Abstract
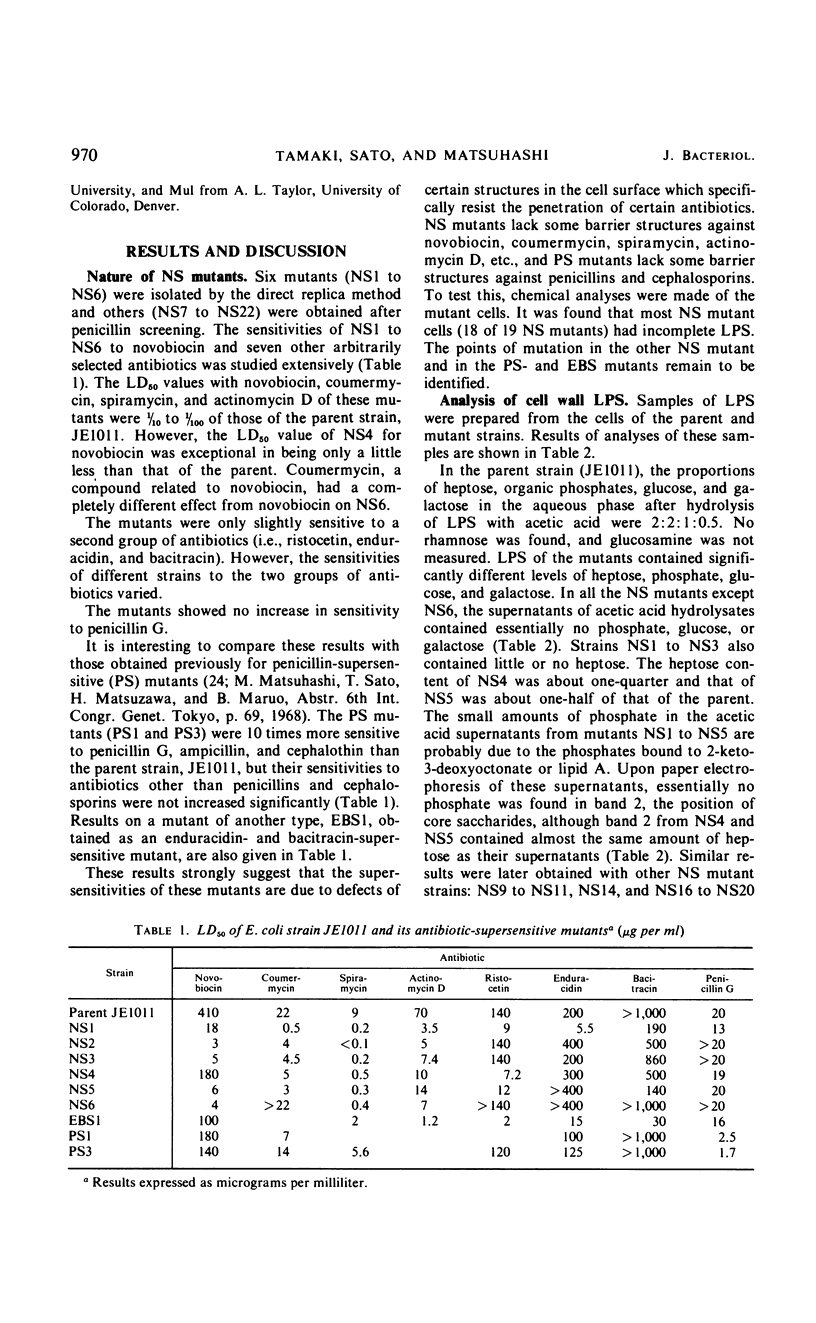
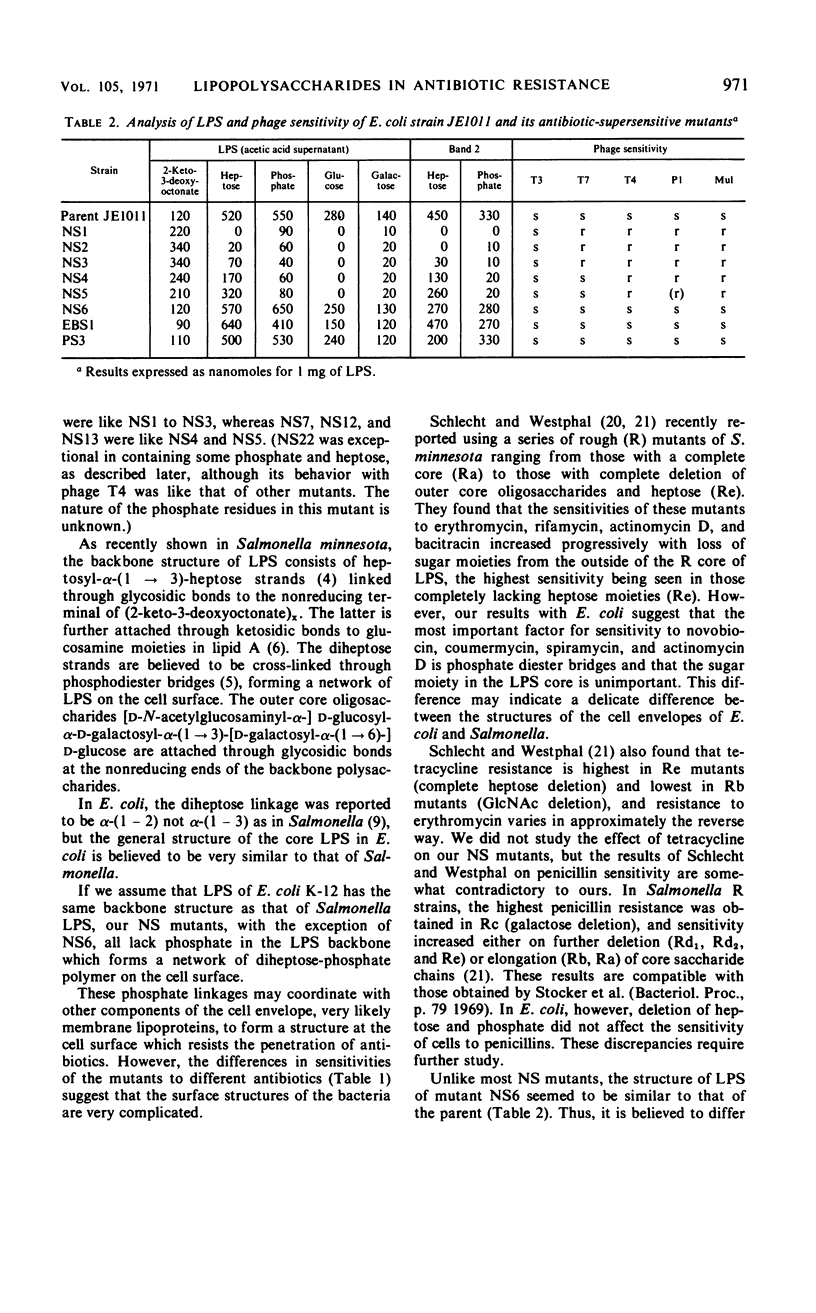
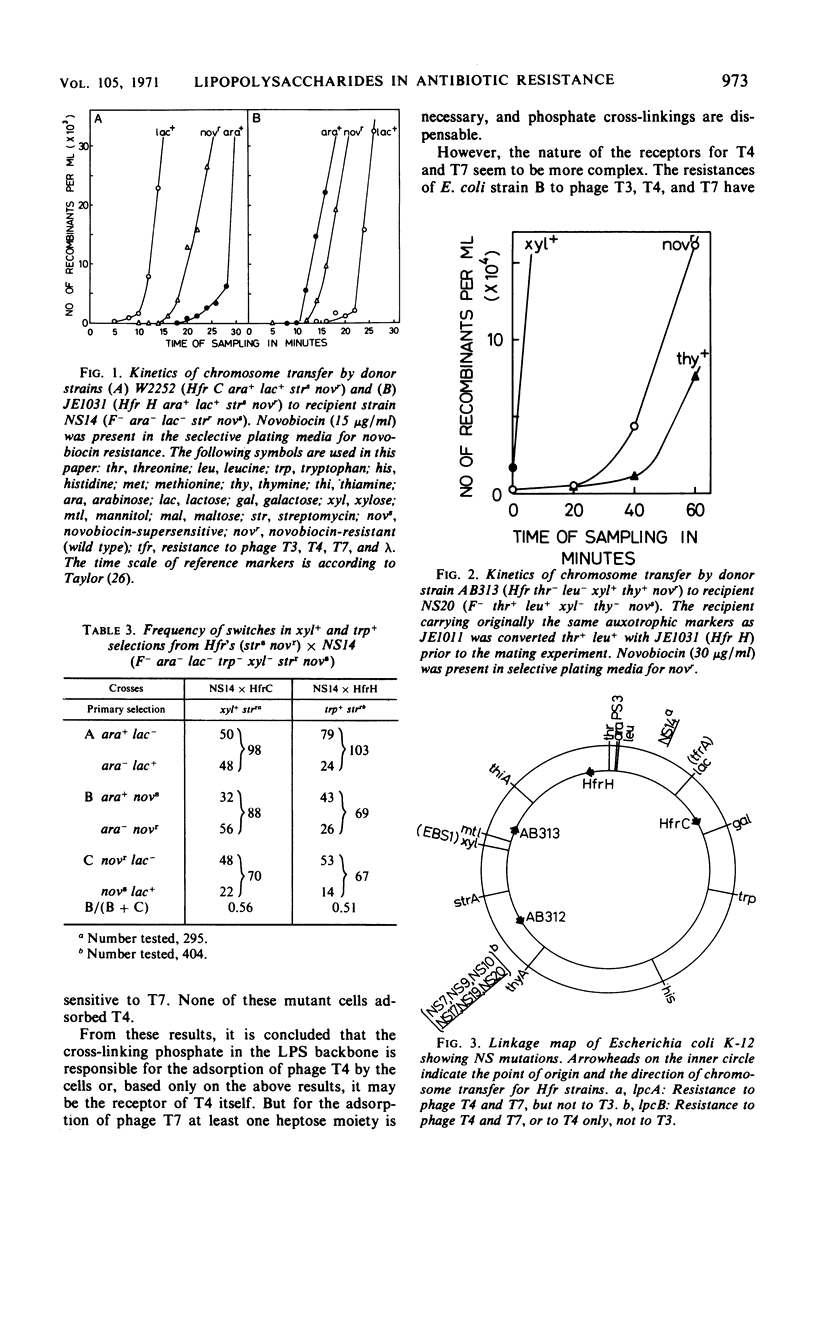
Novobiocin-supersensitive (NS) mutants which could not grow on plates containing 40 μg or less of novobiocin per ml were isolated from Escherichia coli strain JE1011 (derived from E. coli K-12). Most of these NS mutants were found to have incomplete lipopolysaccharides (LPS), and they lack phosphate diester bridges in their backbone structure, with or without total loss of heptose, to which the phosphate diester is linked, and consequently lack external outer-core oligosaccharides. The phosphate diester bridges in the LPS backbone are apparently very important in forming a cell surface structure resistant to the penetration of antibiotics such as novobiocin, spiramycin, and actinomycin D. NS mutants, with incomplete LPS, lacking phosphates in their backbone structure were found to be resistant to phage T4, and those which also lacked heptose were resistant to phages T4 and T7. In contrast to the generally accepted idea that resistances to phages T3, T4, and T7 are linked genetically, no NS mutant was found to be resistant to T3. The possible structures of the receptors for T4 and T7 are discussed. The positions of novobiocin-supersensitive genes on the chromosome of several of the NS mutants defective in LPS were mapped. The genes were designated lpcA (between ara and lac) and lpcB (between 55 min and 60 min). The latter seemed to be a group of several related genes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 3. The linkage of the heptose units. Eur J Biochem. 1968 Mar;4(1):126–133. doi: 10.1111/j.1432-1033.1968.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Dröge W., Ruschmann E., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 4. Phosphate groups linked to heptose units and their absence in some R lipopolysaccharides. Eur J Biochem. 1968 Mar;4(1):134–138. doi: 10.1111/j.1432-1033.1968.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 6. Investigations on the structure of the lipid A component. Eur J Biochem. 1969 Jan;7(3):370–379. doi: 10.1111/j.1432-1033.1969.tb19618.x. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kuriki Y., Kurahashi K. Polymannoheptose isolated from cell wall of uridine diphosphate glucose pyrophosphorylase-less mutant of Escherichia coli K 12. J Biochem. 1965 Sep;58(3):308–311. doi: 10.1093/oxfordjournals.jbchem.a128205. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Mühlradt P., Risse H. J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants 5. Evidence for a phosphorylating enzyme in lipopolysaccharide biosynthesis. Eur J Biochem. 1968 Apr 3;4(2):139–145. doi: 10.1111/j.1432-1033.1968.tb00184.x. [DOI] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risse H. J., Dröge W., Ruschmann E., Lüderitz O., Westphal O., Schlosshardt J. Eine neue Gruppe von Salmonella R-Mutanten. Serologische und biochemische Analyse des Heptosekerns von Lipopolysacchariden aus Salmonella minnesota- und Salmonella ruiru-Mutanten. Eur J Biochem. 1967 Apr;1(2):216–232. doi: 10.1111/j.1432-1033.1967.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Antibiotica-Empfindlichkeit bei S- und R-Formen von Salmonella minnesota. Naturwissenschaften. 1968 Oct;55(10):494–495. doi: 10.1007/BF00599721. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 4. Typisierung von S. minnesota-R-Mutanten mittels Antibiotica. Zentralbl Bakteriol Orig. 1970 Apr;213(3):356–380. [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y. Mutants of Escherichia coli sensitive to methylene blue and acridines. Genet Res. 1966 Feb;7(1):1–11. doi: 10.1017/s0016672300009423. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Watanabe T., Arai T., Hattori T. Effects of cell wall polysaccharide on the mating ability of Salmonella typhimurium. Nature. 1970 Jan 3;225(5227):70–71. doi: 10.1038/225070a0. [DOI] [PubMed] [Google Scholar]



