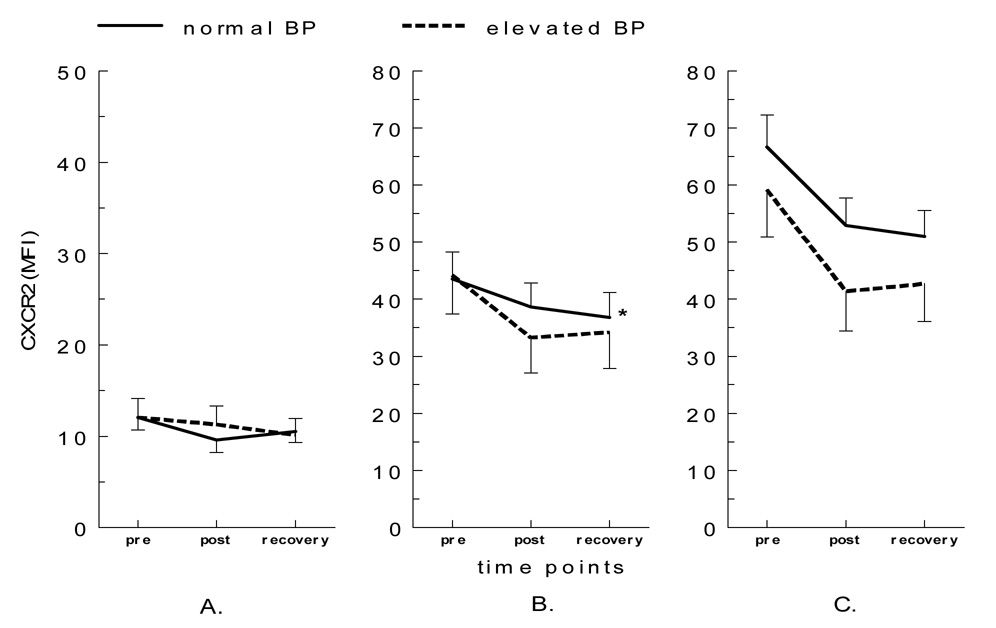
The exercise-by-group interaction for the expression levels of CD62L was significant for both CD16+ [F(1,38)= 4.34, p< 0.05] and CD16− [F(1,38)= 4.46, p< 0.05] monocytes such that a decrease in CD62L expression was greater pre to post exercise in the EBP group compared to the NBP group (Figure 4). Also, increases in CD11b expression were greater in the EBP group after exercise in all three subpopulations, but approached significance only in CD16− monocytes [F(1,38)= 3.51, p= 0.07]. As for chemokine receptors, a decrease in CXCR2 expression was greater in the EBP group for CD16+ monocytes [F(1,38)= 4.01, p= 0.05], but its decrease was smaller on CD16++ monocytes in the EBP group compared to the NBP group [F(1,38)= 3.55, p= 0.07] (Figure 5). Changes in the expression of CCR5, HLA-DR, or CD69 on monocyte subsets were not different between the two BP groups. Subjects who were taking or not taking (before tapering) anti-hypertensive medication(s) did not show significant difference in monocytes subsets or CAM expression in response to exercise.
Figure 4.
CD62L expression levels on monocyte subsets before and after exercise in elevated vs. normal BP groups (A. CD16++CD14+, B. CD16+CD14++, and C. CD16−CD14++). The post-exercise decrease in CD62L expression levels on CD16+CD14+ was greater for individuals with elevated BP (p< 0.05).
Figure 5.
CXCR2 expression on monocyte subsets before and after exercise in the elevated vs. normal BP groups (A. CD16++CD14+, B. CD16+CD14++, and C. CD16−CD14++). The post-exercise decrease in CXCR2 levels on CD16+CD14++ was greater for individuals with elevated BP (p= 0.05).
Exercise-by-BP group interaction was not significant for % or number of the three monocyte subsets. Although not statistically significant, the EBP group (76%) showed a greater increase in CD16+ monocyte numbers compared to the NBP group (42%). Meanwhile, an increase in CD16++ monocyte numbers was smaller in the EBP group (19%) compared to the NBP group (29%). CD16− monocytes increased by 38% for the EBP and 32% for the NBP group. There was no effect of antihypertensive medication(s), which was tapered off three weeks prior to the participation in the study, on monocyte subsets or surface expression of CAM, chemokine receptors, and activation markers.
Discussion
The heterogeneity of circulating monocyte populations has been emphasized in the literature but their phenotypic and functional characteristics need further investigation. We showed that pre to post exercise changes in percents and numbers of monocyte subpopulations based on CD16 expression levels differ, implying that monocyte subsets in blood traffic differently in response to a physical stressor. That is, demargination (traffic to peripheral blood) of the CD16+ monocytes was significantly greater than that of CD16− monocytes. Previously, Steppich and colleagues (2000) reported up to a 4.8 fold increase in numbers of CD16+ monocytes after a vigorous anaerobic exercise. In our study the increase in CD16+ cell numbers was about 1.5 fold, which is assumed to be indicative of the moderate exercise intensity employed in our study compared to the vigorous anaerobic exercise in the study by Steppich et al. (2000). Thus, CD16 expressing monocytes, overall, exhibit a larger mobilization response to an acute physical stressor, and it appears to be dose-dependent on the intensity of exercise.
We also found that CAM expression on monocyte subsets in response to exercise varied. Selectin (CD62L) and integrin (CD11b) levels on monocytes with high levels of CD16 (CD16++) did not change as compared to decreasing CD62L and increasing CD11a on CD16+ and CD16− monocytes. We previously showed that CD62L and CD11a expression on mixed lymphocytes in circulation decreased and increased, respectively, in response to both psychological and physical stressors (Hong et al., 2004, 2005; Mills et al., 2003). Steppich et al. (2000) also reported decreased CD62L expression on CD16+ monocytes after exercise. These results raise the question in the implications of those selectin and integrin expression in association with CD16 levels on circulating monocytes for their trafficking during physical stress. Curiously, exercise-induced changes in CD62L and CD11b expression levels were similar, while the demargination response was different between CD16+ and CD16− monocytes. Although speculative, this indicates that there may be other surface adhesion molecules also playing a role in trafficking of monocytes subsets (based on CD16 expression) in response to a physical stressor. It is beyond the scope of our study, however, to draw a conclusion on monocyte migration to target tissues under stress based on these CAM expression since our investigation was based on monocytes in circulation only. An earlier study showed that expression of an integrin CD11b was greater on peritoneal macrophages (differentiated from CD16− monocytes and migrated to the peritoneum from blood) in patients with renal disease (Brauner et al., 1998).
In addition to selectin and intergrin expression chemokine receptors play an important role in monocyte migration. Unlike CD62L or CD11a levels, the level of chemokine receptors CXCR2 on CD16+ monocytes was smaller compared to those on CD16− monocytes. Previously, CD16+ monocytes in blood were also shown to express less CCR2 (MCP-1 receptor) (Weber et al., 2000). These indicate that CD16+ monocytes in circulation expressed smaller levels of chemokine receptors. Meanwhile, higher levels of HLA-DR expression on CD16+ monocytes, which markedly decreased after exercise, indicate that a greater overall activation state of CD16+ compared to CD16− monocytes and that CD16+ monocytes traffic to circulation under stress exhibit smaller activation. This finding agrees with the previous findings showing high HLA-DR expression on CD16 expressing DC-like monocytes that possess high antigen presenting capacity and are shown to be lymph-node migrating DCs upon activation (Randolph et al., 2002). Differentiation and maturation of CD16+ monocytes to DCs are activated by cytokines and CAMs (Li et al., 2003), which are elevated during acute physical stress, and their migration to lymph nodes is critical in subsequent T cell activation. Taken together, it can be speculated, but remains to be determined, that CD16− monocytes with higher levels of chemokine receptors may preferentially migrate to activated endothelium (e.g., airway, lungs, peritoneum), whereas DC-like monocytes (CD16+) with less chemokine receptors but increased HLA-DR may be mobilized to circulation and then, lymph nodes under stress.
We found only a small effect of elevated BP on monocyte subset demargination and chemokine receptor expression. Expression of HLA-DR and CXCR2 indicated that demarginated CD16++ monocytes after exercise expressed higher levels of activation markers and chemokine receptors in individuals with elevated BP. Since there was no statistically significant difference in CD16 expressing monocyte mobilization between elevated and normal BP individuals, it remains to be clarified whether this greater response of chemokine receptor expression of CD16+ monocytes to acute stress in EBP individuals significantly influences their migration to peripheral blood or lymph nodes. The literature on CD16+ monocyte trafficking and hypertension is largely limited, but general monocytosis is shown to be associated with cardiovascular disease (CVD), even at rest (Reed and Hendley, 1994). We previously showed greater increases in cytokine levels and adhesion of immune cells after exercise in hypertensive patients, implying higher cellular activation in response to exercise in individuals with high BP (Mills et al., 2000). This implies the association between elevated CAM expression on monocytes and high BP, although the nature of this association and its clear clinical implications remain to be further elucidated. The significance of the monocytes’ role in hypertension is further shown by the evidence that they express higher levels of angiotensin converting enzyme (ACE) which plays a major role in increasing oxidative stress, endothelial cytokine production, and leukocyte adhesion to the vascular endothelium, compared to other leukocyte subsets (Dzau et al., 2002). Ulrich and colleagues (2006) showed that dialysis patients with CVD exhibited higher ACE expression on monocytes compared to healthy controls or patients with no CVD and that CD16+ monocytes exhibit highest ACE expression compared to CD16− monocytes.
In summary, we showed that CD16+ monocytes traffic more readily to circulation in response to an acute physical stressor compared to CD16− monocytes. In addition, our findings provide additional information to the current literature on the surface molecule profile of CD16+ vs. CD16− monocytes under stress. However, the clinical implications of these differential monocyte migration patterns in individuals with elevated BP remain to be unclear. This might have been the result of our use of moderate exercise intensity and/or the mild to moderate BP elevation in the study population. The study is limited by a small sample size, thus insufficient power, which may have affected the statistical significance. Also, there is some imbalance in sample-sizes between the BP groups that may have also affected statistical power. Future studies in individuals with more established hypertension or varying the intensity of the stressor will likely further clarify the effect of high BP on monocyte responses under acute stress. The clinical implications of heterogeneous migratory responses in monocyte subsets to stressors merit further investigation.
Acknowledgements
Authors express appreciation to the Immunogenetics Laboratory at the Veterans Affairs Medical Center San Diego and UCSD GCRC Flow Cytometry Core Laboratory for their technical support with the flow cytometric analyses.
Grants
This work was supported by grants MO1-RR00827-25 and RO1 HL57265-05 from the National Institutes of Health.
Footnotes
In this larger hypertension and cell adhesion study other immune cell populations other than monocytes were assessed using flow cytometry, including lymphocyte subsets and CAM expression that is important in lymphocyte trafficking. The lymphocyte data have been published elsewhere (Hong et al., 2005).
We categorized “normal” as screening BP ≤ 129 and 84 mmHg and “elevated” as BP ≥ 130 and/or 85 mmHg (when either of SBP or DBP satisfied the criteria) based on the 2003 Guidelines for Management of Hypertension by the European Society of Hypertension (Guidelines Committee, 2003). Thus, the “elevated BP” group included those with SBP/DBP ranges of 130–166/66–100 mmHg (designated as “high normal” and “mild hypertension” by the European Society Hypertension Guidelines). The “normal BP” group included individuals who would be categorized as “optimal” and “normal” according to the European Society Hypertension Guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ancuta P, Weiss L, Haeffner-Cavaillon N. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur. J. Immunol. 2000;30(7):1872–1883. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Black HR. The burden of CVD: following the link from hypertension to myocardial infarction and heart failure. Am. J. Hypertens. 2003;16(9) Suppl 1:4–6. doi: 10.1016/s0895-7061(03)00969-5. [DOI] [PubMed] [Google Scholar]
- Blann AD, Tse W, Maxwell SJR, Waite MA. Increased levels of the soluble adhesion molecule E-selectin in essential hypertension. J. Hypertens. 1994;12:925–928. [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehab. Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Bosch JA, Berntson GG, Cacioppo JT, Marucha PT. Differential mobilization of functionally distinct natural killer subsets during acute psychologic stress. Psychosom. Med. 2005;67(3):366–375. doi: 10.1097/01.psy.0000160469.00312.8e. [DOI] [PubMed] [Google Scholar]
- Brauner A, Lu Y, Halldén G, Hylander B, Lundahl J. Difference in the blood monocyte phenotype between uremic patients and healthy controls: its relation to monocyte differentiation into macrophages in the peritoneal cavity. Inflammation. 1998;22(1):55–66. doi: 10.1023/a:1022395723972. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Clanchy FI, Holloway AC, Lari R, Cameron PU, Hamilton JA. Detection and properties of the human proliferative monocyte subpopulation. J. Leukoc. Biol. 2006;79(4):757–766. doi: 10.1189/jlb.0905522. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107(5):690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J. Am. Coll. Cardiol. 2004;44(9):1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37(2):247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- Ellery PJ, Crowe SM. Phenotypic characterization of blood monocytes from HIV-infected individuals. Methods Mol. Biol. 2005;304:343–353. doi: 10.1385/1-59259-907-9:343. [DOI] [PubMed] [Google Scholar]
- Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+) / CD16(+) blood monocytes. Eur. J. Immunol. 2001;31(1):48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Guidelines Committee. 2003 European Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003;21(6):1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- Hong S, Farag N, Nelesen RA, Ziegler MG, Mills PJ. Changes in peripheral leukocyte distribution and adhesion molecule expression after laboratory speech stressor and acute exercise. J. Psychosom. Res. 2004;56(3):363–370. doi: 10.1016/S0022-3999(03)00134-X. [DOI] [PubMed] [Google Scholar]
- Hong S, Johnson T, Farag N, Guy H, Matthews S, Ziegler MG, Mills PJ. The effect of fitness on leukocyte trafficking and adhesion molecule expression in response to moderate exercise challenge. J. Appl. Physiol. 2005;98:1057–1063. doi: 10.1152/japplphysiol.00233.2004. [DOI] [PubMed] [Google Scholar]
- Kher N, Marsh JD. Pathobiology of atherosclerosis--a brief review. Semin. Thromb. Hemost. 2004;30(6):665–672. doi: 10.1055/s-2004-861509. [DOI] [PubMed] [Google Scholar]
- Li G, Kim YJ, Mantel C, Broxmeyer HE. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J. Immunol. 2003;171(2):669–677. doi: 10.4049/jimmunol.171.2.669. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Farag N, Hong S, Kennedy BP, Berry CC, Ziegler MG. Immune cell CD62L and CD11a expression in response to a psychological stressor in human hypertension. Brain Behav. Imm. 17(4):260–267. doi: 10.1016/s0889-1591(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 2004;157(1–2):93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schäkel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J. Exp. Med. 2002;196(4):517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JP, Hendley ED. Blood cell changes in spontaneously hypertensive rats are not all associated with the hypertensive phenotype. J. Hypertens. 1994;12(4):391–399. [PubMed] [Google Scholar]
- Sanchez-Torres C, Garcia-Romo GS, Cornejo-Cortes MA, Rivas-Carvalho A, Sanchez-Schmitz G. CD16+ and CD16− human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int. Immunol. 2001;13(12):1571–1581. doi: 10.1093/intimm/13.12.1571. [DOI] [PubMed] [Google Scholar]
- Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am. J. Physiol. Cell. Physiol. 2000;279(3):C578–C586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- Szaflarska A, Baj-Krzyworzeka M, Siedlar M, Weglarczyk K, Ruggiero I, Hajto B, Zembala M. Antitumor response of CD14+/CD16+ monocyte subpopulation. Exp. Hematol. 2004;32(8):748–755. doi: 10.1016/j.exphem.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Ono S, Majima T, Efron PA, Kinoshita M, Hiraide H, Moldawer LL, Mochizuki H. Differential toll-like receptor expression after ex vivo lipopolysaccharide exposure in patients with sepsis and following surgical stress. Clin. Immunol. 2006;119(2):180–187. doi: 10.1016/j.clim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Ulrich C, Heine GH, Garcia P, Reichart B, Georg T, Krause M, Kohler H, Girndt M. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol. Dial. Transplant. 2006;21(6):1596–1602. doi: 10.1093/ndt/gfl008. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yasunari K, Nakamura M, Maeda K. Carotid artery intima-media thickness and reactive oxygen species formation by monocytes in hypertensive patients. J. Hum. Hypertens. 2006;20(5):336–340. doi: 10.1038/sj.jhh.1001990. [DOI] [PubMed] [Google Scholar]
- Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 2000;67(5):699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- Zapolska-Downar D, Siennicka A, Chelstowski K, Widecka K, Goracy I, Halasa M, machalinski B, Naruszewicz M. Is there an association between angiotension-convesrting enzyme gene polymorphism and functional activation of monocytes and macrophage in young patients with essential hypertension? J Hypertens. 24(8):1565–1573. doi: 10.1097/01.hjh.0000239292.32883.38. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol. Today. 1996;17(9):424–428. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]