Abstract
AIMS
A number of factors have been hypothesized to increase the risk of amiodarone-induced pulmonary toxicity (AIPT). This study aimed to confirm these risk factors and determine whether a cohort of tertiary hospital patients diagnosed with AIPT demonstrated comparable characteristics.
METHODS
Phase I of this study involved compilation of a database of adverse reactions to amiodarone reported to the Australian and US drug agencies, and identification of risk factors for AIPT using logistic regression analysis. In Phase II, AIPT cases were identified via a retrospective review of medical records of patients discharged from Fremantle Hospital and Health Service, Western Australia (FHHS) between 2000 and 2005 with diagnosed interstitial lung disease. Data were collected regarding these patients’ risk factors for AIPT and compared with those previously identified in Phase I.
RESULTS
A total of 237 cases of AIPT were identified from agency data. Patients aged > 60 years and those on amiodarone for 6–12 months (odds ratio 18.28, 95% confidence interval 6.42, 52.04) were determined to be at the highest risk of AIPT. Australian data also suggested increased risk in patients who had received cumulative doses of 101–150 g. The seven AIPT cases identified among the FHHS patients were all at high risk of AIPT based on their age and duration of amiodarone therapy.
CONCLUSION
Contrary to previous findings, only patient age and the duration of amiodarone therapy were confirmed as significant risk factors for AIPT. Targeted monitoring of these patients may facilitate early identification and management of AIPT.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
A number of factors have been hypothesized to increase the risk of amiodarone-induced pulmonary toxicity (AIPT), although there remains some controversy in the literature as to their relative significance.
This study aimed to clarify this situation to permit better characterization of patients at risk of AIPT and thus guide the development of guidelines for the monitoring for AIPT in patients receiving the drug.
WHAT THIS STUDY ADDS
Via compilation and analysis of a database of 237 AIPT cases, it was demonstrated that only patient age and duration of therapy significantly affected the risk of AIPT.
A small cohort of hospital AIPT patients demonstrated these ‘at-risk’ characteristics, suggesting that targeted monitoring of patients aged >60 years and those on amiodarone for 6–12 months may enhance the safety of these patients and minimize their risk of morbidity and mortality secondary to AIPT.
Keywords: amiodarone, interstitial lung diseases, risk factors
Introduction
Amiodarone-induced pulmonary toxicity (AIPT), although rare, is a potentially life-threatening adverse reaction [1]. Recent studies have reported that the incidence of AIPT ranges between 5 and 13% [2–5]. Mortality rates due to AIPT have been reported to range from 10 to 23% [2].
Despite its clinical importance, there remain some unanswered questions regarding AIPT. Studies have reported that the daily amiodarone dose, cumulative dose, duration of therapy, patient age and the presence of pre-existing lung disease are associated with an increased risk of AIPT, although these findings are not universally accepted [6–10]. The pathophysiology of, and precise diagnostic criteria for AIPT are still under investigation, its optimal management has yet to be defined, and monitoring guidelines have not been universally implemented [11].
In 2002, the Australian Adverse Drug Reaction Advisory Committee (ADRAC) reported that although amiodarone is indicated only for severe cardiac arrhythmias, its use had doubled between 1989 and 1994 [12]. Furthermore, the number of amiodarone prescriptions dispensed annually in Australia increased almost fourfold from 1994 to 2003 [13], suggesting that amiodarone use is likely to continue to increase in the future. The increasing use of amiodarone, coupled with the potentially fatal outcome of AIPT, results in an increasing need for targeted monitoring of those patients at highest risk of its development to facilitate its early identification and management, to minimize the associated risk of morbidity and mortality. Conclusive identification of the risk factors for AIPT is thus essential.
This study aimed to confirm, using a large database of AIPT cases reported to ADRAC and the United States Center for Drug Evaluation and Research (CDER), whether daily amiodarone dose, cumulative dose, duration of therapy and the age of the patient were risk factors for AIPT; and to reinforce the clinical relevance of these results by determining whether a cohort of tertiary hospital patients diagnosed with AIPT demonstrated the identified risk factors.
Methods
This study consisted of two phases. Phase I involved identification of cases of adverse reactions to amiodarone, particularly AIPT, reported to ADRAC and the CDER, and a comprehensive review and analysis of these reports to identify and explore the risk factors for AIPT. Phase II comprised a retrospective review of the medical records of patients discharged from Fremantle Hospital and Health Service, Western Australia (FHHS) with a diagnosis of interstitial lung disease (ILD) to isolate cases of AIPT. Data were collected regarding these patients’ risk factors for AIPT to validate the findings from Phase I of the study.
Phase I: review of drug agency reports
Details of all case reports of adverse reactions to amiodarone held by ADRAC as of August 2006 were requested by mail. The earliest case report dated from 1983. Data from the CDER were available from their website (http://www.fda.gov/cder/aers/extract.htm). The data were accessed during the period March–July 2006. At that time, data available from the website were quarterly data files from 2004 to 2005. A total of eight quarters were reviewed.
Data were collected regarding the age of the patient at the time of the reaction, duration of therapy, daily dose and the adverse reactions reported. The cumulative dose was estimated by multiplying the recorded daily dose by the duration of therapy.
AIPT was defined as any of the following possible clinical presentations, as described by the reporter of the reaction: ILD, pulmonary fibrosis, pulmonary infiltrates, pleural effusion, alveolitis fibrosis, pneumonitis, bronchiolitis obliterans organizing pneumonia and cryptogenic organizing pneumonia. Patients with the above pulmonary presentations were classified as ‘AIPT patients’ (henceforth referred to as the ‘AIPT group’), whereas those who were not reported as experiencing the above complications but had demonstrated other amiodarone-related toxicities were considered to be ‘non-AIPT patients’ (i.e. the ‘non-AIPT group’).
Data from both ADRAC and the CDER were analysed using independent samples t-testing and univariate binary logistic regression analysis to compare the AIPT and non-AIPT groups and determine whether the previously identified variables (patient age, duration of amiodarone therapy, daily dose and cumulative dose) were unique risk factors for AIPT. Multiple logistic regression analysis was then conducted on the combined data from both agencies in order to identify the significant risk factors for AIPT within this larger population. The non-AIPT group was used as the comparator group as it was methodologically impossible to isolate a control population of Australian and American patients taking amiodarone without developing any form of amiodarone-related toxicity. P-values < 0.05 were considered statistically significant for all analyses.
Phase II: retrospective review of medical records
FHHS is a publicly funded tertiary teaching hospital with approximately 500 beds situated in Fremantle, Western Australia. The medical records of all patients discharged between January 2000 and December 2005 with International Classification of Diseases 9 and 10 (ICD-9 and ICD-10) discharge codes consistent with ILD were requested. The codes selected were 508.8 (respiratory conditions due to other specified external agents), J70.2 (acute drug-induced interstitial lung disorders), J70.3 (chronic drug-induced interstitial lung disorders), J70.4 (drug-induced interstitial lung disorders, unspecified) and J84.1 (idiopathic pulmonary fibrosis) in an attempt to detect the maximum number of patients diagnosed with AIPT, or patients with a history of amiodarone use and a diagnosis of idiopathic pulmonary fibrosis which may have been unrecognized AIPT. A retrospective review of these patients’ medical records was undertaken and data collected regarding the previously identified variables. Descriptive statistics were calculated and compared with the findings from the drug agency data to identify apparent trends.
Ethical approval for the study was granted by the Human Research Ethics Committees of Curtin University of Technology, and the South Metropolitan Area Health Service, Western Australia.
Results
Phase I: review of drug agency reports
Of the 1020 cases of amiodarone-induced toxicity reported to ADRAC from 1983 to August 2006, the most commonly reported adverse reaction was thyroid disorders (225; 22%), followed by skin reactions such as photosensitivity (141; 13.8%). As displayed in Figure 1, pulmonary toxicity was the third most common adverse reaction, with 116 (11.4%) cases. Pulmonary fibrosis (55; 47.4%) was the most frequent manifestation of AIPT reported to ADRAC, with pulmonary infiltrates reported in 31 cases (26.7%) and 13 patients identified with ILD (11.2%). Pleural effusion and alveolitis fibrosis were each reported in seven patients (6%).
Figure 1.
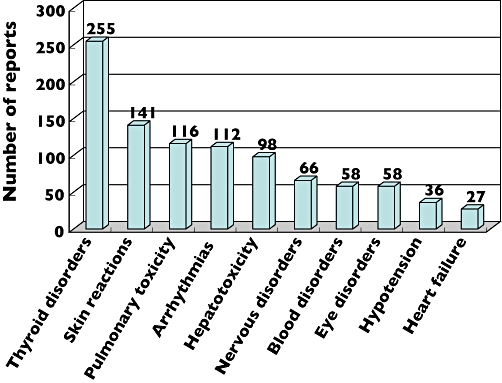
Adverse reactions to amiodarone reported to the Adverse Drug Reaction Advisory Committee from 1983 to August 2006
From January 2004 to December 2005, the CDER received reports of 1196 patients developing adverse reactions related to amiodarone therapy, and of these there were 121 (10.1%) patients with pulmonary toxicity. As seen in Figure 2, the frequency of reported adverse reactions varied from the ADRAC data, with arrhythmias the most commonly reported adverse reaction. Blood disorders and thyroid disorders were second (213; 17.8%) and third (156; 13%), respectively, while AIPT was fourth (121; 10%). Of these 121 AIPT cases, pulmonary toxicity presented most frequently as ILD (38; 31.4%), pulmonary fibrosis (31; 25.6%) and pleural effusion (30; 24.8%).
Figure 2.
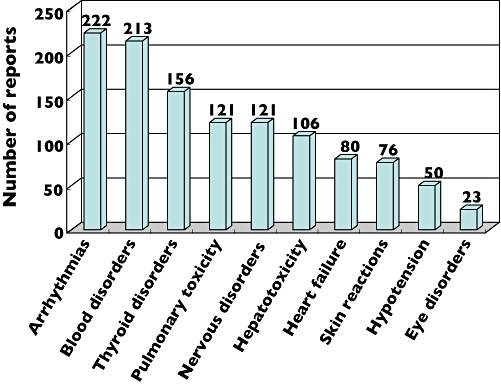
Adverse reactions to amiodarone reported to the Center for Drug Evaluation and Research during 2004 and 2005
Table 1 displays the descriptive statistics and the results of the t-test analyses for the ADRAC and CDER datasets. Among the cases reported to ADRAC, statistically significant differences between the AIPT and non-AIPT groups were demonstrated for all variables. Patients with AIPT were older, had a longer duration of therapy and a lower daily amiodarone dose, but a higher cumulative dose than the non-AIPT group. Analysis of the CDER data revealed significant differences between the AIPT and non-AIPT groups only for patient age and the duration of amiodarone therapy, with AIPT patients being older and having a longer duration of therapy.
Table 1.
Descriptive statistics and t-test analyses of the AIPT and non-AIPT groups in the ADRAC and CDER datasets
| ADRAC data | CDER data | |||||
|---|---|---|---|---|---|---|
| Variable | AIPT [mean (95% CI)] | Non-AIPT [mean (95% CI)] | P | AIPT [mean (95% CI)] | Non-AIPT [mean (95% CI)] | P |
| Age (years) | 73.4 | 69.2 | <0.001* | 73.4 | 70.6 | 0.010* |
| (71.4, 75.5) | (68.3, 70.1) | (71.4, 75.4) | (69.7, 71.4) | |||
| Duration (days) | 498.9 | 204.4 | 0.001* | 654.5 | 326.7 | 0.011* |
| (340.1, 657.6) | (164.2, 244.6) | (415.5, 893.6) | (251.1, 402.2) | |||
| Daily dose (mg) | 302.9 | 357.4 | 0.037* | 445.6 | 451.6 | 0.914 |
| (256.4, 349.4) | (335.9, 378.9) | (340.8, 550.3) | (403.7, 499.5) | |||
| Cumulative dose (g) | 150.3 | 60.1 | 0.008* | 252.3 | 122.9 | 0.093 |
| (87.2, 213.3) | (40.3, 80.0) | (104.1, 400.5) | (86.0, 159.7) | |||
Statistically significant differences between the AIPT and non-AIPT groups (P < 0.05). AIPT, amiodarone-induced pulmonary toxicity; ADRAC, Adverse Drug Reaction Advisory Committee; CDER, Left for Drug Evaluation and Research.
Univariate logistic regression analysis was conducted for each potential risk factor. The ADRAC data confirmed age, duration of therapy and cumulative amiodarone dose as significant risk factors for AIPT, but not daily dose. The risk of AIPT in those aged in their 80s was almost fourfold higher than those aged ≤ 60 years [odds ratio (OR) 3.92, 95% confidence interval (CI) 1.73, 8.89]. AIPT risk increased significantly in those who were on amiodarone for >1 month, with the highest risk for those who were on therapy for 6–12 months. Patients in this group were 33.68 (95% CI 7.53, 150.66) times more likely to develop AIPT than those who were on amiodarone for <2 weeks. Compared with those patients who had received <10 g of amiodarone, AIPT risk gradually increased with increasing cumulative dose and reached a plateau in the 101–150 g and >150 g groups (OR 10.29, 95% CI 3.42, 30.92 and 9.50, 95% CI 3.82, 23.67, respectively).
Univariate analysis of the CDER data confirmed only that a longer duration of amiodarone therapy increased the risk of AIPT; however, the magnitude of the increased risk was lower. In patients receiving >6 months’ therapy, AIPT risk was increased approximately sixfold for the CDER cases vs. 30-fold for the ADRAC cases.
The results of the multiple logistic regression analysis of the combined data from ADRAC and CDER are displayed in Table 2. When the data source (i.e. ADRAC vs. CDER) was included in the model for analysis, it failed to influence the risk of AIPT (P = 0.310), which supported the decision to combine the two data sources. Multiple logistic regression confirmed age (P = 0.035) and duration of amiodarone therapy (P < 0.001), but not daily dose or cumulative dose, as significant risk factors for AIPT. The risk of AIPT was increased almost three times in every age group over the age of 60 years compared with those aged ≤ 60 years. As in the univariate analyses of the individual datasets, duration of amiodarone therapy was a significant risk factor for AIPT after >1 month of amiodarone therapy, with the highest risk once again seen in those who had received amiodarone for 6–12 months.
Table 2.
Multiple logistic regression analysis of combined data
| Variable | Variable groups | Odds ratio (95% CI) | P |
|---|---|---|---|
| Age (years) | ≤60 | 1 | 0.035 |
| 61–70 | 2.66 | 0.008 | |
| (1.29, 5.47) | |||
| 71–80 | 2.58 | 0.011 | |
| (1.25, 5.32) | |||
| ≥81 | 2.75 | 0.035 | |
| (1.07, 7.07) | |||
| Duration (days) | 0–14 | 1 | <0.001 |
| 15–30 | 2.16 | 0.312 | |
| (0.49, 9.55) | |||
| 31–180 | 6.81 | <0.001 | |
| (2.47, 18.74) | |||
| 181–365 | 18.28 | <0.001 | |
| (6.42, 52.04) | |||
| 366–730 | 11.74 | <0.001 | |
| (3.68, 37.42) |
Phase II: retrospective review of medical records
The characteristics of the seven FHHS patients diagnosed with AIPT are displayed in Table 3. These patients were all aged > 60 years, and six of the seven had been on amiodarone for >6 months. They thus met the criteria for being at increased risk of AIPT, as confirmed by multiple logistic regression analysis.
Table 3.
Characteristics of FHHS AIPT patients
| Age (years) | Duration of therapy (days) | Daily dose | Cumulative dose (g) |
|---|---|---|---|
| 67 | 575 | 200 mg | 119.2 |
| 81 | 111 | 200 mg | 26.2 |
| 75 | 182 | 200 mg | 40.6 |
| 90 | 966 | 200 mg | 200.2 |
| 70 | 2443 | 100 mg | 488.6 |
| 82 | 1790 | 200 mg for 6 months, then 100 mg | 203.0 |
| 93 | ‘Long term’ | 200 mg | Unknown |
FHHS, Fremantle Hospital and Health Service; AIPT, amiodarone-induced pulmonary toxicity.
Discussion
In support of previous reports [6], the current study has demonstrated a wide range of adverse reactions associated with amiodarone therapy. Thyroid disorders were the most frequent adverse reaction reported to ADRAC, whereas in the CDER dataset, arrhythmias were the most frequently reported adverse reaction. One possible explanation for the differences in the adverse reactions reported to the two agencies might be the slightly higher doses of amiodarone recommended in the USA as opposed to Australia [14].
Both univariate logistic regression analysis of the ADRAC data and multiple logistic regression analysis of the combined data confirmed patient age as a significant risk factor for AIPT, with older patients at higher risk of AIPT. This is particularly interesting because, although some authors have claimed that the risk of AIPT is higher in elderly patients [15], other studies have demonstrated that increasing age did not increase the risk of AIPT [1, 16], a finding mirrored by the CDER analysis.
Duration of amiodarone therapy was confirmed as a significant risk factor for AIPT from the univariate logistic regression analysis of both the ADRAC and CDER datasets and multiple logistic regression analysis of the combined data. Risk increased after 1 month and was highest in those who were on amiodarone for 6–12 months. This confirmed the findings of Dusman et al., who claimed that duration of amiodarone therapy was a significant risk factor for AIPT, with the highest risk of developing pulmonary toxicity in the first 12 months after starting on the drug [16].
Although the ADRAC data suggested a trend towards increased risk in patients who had received cumulative amiodarone doses of 101–150 g, this was not supported by the CDER or combined data. This issue requires further clarification. One previous study has suggested that a similar cumulative dose (of 140–230 g) is clinically significant in inducing lung toxicity [10], whereas another author has reported that a cumulative dose of amiodarone as low as 10 g may result in pneumonitis [17]. Other studies, however, have argued that AIPT is more common in patients with high cumulative doses of amiodarone [2, 6, 8, 16]. These conflicting findings may result from the two hypothesized mechanisms of AIPT exhibiting differential cumulative dose dependencies [1].
The relevance of age and duration of therapy as risk factors was demonstrated in a small cohort of AIPT patients from a Western Australian tertiary hospital. According to the multiple logistic regression analysis, the FHHS patients were all at increased risk of AIPT based on their age, and six of the seven had been receiving amiodarone for >6 months at the time of diagnosis of pulmonary toxicity.
This study was not without its limitations. As discussed previously, an ideal control group was not available for the drug agency data, a common problem with retrospective studies. Retrospective studies may also suffer from poor data quality, and that may have influenced the results. Data collected from the drug agencies was based on voluntary case reports; consequently, information was often missing, especially regarding the indication for, and duration of, amiodarone therapy. The CDER records were at times difficult to manipulate, requiring configuration of the data to determine the daily amiodarone dose and the duration of therapy. Determination of the manifestations of AIPT may have been complicated by the use of different terms to describe the observed toxicity. While some reports may have used the term ‘ILD’ (the Medical Subject Heading) to describe the toxicity experienced, others may have used more specific or interchangeable terms; thus it must be accepted that certain inconsistencies in these data were inevitable.
If the patients at high risk of AIPT could be identified, monitoring of these patients could be performed in an appropriate, timely and cost-effective manner, facilitating the early identification and management of AIPT and thus minimizing the risk of AIPT-related morbidity and mortality. This requires final clarification of the risk factors for AIPT, and while the large dataset of AIPT cases compiled during this study has made some progress in that direction, the best hope of truly confirming these risk factors lies with a future long-term prospective cohort study with an appropriate control group.
In conclusion, amiodarone possesses a significant adverse reaction profile, including potentially life-threatening pulmonary toxicity. AIPT remains a significant potential cause of morbidity and mortality, a fact confirmed in a small local population of ILD patients. Although a variety of factors have been suggested to increase the risk of AIPT, this study has confirmed only patient age and duration of amiodarone therapy as significant risk factors. These risk factors were also exhibited by a small group of hospital AIPT patients. This study has thus allowed identification of those patients at the highest risk of pulmonary toxicity, and therefore those requiring closest monitoring. Further prospective studies are needed to clarify definitively the risk factors for AIPT and the appropriate frequency and intensity of monitoring of ‘at-risk’ patients.
D.K.E. acknowledges the financial support of AusAid in the completion of her MPharm project, which formed the basis of this manuscript.
REFERENCES
- 1.Jessurun GAJ, Boersma WG, Crijns HJGM. Amiodarone-induced pulmonary toxicity: predisposing factors, clinical symptoms and treatment. Drug Saf. 1998;18:339–44. doi: 10.2165/00002018-199818050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Oyama N, Yokoshiki H, Kamishima T, Nambu T, Tsutsui H, Miyasaka K. Detection of amiodarone-induced pulmonary toxicity in supine and prone positions – high-resolution computed tomography study. Circ J. 2005;69:466–70. doi: 10.1253/circj.69.466. [DOI] [PubMed] [Google Scholar]
- 3.Handschin A, Lardinois D, Schneiter D, Bloch K, Weder W. Acute amiodarone-induced pulmonary toxicity following lung resection. Respiration. 2003;70:310–2. doi: 10.1159/000072016. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Garcia JL, Garcia-Nieto JC, Ballesta F, Prieto E, Villanueva MA, Gallardo J. Pulmonary mass and multiple lung nodules mimicking a lung neoplasm as amiodarone-induced pulmonary toxicity. Eur J Intern Med. 2001;12:372–6. doi: 10.1016/s0953-6205(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein I, Topilsky M, Segev D, Isakov A, Heller I. Very early onset of acute amiodarone pulmonary toxicity presenting with hemoptysis. Chest. 1997;111:1446–7. doi: 10.1378/chest.111.5.1446. [DOI] [PubMed] [Google Scholar]
- 6.Adverse Drug Reactions Advisory Committee. The multiple toxicities of amiodarone. Aust Adv Drug React Bull. 2005;24:11. [Google Scholar]
- 7.Ott MC, Khoor A, Leventhal JP, Paterick TE, Burger CD. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123:646–51. doi: 10.1378/chest.123.2.646. [DOI] [PubMed] [Google Scholar]
- 8.Hughes M, Binning A. Intravenous amiodarone in intensive care. Intensive Care Med. 2000;26:1730–9. doi: 10.1007/s001340000668. [DOI] [PubMed] [Google Scholar]
- 9.Kharabsheh S, Abendroth CS, Kozak M. Fatal pulmonary toxicity occurring within two weeks of initiation of amiodarone. Am J Cardiol. 2002;89:896–8. doi: 10.1016/s0002-9149(02)02213-0. [DOI] [PubMed] [Google Scholar]
- 10.Ashrafian H, Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120:275–82. doi: 10.1378/chest.120.1.275. [DOI] [PubMed] [Google Scholar]
- 11.Stelfox HT, Ahmed SB, Fiskio J, Bates DW. Monitoring amiodarone's toxicities: recommendations, evidence, and clinical practice. Clin Pharmacol Ther. 2004;75:110–22. doi: 10.1016/j.clpt.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Adverse Drug Reactions Advisory Committee. Amiodarone and pulmonary toxicity. Aust Adv Drug React Bull. 2002 [Google Scholar]
- 13.Dudley J, Robinson M, Main P. Canberra: Australian Statistics On Medicines 2003. Drug Utilization Sub-Committee Secretariat, Australian Government Department of Health and Ageing, 2003. [Google Scholar]
- 14.McEvoy GL, Kester L, Litvak K, Miller J, Welsh OH, editors. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2007. Amiodarone hydrochloride; pp. 1623–35. [Google Scholar]
- 15.Wang T, Charette S, Smith MI. An unintended consequence: fatal amiodarone pulmonary toxicity in an older woman. J Am Med Dir Assoc. 2006;7:510–3. doi: 10.1016/j.jamda.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Dusman RE, Stanton MS, Miles WM, Klein LS, Zipes DP, Fineberg NS, Heger JJ. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82:51–9. doi: 10.1161/01.cir.82.1.51. [DOI] [PubMed] [Google Scholar]
- 17.Polkey MI, Wilson POG, Rees PJ. Amiodarone pneumonitis: no safe dose. Respir Med. 1995;89:233–5. doi: 10.1016/0954-6111(95)90254-6. [DOI] [PubMed] [Google Scholar]


