Abstract
AIMS
To evaluate the association between noncompliance with alendronate and risedronate and the risk of nonvertebral osteoporotic fracture in community-dwelling elderly women.
METHODS
A nested case–control study was conducted using the Quebec administrative health databases. To be included in the cohort, women needed to be aged ≥ 68 years and to have initiated treatment with alendronate or risedronate between 1 January 2002 and 31 March 2005. Cases consisted of all women with an incident nonvertebral osteoporotic fracture occurring ≥ 1 year after initiation of therapy. Each case was matched with up to 20 controls using incidence density sampling, according to age (± 1 year) and follow-up duration. A woman was noncompliant if she had a medication possession ratio (MPR) <80% for total follow-up duration. Rate ratios (RR) for fracture were estimated through conditional logistic regression analysis, adjusting for potential confounders.
RESULTS
Among the 30 259 women included in the cohort, 1036 nonvertebral fracture cases were identified and were matched to 20 069 controls. Compared with women with a MPR ≥ 80%, those with a MPR < 80% had a greater risk of nonvertebral fracture [adjusted RR 1.27, 95% confidence interval (CI) 1.12, 1.44]. Considering hip fracture only, the multivariate model yielded similar results, (adjusted RR 1.28, 95% CI 1.02, 1.61).
CONCLUSIONS
Among community-dwelling elderly women, noncompliance with alendronate or risedronate is associated with an increased risk of nonvertebral fracture.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Compliance with alendronate and risedronate is suboptimal.
Few studies have specifically evaluated the impact of noncompliance with alendronate or risedronate on the incidence of osteoporotic fractures in community-dwelling elderly women.
WHAT THIS STUDY ADDS
Among community-dwelling elderly women, noncompliance [defined as medication possession ratio (MPR) < 80%] with alendronate or risedronate was associated with a 27% increased risk of nonvertebral fracture [rate ratio (RR) 1.27, 95% confidence interval (CI) 1.12, 1.44].
This study is the first to assess the impact of noncompliance with bisphosphonates in a subgroup of women aged > 80 years.
Among women aged > 80 years, MPR < 80% was associated with a 48% greater risk of sustaining a nonvertebral fracture (RR 1.48, 95% CI 1.19, 1.85), compared with women with a MPR ≥ 80%.
Keywords: administrative databases, alendronate, noncompliance, osteoporosis, osteoporotic fractures, risedronate
Introduction
Osteoporotic fractures have extensive clinical and economic consequences, and are a major public health concern. The estimated worldwide number of new osteoporotic fractures for the year 2000 was 9.0 million, of which 1.6 million were at the hip, 1.7 million were at the distal forearm and 1.4 million were clinical vertebral fractures [1].
The important burden of nonvertebral fractures highlights the need for osteoporosis therapies with efficacy that extends beyond the spine. Alendronate and risedronate have both shown significant effect on vertebral and nonvertebral fracture reduction [2]. In clinical trials, significant reductions in the incidence of nonvertebral fractures were apparent after 6 months of treatment for risedronate [3] and 12 months for alendronate [4].
A major concern about alendronate and risedronate is the lack of medication compliance [5, 6]. Compliance can be defined as the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen. It is usually measured over a period of time and reported as a percentage [7]. Compliance differs from persistence, which is defined as the duration of time from initiation to discontinuation of therapy. A systematic review of 14 observational studies has reported that, for a 1-year period, on average, patients being prescribed a daily regimen of alendronate or risedronate were exposed to their treatment only between 46 and 64% of the time, whereas patients receiving a weekly regimen were exposed between 58 and 76% of the time [5].
Noncompliance with antiresorptive therapies has been associated with a 16–50% increased risk of fracture [8–13]. However, few studies have specifically evaluated the impact of noncompliance with alendronate or risedronate. Furthermore, none of these studies evaluated the impact of noncompliance in the subgroup of elderly women. This is an appreciable knowledge gap, given that this age group is at high risk of sustaining osteoporotic fractures [14].
The aim of this population-based study was to evaluate the association between noncompliance with alendronate or risedronate and the incidence of nonvertebral osteoporotic fractures among community-dwelling elderly women in the province of Quebec, Canada.
Methods
A nested case–control study was conducted using the linked administrative health databases of the province of Quebec [referred to as the Régie de l’Assurance Maladie du Québec (RAMQ) and MED-ECHO databases]. It has been shown that a nested case–control study design leads to practically the same results as those that would be obtained if the whole cohort was used, but at greater efficiency [15]. The study protocol was approved by the Commission d’Accès à l’Information du Québec and by the University of Montreal Research Ethics Committee.
Data source
The RAMQ databases are claims-based and contain four types of files which can be linked via a unique patient identification number included in each file. The patient identification number is scrambled to ensure patient confidentiality. The medical services file contains fee-for-service claims for inpatient and outpatient medical services supplied to all residents of Quebec. It includes demographic information (gender, age) as well as the nature of the medical act, date, location (office, emergency room, hospital), and associated diagnoses coded according to the International Classification of Diseases, ninth revision (ICD-9). The pharmaceutical file contains data on all drugs listed in the RAMQ drug formulary that are dispensed to community-dwelling patients who are enrolled in the public drug plan. The pharmaceutical file includes the generic name, the drug identification number, strength, form, quantity, date and duration of therapy, as indicated by the community pharmacist who filled the prescription. Drugs received during a hospitalization are not recorded in the database. Between 2002 and 2006, >90% of Quebec citizens aged ≥ 65 years were covered by the RAMQ drug plan [16]. The admissibility file lists the date of the beginning and end of eligibility to the RAMQ drug plan. Finally, the beneficiary file contains information about the date of birth and death, if applicable. The RAMQ databases have been frequently used for pharmacoepidemiological studies. The combination of diagnostic and procedure codes in the medical services file has been shown to be a sensitive indicator of fractures in the elderly [17], and data on medications recorded in the pharmaceutical file have been found to be comprehensive and valid [18].
The MED-ECHO database contains information about acute-care hospitalizations and was used to obtain length of hospital stay, if applicable.
Study cohort
From the RAMQ pharmaceutical file, a total of 38 343 women having filled an initial prescription of alendronate (10 mg once daily or 70 mg once weekly) or risedronate (5 mg once daily or 35 mg once weekly) between 1 January 2002 and 31 March 2005 were identified. To be considered new users, women needed to have not filled any prescription of bisphosphonates (alendronate, risedronate, etidronate), raloxifene, calcitonin or hormone replacement therapy at least 2 years prior to initiation of alendronate or risedronate. The date of entry in the cohort was the date of the first dispensing of alendronate or risedronate. To be eligible in the cohort, women needed to be at least 68 years old at cohort entry and to have been continuously covered by the RAMQ drug plan during the 2 years prior to cohort entry.
To avoid entering into the cohort women with fracture risk not related to osteoporosis, women having one of the following diseases in the 2 years before cohort entry were excluded: (i) malignant neoplasm or neoplasms of uncertain behaviour (ICD-9 codes 140–195, 196–198, 199, 200–208, 235–238, 239 and 255.6 or having received a prescription of pamidronate or clodronate); (ii) Paget's disease (ICD-9 codes 731.0, 731.1 or drug markers); (iii) osteomalacia (ICD-9 code 268.2); (iv) Cushing's syndrome (ICD-9 code 255.0 or a medical procedure code for adrenalectomy); (v) hyperthyroidism (ICD-9 code 242 or drug markers); (vi) primary hyperparathyroidism (ICD-9 code 252.0 or medical procedure codes for exploration or excision of the parathyroid glands); (vii) celiac sprue (ICD-9 code 579.0); (viii) impaired renal function (ICD-9 code 581–589, medical procedure codes for haemodialysis or peritoneal dialysis, or drug markers); and (ix) solid organ transplant (medical procedure code for a lung, cardiac, hepatic or kidney transplant). Women having sustained fractures likely to be secondary to serious trauma [multiple fractures (ICD-9 codes 804, 819, 828) or vertebral fracture with spinal cord injury (ICD-9 code 806)] within 2 years prior to cohort entry were also excluded. Women with other types of prior fractures were not excluded. Women were followed-up into the cohort until occurrence of an exclusion criterion, admission in a long-term public healthcare institution, end of RAMQ drug plan enrolment, switch to another type of antiresorptive agent or prescription of an additional agent, death, or the end of the study period (31 March 2006), whichever occurred first.
Identification of cases and controls
In order to allow a time-window for the onset of bisphosphonate effectiveness [3, 4], cases consisted of all women in the study cohort who had had an incident nonvertebral fracture at least 1 year after initiation of bisphosphonate therapy. In agreement with a recent methodological review [19], fractures occurring during the first year of therapy were assessed for both cases and controls and considered as potential confounders, since they could not be associated with noncompliance. Fractures were identified by the presence of either an ICD-9 code or a medical procedure code from the medical services file. The estimated sensitivity of the use of either diagnostic or procedure code in RAMQ medical file to detect any fracture is 85.0% [17]. The highest sensitivity is for the detection of hip fractures (97.2%) [17]. Fracture sites selected were hip (ICD-9 codes 820.0, 820.2, 820.8), pelvis (ICD-9 codes 808.0, 808.2, 808.4, 808.8), rib (ICD-9 code 807.0), humerus (ICD-9 codes 812.0, 812.2, 812.4), radius or ulna (ICD-9 codes 813.0, 813.2, 813.4), clavicle (ICD-9 code 810.0), carpal or metacarpal bones (ICD-9 codes 814.0, 815.0), ankle (ICD-9 codes 824.0, 824.2, 824.4, 824.6, 824.8), tarsus or metatarsus (ICD-9 code 825.2). Vertebral fractures were not included because more than two-thirds of them do not come to clinical attention [20] and thus would not be captured in the RAMQ databases. The date of the first claim related to an incident nonvertebral fracture at least 1 year after initiation of bisphosphonate therapy was defined as the index date.
To avoid the identification of prevalent hip fractures as incident cases, women were considered to have a new fracture if they incurred a claim at least 6 months after a previous claim for a hip fracture. For the other nonvertebral fractures, women were considered to have a new fracture if they incurred a claim at least 3 months after a previous claim for the same fracture type.
For each case, up to 20 controls were randomly selected from the study cohort, using incidence density sampling [21]. The maximum number of controls per case was fixed at 20 in order to increase the statistical power of the study [22]. Controls were matched to cases according to age (± 1 year). The index date of the controls was the date resulting in the same number of days of follow-up (based on the number of days between date of entry in the cohort and date of fracture) as their respective case. All risk sets that included at least one case and one matched control were retained for further analyses.
Exposure ascertainment
Within each risk set, compliance was measured by calculating the medication possession ratio (MPR), which was defined as the number of days’ supply of medication received divided by the number of days between date of entry in the cohort and index date [23]. Thus, the time period over which compliance was assessed was the same for cases and controls. For prescriptions extending beyond the index date, days’ supply was truncated at the index date. Since drugs dispensed in hospitals are not recorded in RAMQ databases, if a woman had an acute-care hospitalization between the date of entry in the cohort and index date, compliance was assumed to be 100% during hospital stay if the woman had filled a prescription < 2 months prior to admission. The supply of medication dispensed prior to hospital admission was carried over to the post-discharge period. If MPR exceeded 100%, it was truncated at 100%.
For the main analyses, MPR was evaluated as a dichotomous variable, using a threshold of MPR < 80% to identify noncompliant women. This threshold was selected since it has been used in several other studies evaluating compliance with osteoporosis therapies [8, 10, 12]. Due to the arbitrary nature of this threshold, a sensitivity analysis was also conducted, using different ranges of MPR in order to evaluate the effect of decreasing levels of compliance.
Potential confounders
Several potential confounders which can be measured using the RAMQ databases were considered. These included: type of bisphosphonate received at cohort entry (alendronate vs. risedronate); noncompliance with calcium and vitamin D supplements (defined as a MPR < 80% during follow-up); low income [24] (receiving maximum guaranteed income supplement from the Canadian government); a procedure code for a bone mineral density testing (BMD) [25]; a diagnosis of osteoporosis [25] (ICD-9 code 733.0); a prior osteoporotic fracture [14]; risk factor for falls [26], such as a history of prior accidental fall (ICD-9 codes E880, E884, E885, E886, E888), Parkinson's disease (ICD-9 code 332.0 or drug markers), orthostatic hypotension (ICD-9 code 458.0 or drug markers), epilepsy (ICD-9 code 345), blindness (ICD-9 code 369) or specific neurological or gait abnormalities (ICD-9 codes 340, 342, 344, 358, 359, 781.2, 781.3); dementia [27] (ICD-9 code 290, 294, 331, 334–335 or donepezil, rivastigmine, galantamine or memantine); rheumatoid arthritis [14] (ICD-9 code 714.0 or drug markers); hypertension [28][ICD-9 codes 401–404 or use of calcium channel blockers or β-blockers without markers of coronary artery disease, thiazides diuretics or angiotensin converting enzyme inhibitors (ACEi) without furosemide]; hyperlipidaemia [29] (ICD-9 code 272 or drug markers); diabetes mellitus [30] (ICD-9 code 250 or drug markers); congestive heart failure [28] (ICD-9 code 428 or use of furosemide with digoxin, ACEi, sprironolactone or β-blockers); coronary artery disease [28] (ICD-9 codes 410–414, procedure codes such as coronary artery bypass surgery or angioplasty, nitrates); cerebrovascular disease [28] (ICD-9 codes 430–438, medical procedure or drug markers); peripheral vascular disease [28] (440–447, or medical procedure for noncoronary angioplasty or use of pentoxyfylline). All these variables were assessed in the year prior to cohort entry and during follow-up, except for low income (at cohort entry) and prior osteoporotic fracture (within 2 years prior to cohort entry and during the first year following cohort entry).
As used in our previous published methodology [6], patient overall health status and opportunity for follow-up were assessed through the number of different therapeutic classes (according to the American Hospital Formulary Service classification) dispensed and outpatient medical visits, as well as any hospitalization during the year prior to index date.
Finally, the use of medications known to increase the risk of fracture was assessed, such as oral glucocorticosteroids [14] (≥5 mg of prednisone equivalent per day for at least 3 months during the year prior to index date), chronic use of anticonvulsants (phenytoin, phenobarbital, carbamazepine, or valproic acid) [31], anticoagulants [32] (vitamin K antagonists, standard or low-molecular-weight heparin), or proton pump inhibitors [33] during the year prior to the index date, as well as current use of opiates [34], benzodiazepines [35] or antidepressants [34]. Current use was defined as at least one prescription dispensed within 30 days prior to index date.
Statistical analyses
Characteristics of fracture cases and controls were compared using χ2 test and Student's t-test, respectively, for categorical and continuous variables. Wilcoxon's rank sum test was used to compare medians for continuous variables with non-normal distributions. Conditional logistic regression models were used to determine crude and adjusted rate ratios (RRs) for nonvertebral fracture in association with MPR < 80%. All potential confounders previously listed were entered in the model and subjected to backward elimination [36] using a criterion of P ≤ 0.100 for retention in the final model.
Several sensitivity analyses were conducted to assess the robustness of the results. First, the MPR was re-defined according to three mutually exclusive categories (MPR < 50%, ≥50% to <90%, or ≥90%). A Cochran–Armitage test was also performed to evaluate if there was a trend in nonvertebral fracture risk across these three categories of MPR. Second, another nested case–control analysis was conducted in which only hip fractures were considered. For this analysis, other types of nonvertebral fractures occurring after 1 year of treatment were considered as prior osteoporotic fractures and were assessed as potential confounders in the model. Third, some potentially confounding variables, such as smoking or body mass index, are not recorded in the RAMQ databases, and hence could not be considered in this study. The rule-out approach sensitivity analysis [37, 38] was therefore used to assess the extent necessary for such residual confounding to explain fully the observed association between noncompliance and the risk of nonvertebral osteoporotic fracture.
All reported P-values are two-sided, with a significance level of 0.05, and 95% confidence intervals (CIs). All analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
After applying inclusion and exclusion criteria, the final study cohort consisted of 30 259 women. The mean age at cohort entry was 77.1 ± 6.1 years and the mean duration of follow-up in the cohort was 703 ± 392 days. A total of 1038 incident cases of nonvertebral fractures having occurred at least 1 year after cohort entry were identified. Of these cases, two were excluded from the nested case–control analysis because they could not be matched to any control, leading to a total of 1036 cases and 20 069 controls.
Characteristics of cases and controls
Among cases, the distribution of fracture sites was hip (n = 287, 27.7%), distal forearm (n = 65, 6.3%) and other nonvertebral sites (n = 684, 66.0%). Hip fractures were more frequent among older women; they accounted for 35.8% of nonvertebral fractures in women aged > 80 years, compared with 23.6 % in women aged ≤ 80 years (P < 0.001). As presented in Table 1, among cases, 58.2% of women had started on alendronate, compared with 57.9% of controls (P = 0.268). Switching from one bisphosphonate to the other was uncommon. Among cases, 7.3% of women starting therapy with alendronate switched to risedronate some time during follow-up, whereas 11.8% of women starting therapy with risedronate switched to alendronate. Among controls, these values were 7.2% and 8.3%, respectively. About 70% of cases and controls were dispensed calcium and vitamin D supplements at the time of cohort entry. We found that MPR with calcium and vitamin D supplements was low, for both cases and controls: 60.1% of cases and 59.9% of controls had a MPR < 80% (P = 0.859). Interestingly, among women having had a MPR < 80% with alendronate and risedronate, 81.6% of them also had a MPR < 80% with calcium and vitamin D supplements, whereas 44.8% of women having shown a MPR ≥ 80% with alendronate had a MPR < 80% with calcium and vitamin D supplements (P < 0.001).
Table 1.
Characteristics of cases and controls (n = 21 105)
| Cases (n = 1036) | Controls (n = 20 069) | P | |
|---|---|---|---|
| Age (years) at cohort entry, mean ± SD | 78.1 ± 6.1 | 78.0 ± 6.0 | 0.280 |
| Days of follow-up (from cohort entry to index date) | |||
| Mean ± SD | 688.4 ± 251.8 | 684.4 ± 248.2 | 0.610 |
| Median (IR) | 636 (479–854) | 629 (478–850) | |
| Bisphosphonate prescribed at cohort entry | 0.268 | ||
| Alendronate 10 mg once daily | 12.2 | 11.2 | |
| Alendronate 70 mg once weekly | 46.0 | 46.7 | |
| Risedronate 5 mg once daily | 17.7 | 16.0 | |
| Risedronate 35 mg once weekly | 24.2 | 26.1 | |
| Supplements prescribed at cohort entry (within 1 month before or after cohort entry) | |||
| Calcium supplements | 70.5 | 68.3 | 0.151 |
| Vitamin D supplements | 69.0 | 69.0 | 0.988 |
| Low income at cohort entry | 9.3 | 8.7 | 0.529 |
| BMD test* | 60.4 | 68.2 | <0.001 |
| Diagnostic code for osteoporosis* | 58.1 | 58.3 | 0.895 |
| Prior osteoporotic fracture† | 28.7 | 14.9 | <0.001 |
| Health condition* | |||
| Risk factor for falls | 8.8 | 5.9 | <0.001 |
| Dementia | 13.8 | 7.5 | <0.001 |
| Rheumatoid arthritis | 6.0 | 5.5 | 0.470 |
| Hypertension | 69.2 | 69.9 | 0.636 |
| Hyperlipidaemia | 36.0 | 40.4 | 0.005 |
| Diabetes mellitus | 16.8 | 15.3 | 0.184 |
| Congestive heart failure | 13.6 | 11.4 | 0.030 |
| Coronary artery disease | 33.1 | 28.9 | 0.004 |
| Cerebrovascular disease | 9.4 | 7.3 | 0.011 |
| Peripheral vascular disease | 8.7 | 5.8 | <0.001 |
| Number of different therapeutic classes‡, median (IR) | 8.0 (5.0–11.0) | 8.0 (5.0–10.0) | <0.001†† |
| Number of outpatient medical visits‡, median (IR) | 8.0 (5.0–13.0) | 7.0 (4.0–11.0) | 0.028†† |
| Hospitalization‡ (at least once) | 28.3 | 19.4 | <0.001 |
| Medication use | |||
| Chronic (during the year prior to index date) | |||
| Oral glucocorticosteroids§ | 3.8 | 3.7 | 0.950 |
| Anticonvulsants | 2.0 | 1.1 | 0.005 |
| Anticoagulants | 4.8 | 3.6 | 0.041 |
| Proton pump inhibitors | 18.2 | 14.6 | 0.002 |
| Current¶ | |||
| Opiates | 8.4 | 2.7 | <0.001 |
| Benzodiazepines | 27.9 | 25.6 | 0.094 |
| Antidepressants | 18.0 | 11.2 | <0.001 |
In the year before cohort entry or during follow-up.
Within 2 years prior to cohort entry and during the first year of treatment (as well as during follow-up for vertebral fractures).
During the year prior to the index date.
A daily dose ≥5 mg of prednisone equivalent during at least 3 months.
At least one prescription dispensed within 30 days prior to index date.
Wilcoxon rank sum test. Low income = receiving maximum guaranteed income supplement from the Canadian government. Anticonvulsants = phenytoin, phenobarbital, carbamazepine, valproic acid. Anticoagulants = vitamin K antagonists, standard or low-molecular-weight heparin. Risk factor for falls = history of prior accidental fall (ICD-9 codes E880, E884, E885, E886, E888), Parkinson's disease (ICD-9 code 332.0 or drug markers), orthostatic hypotension (ICD-9 code 458.0 or drug markers), epilepsy (ICD-9 code 345 or drug markers), blindness (ICD-9 code 369) or specific neurological or gait abnormalities (ICD-9 codes 340, 342, 344, 358, 359, 781.2, 781.3). SD, standard deviation; IR, interquartile range. Values are percentages, unless stated otherwise.
As shown in Table 1, cases were frailer and presented with more risk factors for fracture than controls. Compared with controls, they were almost twice as likely to have sustained a prior osteoporotic fracture. Cases were also almost twice more likely than controls to suffer from dementia and presented more frequently with other comorbidities such as risk factors for falls, or cardiovascular disease. Finally, compared with controls, cases were more likely to have had exposure to medications known to increase fracture risk during the year prior to index date, except for oral glucocorticosteroids and benzodiazepines.
Association between noncompliance with alendronate and risedronate and risk of nonvertebral fracture
More cases had a MPR < 80% during follow-up compared with controls (respectively, 45.7% and 40.7%, P = 0.002). The association between noncompliance and risk of nonvertebral fracture remained statistically significant after adjusting for potential confounders (RR 1.27, 95% CI 1.12, 1.44) (Table 2). When the analysis was restricted to women aged > 80 years (n = 6891, 349 cases, 6542 controls), the association between noncompliance and risk of nonvertebral fracture was even greater (RR 1.48, 95% CI 1.19, 1.85).
Table 2.
Crude and adjusted rate ratios of nonvertebral osteoporotic fracture in association with MPR < 80% (n = 21 105)
| Crude RR (95% CI) | Adjusted* RR (95% CI) | |
|---|---|---|
| MPR ≥ 80% (n = 12 455) | Reference | Reference |
| MPR < 80% (n = 8 650) | 1.22 (1.08, 1.39) | 1.27 (1.12, 1.44) |
| BMD test† | 0.71 (0.62, 0.82) | 0.80 (0.68, 0.93) |
| Diagnostic code for osteoporosis† | 1.01 (0.89, 1.15) | 1.24 (1.07, 1.44) |
| Prior osteoporotic fracture‡ | 2.31 (2.00, 2.66) | 2.09 (1.80, 2.42) |
| Health condition | ||
| Dementia† | 1.97 (1.64, 2.39) | 1.57 (1.28, 1.91) |
| Hyperlipidaemia† | 0.83 (0.73, 0.95) | 0.85 (0.74, 0.97) |
| Coronary artery disease† | 1.22 (1.07, 1.39) | 1.13 (0.98, 1.31) |
| Peripheral vascular disease† | 1.58 (1.26, 1.98) | 1.42 (1.12, 1.79) |
| Hospitalization§ (at least once) | 1.62 (1.41, 1.86) | 1.36 (1.17, 1.58) |
| Medication use (in the year before the index date) | ||
| Anticonvulsants | 1.72 (1.09, 2.73) | 1.67 (1.04, 2.67) |
| Opiates¶ | 3.30 (2.60, 4.19) | 2.77 (2.17, 3.53) |
| Antidepressants¶ | 1.73 (1.47, 2.04) | 1.44 (1.21, 1.71) |
Adjusted for variables in Table 1 that were selected by backward procedure. (The variable MPR < 80% was forced in the model a priori.)
In the year before cohort entry or during follow-up.
Within 2 years prior to cohort entry and during the first year of treatment (as well as during follow-up for vertebral fractures).
In the year before the index date.
At least one prescription dispensed within 30 days prior to index date. CI, confidence interval; MPR, medication possession ratio; RR, rate ratio.
The association between MPR and fracture risk varied according to the number of days of follow-up. Among women with the longest follow-up (>850 days), MPR < 80% was associated with a 48% increased risk of nonvertebral fracture (RR 1.48, 95% CI 1.15, 1.91). Among women with follow-up ≤ 850 days, the association between MPR and nonvertebral fracture risk was lower (RR 1.21, 95% CI 1.04, 1.40).
Risk factors for fracture
As indicated in Table 2, a prior osteoporotic fracture (RR 2.09, 95% CI 1.80, 2.42), dementia (RR 1.57, 95% CI 1.28, 1.91) and long-term use of anticonvulsants (RR 1.67, 95% CI 1.04, 2.67) or opiates (RR 2.77, 95% CI 2.17, 3.53) were important risk factors for nonvertebral fracture. Having a diagnostic code for osteoporosis, having peripheral vascular disease, having been hospitalized, or having used antidepressants were also associated with a 24–44% increased risk of nonvertebral fracture. Finally, women having had a BMD test in the year prior to cohort entry or during follow-up had a 20% lower risk to sustain a fracture, whereas women suffering from hyperlipidaemia had a 15% lower risk.
Sensitivity analyses
When the MPR was redefined according to three categories (MPR < 50%, ≥50% to <90%, or ≥90%), Cochran–Armitage test showed that there was a trend indicating a greater risk of fracture with decreasing compliance (P for trend = 0.001). As presented in Figure 1, in the adjusted model, compared with women with a MPR ≥ 90%, women with a MPR < 50% had a 29% greater risk of fracture (adjusted RR 1.29, 95% CI 1.11, 1.50).
Figure 1.
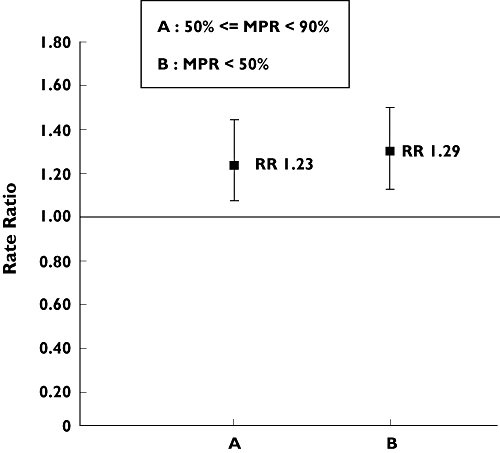
Association between noncompliance and nonvertebral fracture for different ranges of MPR (n = 21 105). RR, rate ratio; MPR, medication possession ratio; reference was MPR ≥ 90% (RR = 1.00). For 50% ≤ MPR < 90%: adjusted RR 1.23 [95% confidence interval (CI) 1.05, 1.44]; for MPR < 50%: adjusted RR 1.29 (95% CI 1.11, 1.50). Adjusted for variables listed in Table 1 that were selected by backward procedure (the variable MPR < 80% was forced in the model a priori): bone mineral density (BMD) test, diagnostic code for osteoporosis, prior osteoporotic fracture, dementia, hyperlipidaemia, coronary artery disease, peripheral vascular disease, prior hospitalization, use of anticonvulsants, use of opiates, use of antidepressants
In another nested case–control analysis, in which only hip fractures were considered as the outcome (n = 6702; 329 cases, 6373 controls), a similar association was found between noncompliance and fracture risk (adjusted RR 1.28, 95% CI 1.02, 1.61) (Table 3). Statistically significant risk factors for fracture were also similar to those observed in the first nested case–control analysis.
Table 3.
Crude and adjusted rate ratios of hip fracture in association with MPR < 80% (n = 6702)
| Crude RR (95% CI) | Adjusted* RR (95% CI) | |
|---|---|---|
| MPR ≥ 80% (n = 3954) | Reference | Reference |
| MPR < 80% (n = 2748) | 1.27 (1.02, 1.59) | 1.28 (1.02, 1.61) |
| BMD test† | 0.53 (0.42, 0.67) | 0.62 (0.48, 0.82) |
| Diagnostic code for osteoporosis† | 0.93 (0.74, 1.17) | 1.27 (0.98, 1.65) |
| Prior osteoporotic fracture‡ | 2.73 (2.16, 3.46) | 2.28 (1.78, 2.91) |
| Health condition | ||
| Dementia† | 3.11 (2.32, 4.18) | 2.14 (1.57, 2.92) |
| Hyperlipidaemia† | 0.59 (0.45, 0.76) | 0.62 (0.48, 0.81) |
| Hospitalization§ (at least once) | 2.48 (1.97, 3.13) | 2.11 (1.66, 2.69) |
| Medication use in the year before the index date | ||
| Opiates¶ | 3.05 (1.97, 4.71) | 2.44 (1.56, 3.82) |
| Antidepressants¶ | 2.07 (1.57, 2.72) | 1.60 (1.20, 2.13) |
Adjusted for variables in Table 1 that were selected by the backward procedure. (The variable MPR < 80% was forced in the model a priori.)
In the year before cohort entry or during follow-up.
Within 2 years prior to cohort entry and during the first year of treatment (as well as during follow-up for vertebral fractures).
In the year before the index date.
At least one prescription dispensed within 30 days prior to index date. CI, confidence interval; MPR, medication possession ratio; RR, rate ratio.
Finally, results of the rule-out approach sensitivity analysis [37, 38] are presented in Figure 2. The area to the right of the curve (zone B) shows combinations of associations between confounder-fracture and confounder-noncompliance that would induce confounding by an unmeasured variable strong enough to have elevated the association between noncompliance and fracture risk from the null value (RR = 1) to the observed point estimate (RR = 1.27). The area to the left of the curve (zone A) represents combinations that would not be strong enough to have biased the observed RR. The prevalence of the confounder in the cohort was assumed to be 20% because it minimized the magnitude of the association needed to invalidate the results. The prevalence of women with a MPR < 80% was set at 41%, as observed among the controls. As shown on the graph, if the association between the unmeasured confounder and the risk of nonvertebral osteoporotic fracture was 3.00, the association between the confounder and noncompliance would have to be of ≥ 2.87 to move the observed association (RR = 1.27) to the null value (RR = 1).
Figure 2.
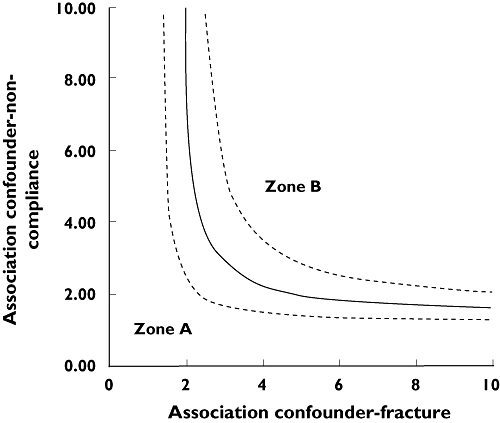
Rule-out approach sensitivity analysis to assess the strength of residual confounding necessary to fully explain the observed association between noncompliance and nonvertebral fractures. The area at the right (zone B) of the curve shows combinations of associations between confounder-fracture and confounder-noncompliance that would induce confounding by an unmeasured variable strong enough to have elevated the association between noncompliance and fracture risk (95% Cl 1.12–1.44, - - -) from the null value (RR = 1) to the observed point estimate (RR = 1.27, ——). The area at the left of the curve (zone A) represents combinations that would not be strong enough to bias the observed RR. The prevalence of the confounder was assumed to be 20% and the prevalence of women with a medication possession ratio (MPR) < 80% was 41%.
Discussion
Within a population of community-dwelling elderly women, noncompliance with alendronate or risedronate was associated with a greater risk of nonvertebral osteoporotic fracture. In the subgroup analysis in which only hip fractures were considered as the outcome, a similar association was found.
This study was the first to assess the impact of noncompliance with bisphosphonates in a subgroup of women aged >80 years. Among women aged >80 years, MPR < 80% was associated with a 48% greater risk of sustaining a nonvertebral fracture, compared with women with a MPR ≥ 80%. These results emphasize the benefits associated with compliance to bisphosphonates in this population at high risk of fracture.
We chose to use a 80% compliance threshold because it had been frequently used in other studies evaluating compliance with osteoporosis medications. When compliance was redefined using different thresholds, consistent with prior research [11–13], there was a trend for a greater risk of fracture with decreasing compliance levels.
These findings are similar to those of other studies having evaluated the association between MPR and osteoporotic fractures [11–13]. Within a cohort of 35 537 women exclusively exposed to alendronate or risedronate (mean age 65.3 years), Siris et al.[12] found that women with a MPR ≥ 80% of the time over a 24-month period had a 20.1% lower risk of nonvertebral fracture, compared with women with a MPR < 80% (P < 0.001). Nevertheless, the authors do not specify if they allowed a minimum time-window for the onset of bisphosphonate effectiveness. In a cohort of 8822 new female users of alendronate or risedronate (mean age 69.4 years), Penning-van Beest et al.[13] reported that, excluding fractures occurring in the first year of follow-up, women with a MPR < 80% had a 50% (adjusted hazard ratio 1.50, 95% CI 1.06, 2.13) increased risk of fracture (vertebral and nonvertebral). It is important to note that in this study, only fractures resulting in hospitalization were considered.
In another study in which about 70% of women were receiving either alendronate or risedronate (mean age was 68.5 years among cases and 67.4 years among controls), Weycker et al.[11] found that women with a MPR ≥ 90% had a 30% lower risk of fracture (vertebral and nonvertebral) compared with women with a MPR < 30% [adjusted odds ratio (OR) 0.70, 95% CI 0.52, 0.93]. The authors allowed only a 90-day period for the onset of therapy effectiveness.
The MPR was used to measure compliance because we believed that this method is more appropriate to capture the cyclical patterns of compliance with osteoporosis medications than measuring compliance as a function of a permissible gap between refills. Indeed, in a large cohort study involving 26 636 new users of an osteoporosis medication (alendronate, calcitonin, oestrogen, raloxifene, or risedronate), Brookhart et al.[39] found that among patients who stopped therapy for at least 60 days, an estimated 30% restarted treatment within 6 months, and 50% restarted within 2 years. However, MPR has been criticized for not being particularly sensitive to the cumulative effects of drug therapy [11]. In order to account for this limit, a subgroup analysis was done in which only women with the longest follow-up were included. Among women followed up for at least 850 days, MPR < 80% was associated with a 48% increased risk of nonvertebral fracture. These results highlight the benefits of long-term exposure to bisphosphonates.
Predictors of noncompliance with alendronate and risedronate are multifactorial and remain inadequately explored. In a cross-sectional survey among 533 women receiving a bisphosphonate, Carr et al.[40] have reported that dissatisfaction with therapy, defined by side-effects or practical problems taking the medication due to too frequent dosing, or difficulty following special instructions for taking bisphosphonates, was associated with a significantly lower risk of compliance (OR 0.65, 95% CI 0.44, 0.97). In another survey in which 1015 women were contacted, McHorney et al.[41] found that women having presented most side-effects (OR 6.78, 95% CI 4.67, 9.86), those with the most sceptical beliefs in drug effectiveness (OR 5.70, 95% CI 3.65, 8.92) or drug safety (OR 2.26, 95% CI 1.49, 3.42) had significantly greater risk of being noncompliant.
This study has some limitations. Considering that ICD-9 and medical procedure codes used to identify some types of fractures had low sensitivity (e.g. ribs 25.5%) [17], the number of nonvertebral fracture cases was probably underestimated. However, the sensitivity analysis in which only hip fractures were considered (sensitivity of 97.2%) yielded similar results. This study included only women having filled at least one prescription of alendronate or risedronate; we had no data on women having received a prescription for these agents and who never filled it. In addition, the assessment of noncompliance was based on supplies of medication, which is an indirect measure of medication-taking behaviour, compared with patient self-reported measures or electronic monitoring devices. However, administrative databases offer many advantages over patient self-reported measures, mainly because they avoid the problem of reporting bias [42]. Moreover, even though electronic monitoring devices offer the advantage of indicating the exact moment when the medication was taken, these devices are expensive and it would therefore be difficult to recruit a sample size as large as what we obtained using administrative databases. Finally, the RAMQ databases did not contain information on potential confounders such as family history of osteoporotic fracture, baseline BMD, body mass index or lifestyle habits. This may have led to residual confounding. However, results from a survey carried out in 9851 postmenopausal women have shown that well-documented risk factors for osteoporotic fractures, such as low BMD (T score < −2.5), early menopause, or family history of osteoporosis were associated with higher compliance with osteoporosis medications [25]. This means that not controlling for those variables would have underestimated the association between noncompliance and fractures, since compliant women may have presented with a higher risk of fracture at baseline compared with noncompliant women. Nevertheless, some less documented lifestyle risk factors for fracture such as smoking, or low level of activity have also been associated with a lower medication compliance [43, 44]. The rule-out approach sensitivity analysis that was conducted showed that, to explain fully the observed association between noncompliance and risk of nonvertebral fracture, the magnitude of the association between these potential confounders and osteoporotic fracture would need to be ≤3.00, whereas the association between these confounders and noncompliance would have be ≤2.87. To the best of our knowledge, associations of such magnitude have not been reported in the literature between lifestyle habits and noncompliance [43–45]. Therefore, we believe that it is unlikely that the association found is solely due to residual confounding.
In summary, among community-dwelling elderly women, noncompliance with alendronate and risedronate was associated with an increased risk of nonvertebral fracture. These findings highlight the need to implement strategies to increase compliance. Bisphosphonates with less frequent dosing have recently been developed, such as once-monthly oral ibandronate [46] and risedronate [47, 48], intravenous ibandronate given every 3 months [49], as well as once-yearly intravenous zoledronic acid [50], which might help to improve patients’ satisfaction with therapy. In addition, it is of great importance that healthcare professionals provide education to women about osteoporosis and the consequences of noncompliance, in order to clarify misconceptions. Frequent monitoring and feedback by healthcare providers may also be helpful in improving compliance.
Competing interests
Y.M. has received a fee for speaking from Merck Frosst. L-G.S-M. has received research grants, honoraria for lectures and/or is a board member of Alliance for better bone health, Proctor & Gamble Pharmaceuticals and Sanofi Aventis Canada Inc., Eli Lilly Inc., Hoffmann-Laroche Limited, Merck Frosst, and Novartis Pharma Inc.
This study was funded by a grant from the Canadian Institutes of Health Research (Ottawa, Ontario, Canada). J.B. is the recipient of a graduate scholarship in pharmaceutical sciences offered jointly by the Rx&D Health Research Foundation and the Faculty of Pharmacy of the University of Montreal. S.P is a research scholar receiving financial support from the Fonds de la recherche en santé du Québec.
REFERENCES
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C. Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570–8. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 3.Harrington JT, Ste-Marie LG, Brandi ML, Civitelli R, Fardellone P, Grauer A, Barton I, Boonen S. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–35. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 4.Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–8. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 5.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–31. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 6.Blouin J, Dragomir A, Ste-Marie LG, Fernandes JC, Perreault S. Discontinuation of antiresorptive therapies: a comparison between 1998–2001 and 2002–2004 among osteoporotic women. J Clin Endocrinol Metab. 2007;92:887–94. doi: 10.1210/jc.2006-1856. [DOI] [PubMed] [Google Scholar]
- 7.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–8. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- 9.McCombs JS, Thiebaud P, Laughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271–87. doi: 10.1016/j.maturitas.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–8. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271–7. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 12.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–22. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 13.Penning-van Beest FJ, Erkens JA, Olson M, Herings RM. Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int. 2008;19:511–17. doi: 10.1007/s00198-007-0466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16:581–9. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 15.Garbe E, Suissa S. Pharmacoepidemiology. In: Pigeot I, editor. Handbook of Epidemiology. Berlin: Springer-Verlag; 2005. pp. 1225–66. [Google Scholar]
- 16.Rapport annuel de gestion 2003–2004. Quebec: Government of Quebec; 2004. Régie de l’assurance maladie du Québec; pp. 113–9. [Google Scholar]
- 17.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–94. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 18.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Silverman SL, Gold DT. Methodological considerations in using claims databases to evaluate persistence with bisphosphonates for osteoporosis. Curr Med Res Opin. 2007;23:2369–77. doi: 10.1185/030079907X226311. [DOI] [PubMed] [Google Scholar]
- 20.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ III. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–7. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 21.Lubin JH, Gail MH. Biased selection of controls for case–control analyses of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 22.Pang D. A relative power table for nested matched case–control studies. Occup Environ Med. 1999;56:67–9. doi: 10.1136/oem.56.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 24.Zingmond DS, SooHoo NF, Silverman SL. The role of socioeconomic status on hip fracture. Osteoporos Int. 2006;17:1562–8. doi: 10.1007/s00198-006-0161-7. [DOI] [PubMed] [Google Scholar]
- 25.Rossini M, Bianchi G, Di MO, Giannini S, Minisola S, Sinigaglia L, Adami S. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int. 2006;17:914–21. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 26.Grisso JA, Kelsey JL, Strom BL, Chiu GY, Maislin G, O’Brien LA, Hoffman S, Kaplan F. Risk factors for falls as a cause of hip fracture in women. The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326–31. doi: 10.1056/NEJM199105093241905. [DOI] [PubMed] [Google Scholar]
- 27.Walter LC, Lui LY, Eng C, Covinsky KE. Risk of hip fracture in disabled community-living older adults. J Am Geriatr Soc. 2003;51:50–5. doi: 10.1034/j.1601-5215.2002.51009.x. [DOI] [PubMed] [Google Scholar]
- 28.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaelsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–62. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 29.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, van der Klift M, Pols HA. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164:146–52. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–8. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 31.Souverein PC, Webb DJ, Weil JG, van Staa TP, Egberts AC. Use of antiepileptic drugs and risk of fractures: case–control study among patients with epilepsy. Neurology. 2006;66:1318–24. doi: 10.1212/01.wnl.0000210503.89488.88. [DOI] [PubMed] [Google Scholar]
- 32.Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF. Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2. Arch Intern Med. 2006;166:241–6. doi: 10.1001/archinte.166.2.241. [DOI] [PubMed] [Google Scholar]
- 33.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–53. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 34.Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, Hanlon JT, Nevitt MC, Whooley MA. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163:949–57. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 35.Wagner AK, Zhang F, Soumerai SB, Walker AM, Gurwitz JH, Glynn RJ, Ross-Degnan D. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–72. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 38.Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, Pahor M, Furberg CD. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–54. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 39.Brookhart MA, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Patrick AR, Mogun H, Solmon DH. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120:251–6. doi: 10.1016/j.amjmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Carr AJ, Thompson PW, Cooper C. Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int. 2006;17:1638–44. doi: 10.1007/s00198-006-0166-2. [DOI] [PubMed] [Google Scholar]
- 41.McHorney CA, Schousboe JT, Cline RR, Weiss TW. The impact of osteoporosis medication beliefs and side-effect experiences on non-adherence to oral bisphosphonates. Curr Med Res Opin. 2007;23:3137–52. doi: 10.1185/030079907X242890. [DOI] [PubMed] [Google Scholar]
- 42.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 43.Kim YS, Sunwoo S, Lee HR, Lee KM, Park YW, Shin HC, Kim CH, Kim DH, Kim BS, Cha HS, Huh BY. Determinants of non-compliance with lipid-lowering therapy in hyperlipidemic patients. Pharmacoepidemiol Drug Saf. 2002;11:593–600. doi: 10.1002/pds.730. [DOI] [PubMed] [Google Scholar]
- 44.Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Pressman AR, Ettinger B. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115:209–16. doi: 10.1016/s0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- 45.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 46.Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65:654–61. doi: 10.1136/ard.2005.044958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delmas PD, Benhamou CL, Man Z, Tlustochowicz W, Matzkin E, Eusebio R, Zanchetta J, Olszynski WP, Recker RR, McClung MR. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. [2008 February 18];Osteoporos Int. 2007 doi: 10.1007/s00198-007-0531-9. [online]. Available at http://www.springerlink.com/content/102828. [DOI] [PubMed] [Google Scholar]
- 48.Delmas PD, McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, Eusebio RA, Beary JF, Burgio DE, Matzkin E, Boonen S. Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Bone. 2008;42:36–42. doi: 10.1016/j.bone.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Delmas PD, Adami S, Strugala C, Stakkestad JA, Reginster JY, Felsenberg D, Christiansen C, Civitelli R, Drezner MK, Recker RR, Bolognese M, Hughes C, Masanauskaite D, Ward P, Sambrook P, Reid DM. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum. 2006;54:1838–46. doi: 10.1002/art.21918. [DOI] [PubMed] [Google Scholar]
- 50.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]


