Abstract
AIMS
Budesonide, unlike fluticasone propionate, undergoes fatty acid esterification in the lungs, and there is a need to characterize fully the distribution and fate of the two drugs after inhalation in humans.
METHODS
This open-label, randomized study was performed in adults undergoing whole lung or lobar resection resulting from lung cancer. Patients were given single 1000-μg doses of both budesonide and fluticasone propionate via dry powder inhalers before surgery. Tissue samples from peripheral and central lung, an ex vivo bronchial brush sample and intercostal muscle, together with plasma samples, were taken during surgery and analysed by liquid chromatography plus tandem mass spectrometry.
RESULTS
Lung tissue samples were obtained from 22 patients at surgery, 1–43 h after drug dosing. Budesonide was detectable from earliest sampling in central and peripheral lung tissue up to 10 h (in six of 22 samples), fluticasone propionate up to 22 h after inhalation (in 16 of 22 samples), and budesonide oleate up to 43 h after inhalation (in 21 of 22 samples). Budesonide, but not fluticasone propionate, was detected in intercostal muscle for up to 10 h after inhalation. Bronchial brush samples showed the presence of fluticasone propionate for up to 18 h, suggesting the presence of undissolved drug powder particles in the airway lumen.
CONCLUSION
Sustained retention of esterified budesonide in the lungs supports the prolonged duration of action of budesonide and suitability for once-daily administration.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
In vitro studies with human bronchial epithelial cells have shown that budesonide undergoes rapid, extensive and reversible intracellular esterification, a finding that is believed to contribute to retention and prolonged duration of action.
A case report has suggested retention of budesonide in the lungs of patients undergoing surgical resection.
This study evaluated, for the first time, the esterification (and distribution) of inhaled budesonide and fluticasone propionate in vivo in human lung.
WHAT THIS STUDY ADDS
This study has unequivocally shown that budesonide undergoes esterification in human lungs in vivo.
Budesonide was detectable in central and peripheral lung tissue for 10 h, and budesonide oleate up to 43 h after inhalation.
The sustained retention of budesonide esters in the lungs probably contributes to the prolonged duration of action and once-daily efficacy of budesonide.
Keywords: budesonide, esterification, fluticasone propionate, human, in vivo
Introduction
Corticosteroids are noted for their anti-inflammatory effects in a variety of disease states, but efforts to develop agents that have selective intracellular actions on inflammatory but not on endocrine processes have as yet not been successful. Avoidance or minimization of unwanted endocrine effects with anti-inflammatory corticosteroids has therefore been achieved by targeting affected tissues directly by using local delivery. As a result, topical corticosteroids, delivered by inhalation devices, are now regarded as the most effective form of treatment for asthma and rhinitis [1, 2].
The aim of inhaled administration of corticosteroids in respiratory disease is to achieve high local concentrations of active drug in the lungs while limiting systemic exposure. Thus, inhaled corticosteroids should preferentially combine a high fraction of the dose that reaches the airways with a low swallowed fraction [3]. Additionally, inhaled corticosteroids should achieve high levels of airway binding with long duration of retention in the airway, rapid systemic elimination, and low extrapulmonary tissue retention to minimize systemic load [4].
Budesonide is an inhaled corticosteroid developed for its high ratio of topical to systemic activity [5, 6]. The clinical properties and pharmacokinetic characteristics of this agent have been investigated extensively, and research and clinical experience have demonstrated that budesonide is effective when given once daily in mild asthma [7, 8]. These early observations of prolonged duration of action were followed by tracheal superfusion and inhalation experiments in animals, which showed the retention of budesonide in airway tissue to be markedly longer than might be expected on the basis of the drug's lipophilicity alone [9]. Prolongation of local retention of budesonide fatty acid esters is a promising potential explanation for the sustained efficacy of budesonide after once-daily inhalation, particularly as these observations conflict with what might be expected in light of the relatively short plasma elimination half-life of the drug (around 4.5 h) [10].
Kinetic studies in animals have shown that budesonide undergoes rapid, extensive and reversible intracellular esterification with long-chain fatty acids in airway tissue [9, 11]. After inhalation or intratracheal instillation of radioactive budesonide, approximately 80% of bound radioactivity is found as esters (predominantly oleate) within 20 min [9]. Very high rates of esterification have also been noted in in vitro studies with human bronchial epithelial cells [12]. This is a novel finding with budesonide and contrasts with fluticasone propionate, which does not undergo esterification [11]. Indeed, although fluticasone propionate has a considerably longer plasma elimination half-life than budesonide (12.5 h) [10], it has been suggested to be unsuitable for once-daily administration [13]. Recent animal studies have suggested that esterification may also occur for ciclesonide [14].
The clinical relevance of these findings is underlined by reports of marked retention of budesonide in the lungs of patients undergoing surgical resection – concentrations of budesonide in lung tissue were approximately nine times higher than those in plasma when simultaneously obtained between 1 and 6 h after inhalation [15]. Retention of fluticasone propionate in lung tissue has also been shown [16], however, with predominant deposition in central airways in patients with viscous mucus in airways or poor lung function.
These apparent contrasting data illustrate the need to characterize fully the central and peripheral lung deposition patterns of these two corticosteroids, and to determine the presence and relative proportions of fluticasone propionate, budesonide and its fatty acid conjugates in lung tissue, skeletal muscle, and plasma. The present study was performed to address these issues by examining tissue and plasma samples from patients with lung cancer undergoing surgical resection who had received budesonide and fluticasone propionate via dry powder inhalation devices prior to surgery.
Methods
Patients and study design
This was an open-label, single-dose, randomized study performed in patients scheduled to undergo whole lung or lobar resection for lung cancer. The study was planned to enrol 25 patients to ensure an evaluable cohort of at least 20 patients. Patients were excluded if they had hypersensitivity to corticosteroids, were pregnant (known or likely) or lactating, had evidence of other major illness or organ dysfunction that might affect study outcomes, were taking other steroid medication (including hormonal contraception), had taken part in another study in the preceding 3 months, or had a condition that might affect the disposition of study drugs.
At study entry, all patients underwent physical examination and pulmonary function tests. Routine perfusion and ventilation scans were performed in order to establish that the resected lung tissue was well ventilated and perfused. Lung function was measured in terms of forced expiratory volume in 1 s (FEV1), and vital capacity (VC). Training in the use of dry powder inhalers (Turbuhaler® and Diskus®) was also given. Written and informed consent was obtained from all participants, and the local medical ethics committee approved the study.
Study drug administration
Drug doses were based on the highest dosages recommended in clinical practice in order to facilitate the detection of fluticasone propionate and budesonide and its esters. Tissue samples from two patients who did not receive any study drugs were also taken for use in assay development and to act as bioanalytical controls. Each patient inhaled single 1000-μg doses of both budesonide (five inhalations of 200 μg) and fluticasone propionate (four inhalations of 250 μg) in random order within 5 min of each other from 1 to 43 h before surgery. The inhalation times were planned prior to surgery (1–6, 6–12, 12–18, 18–24 or >24 h before surgery).
Other medication
Concomitant medication was used as necessary for each patient at the investigator's discretion and was recorded. Medication used routinely for surgery included paracetamol, the synthetic opioid sufentanil, epidural bupivacaine, prophylactic antibiotic therapy with cefazolin, the antiemetic ondansetron, and an inhaled short-acting β2-agonist.
Tissue and plasma sample analyses
A small sample of intercostal muscle was taken immediately after the chest was opened, and from unaffected and well-ventilated parts of the resected lung lobe samples of central and peripheral tissue were taken. In the resected material, large airways were gently brushed to obtain superficial bronchial brush samples. Plasma samples were taken immediately before drug inhalation, 20 min thereafter, 24 h after drug inhalation and at the time of ligation of the pulmonary artery. Thus, at this time further blood flow through the parts which were later resected was stopped. Samples were centrifuged at 1300 g for 10 min and immediately frozen at −70°C until analysis. Extracts were analysed by liquid chromatography plus tandem mass spectrometry with atmospheric pressure chemical ionization.
The tissue (approximately 0.8 g) was extracted in Teflon vessels with 3 ml of 99.5% ethanol containing internal standards for budesonide, its fatty esters budesonide palmitate and budesonide oleate, and for fluticasone propionate. The tissue was homogenized together with a steel ball in a MicroDismembrator U at 2000 r.p.m. for 4–8 min. The samples were then extracted in a microwave oven for 30 min at 90°C.
After extraction, the samples were allowed to cool down before mixing on a vortex-mixer and centrifuged for 5 min at 3600 g. The vessels were put in a freezer at −20°C to precipitate overnight. The day after, the samples were once more centrifuged for 15 min at 3600 g, and 1 ml of the supernatant was transferred to injection vials and 900 μl was injected to the liquid chromatograph system, where the analytes were cleaned up and fractions collected. Three hundred microlitres of each fraction was transferred to injection vials for injection on the LC-MS/MS system. The bronchial brush specimen was shaken into 4 ml of culture medium and immediately frozen and stored at −80°C. The samples were then treated and analysed as for the other tissue samples. For the plasma samples, internal standards were added to the samples (1 ml) and the samples were transferred to Isolut MFC C18 solid-phase extraction columns. The samples were washed with water/methanol and eluted with acetonitrile. The extracts were evaporated to dryness and reconstituted with 200 μl methanol prior to injection to the liquid chromatography system.
The timing of fraction collection was verified before and after the run with a radiolabelled solution (3H-budesonide, 3H-BP, 3H-fluticasone propionate) on the LC-FlowOne-system. The extracted sample (900 μl) was injected and 2.5 min fractions were collected around the retention time for budesonide/fluticasone propionate and the budesonide esters, respectively. The two purified fractions were then pooled for further analysis. Quantification was carried out using a Thermo Finnigan TSQ7000 mass spectrometer equipped with Thermo Finnigan Atmospheric Pressure Ionization interface (API II) using APCI negative ion mode. A gradient high-performance liquid chromatography system with ethanol/water/acetic acid as mobile phase was used and analysis was done in selected reaction monitoring (SRM)-mode using argon as the collision gas. The quantification was based on the use of internal standards. The lower limit of quantification (LOQ) in the extract was 1.0 pmol for budesonide and fluticasone propionate, and 0.5 pmol for the budesonide esters. For a typical sample of 0.8 g this corresponds to 1.25 and 0.625 pmol g−1 tissue, respectively. For plasma samples, the LOQ for fluticasone propionate was 0.01 nmol l−1 and that for budesonide was 0.025 nmol l−1. Unfortunately, budesonide esters in plasma could not be assessed accurately at the expected levels.
The accuracy of the analytical techniques used was on average 99%, 97%, 101% and 89% for budesonide, fluticasone propionate, budesonide palmitate and budesonide oleate, respectively. The method typically generates and combines within- and between-day variations, and the total coefficient of variation for the tissue samples was on average 7.6%, 12.6%, 8.3% and 17.8% for budesonide, fluticasone propionate, budesonide palmitate and budesonide oleate, respectively.
Budesonide levels below the LOQ were arbitrarily assigned 1/2 the LOQ to prevent losing the information that corticosteroid levels were low. Mean and SD are shown, including these arbitrary data.
Tolerability
Adverse events were also monitored, with a serious event being defined as one that resulted in death, was life-threatening, required or prolonged hospitalization, resulted in persistent or significant disability or incapacity, or was a congenital anomaly or birth defect. Only serious adverse events or those that resulted in withdrawal from the study were reported.
Results
Of 28 patients randomized, 22 were men and six were women. Their average age was 61.4 years, with a range of 43–79 years. All were White. Two patients were controls (i.e. they did not receive study medication). Lung tissue samples after corticosteroid inhalation were obtained from 22 patients, all of whom provided data for both study drugs. In the other four patients the operation was cancelled (n = 2), postponed for 1 week (n = 1) or prednisone treatment was given preoperatively. Most of the patients were ex-smokers with a significant smoking history (Table 1). Median FEV1 was 2.62 l (range 1.46–5.1 l), equivalent to 89% predicted (range 55–130%).
Table 1.
Demographic and disease data (randomized patients)
| Characteristic | Category | All patients |
|---|---|---|
| Gender | Male | 22 |
| Female | 6 | |
| Age (years) | Mean | 61.4 |
| Range | 43–79 | |
| BMI (kg m−2) | Mean | 26.4 |
| Range | 19–43 | |
| Smoking status | Never | 1 |
| Previously | 17 | |
| Occasional | 1 | |
| Habitual | 9 | |
| Pack-years | Median | 40 |
| Range | 0–80 | |
| FEV1 (l) | Mean | 2.692 |
| Range | 1.46–5.1 | |
| FEV1 (% predicted) | Mean | 89.1 |
| Range | 55–130 |
BMI, body mass index; FEV1, forced expiratory volume in 1 s.
The number of patients sampled at each preplanned inhalation time prior to surgery was 1–6 h (n = 6), 6–12 h (n = 3), 12–18 h (n = 5), 18–24 h (n = 6), and >24 (n = 2).
Tissue and plasma levels
Tissue concentrations of budesonide, budesonide oleate and fluticasone propionate are shown in Figure 1. Budesonide was detectable in central and peripheral lung tissue for about 10 h after inhalation (in tissue from six of the 22 patients), and budesonide oleate was measurable from the time of the earliest samples to the latest at 43 h after inhalation and was detectable in 21 of the 22 patients (not detectable in the patient sampled at 29 h). Budesonide palmitate was found in a single sample of central lung tissue, but not in any peripheral lung tissue samples. Budesonide, the parent compound, was present in intercostal muscle samples for up to 10 h after dosing (seven of the 22 patients), but at lower concentrations than those found in lung tissue (Table 2). Budesonide oleate and fluticasone propionate could not be detected in intercostal muscle samples. Only one bronchial brush sample (taken 2 h after inhalation) was found to contain budesonide and budesonide oleate. Fluticasone propionate was detectable in central and peripheral lung tissue for 22 h (in 16 of the 22 patients), and six bronchial brush samples showed measurable quantities of fluticasone propionate for up to 18 h after drug inhalation. Concentrations of budesonide and fluticasone propionate in plasma declined over time. With three data points per patient, individual elimination data could not be accurately calculated. However, from the mean plasma concentrations, elimination half-lives of budesonide and fluticasone propionate of approximately 3 and 14 h, respectively, were estimated. Plasma concentrations of fluticasone propionate could be followed for approximately 24 h in most patients, and plasma concen trations of budesonide remained above the LOQ for at least 24 h after inhalation.
Figure 1.

Concentrations of (a) budesonide, (b) budesonide oleate and (c) fluticasone propionate in central and peripheral lung tissue, in brush samples and intercostal muscle from the time of inhalation to 22 h afterwards. A sample obtained at 29 h had no detectable corticosteroid levels, a further sample at 43 h contained detectable budesonide-oleate in peripheral lung tissue. Tissue concentrations are shown on logarithmic scales. Dotted line represents limit of quantification. Bronchial, (○); Central, (□); Peripheral, (▵); Muscle, (+)
Table 2.
Tissue concentrations of budesonide, budesonide oleate and fluticasone propionate, obtained 1–43 h after a single inhalation
| Mean tissue concentrations, pmol g−1 | Compound | ||
|---|---|---|---|
| [number of values above LOQ] (range) | Budesonide | Budesonide oleate | Fluticasone propionate |
| Interval 1–6 h (n = 6) | |||
| Brush | 1.31 [1] (BQL-4.73) | 0.84 [1] (BQL-3.48) | 0.82 [1] (BQL-1.78) |
| Central | 17.15 [5] (BQL-61.70) | 17.73 [6] (2.41–58.60) | 87.27 [5] (BQL-459.00) |
| Peripheral | 4.33 [5] (BQL-14.60) | 3.96 [6] (2.02–9.34) | 18.49 [6] (2.07–40.30) |
| Muscle | 3.08 [6] (1.68–6.45) | BQL [0] | BQL [0] |
| Interval 6–12 h (n = 3) | |||
| Brush | BQL [0] | BQL [0] | 173.48 [2] (BQL-506.00) |
| Central | 0.86 [1] (BQL-1.33) | 1.85 [3] (0.64–2.88) | 5.99 [2] (BQL-12.00) |
| Peripheral | 1.21 [1] (BQL-2.37) | 2.41 [3] (0.98–4.74) | 10.47 [3] (3.76–21.20) |
| Muscle | 0.98 [1] (BQL-1.70) | BQL [0] | BQL [0] |
| Interval 12–18 h (n = 5) | |||
| Brush | BQL [0] | BQL [0] | 2.00 [2] (BQL-6.84) |
| Central | BQL [0] | 2.20 [0] (BQL-4.35) | 27.80 [4] (BQL-96.30) |
| Peripheral | BQL [0] | 2.24 [5] (0.68–3.31) | 8.56 [4] (BQL-26.70) |
| Muscle | BQL [0] | BQL [0] | BQL [0] |
| Interval 18–24 h (n = 6) | |||
| Brush | BQL [0] | BQL [0] | BQL [0] |
| Central | BQL [0] | 1.94 [4] (BQL-5.25) | 0.94 [1] (BQL-2.51) |
| Peripheral | BQL [0] | 2.18 [6] (0.98–4.76) | 2.06 [3] (BQL-4.59) |
| Muscle | BQL [0] | BQL [0] | BQL [0] |
| Interval >24 h (n = 2) | |||
| Brush | BQL [0] | BQL [0] | BQL [0] |
| Central | BQL [0] | BQL [0] | BQL [0] |
| Peripheral | BQL [0] | 0.50 [1] (BQL-0.68) | BQL [0] |
| Muscle | BQL [0] | BQL [0] | BQL [0] |
BQL, below the lower limit of quantification.
Analysis of individual plasma profiles showed greater interindividual variability for fluticasone propionate than for budesonide (Figure 2). Evaluation of fluticasone propionate curves revealed two apparent anomalies: in one patient, the plasma concentration of drug at ligation of the pulmonary artery (22 h after dosing) was 1 nmol l−1, which was much greater than the corresponding value in any other patient; moreover, one further patient had a 24-h plasma concentration of 0.285 nmol l−1 despite having a value below the LOQ for fluticasone propionate at ligation (23.5 h after dosing).
Figure 2.
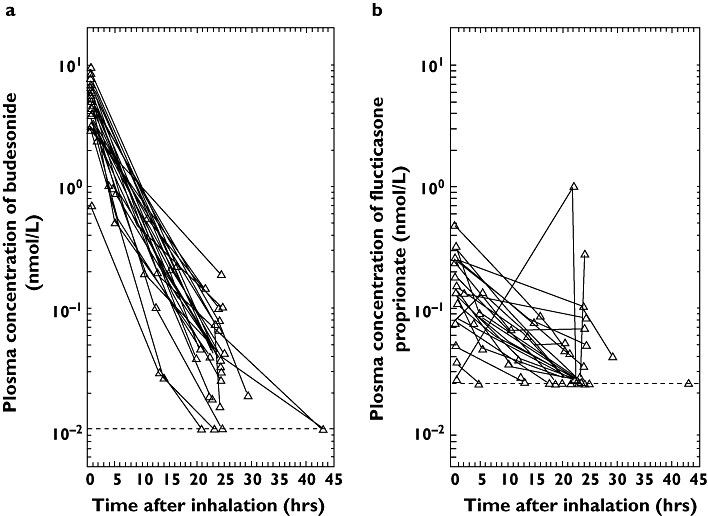
Individual plasma concentration–time curves for (a) budesonide and (b) fluticasone propionate. Dotted line represents limit of quantification
Tolerability
There were no serious adverse events, and none of the 26 patients who received study medication was withdrawn.
Discussion
This is the first study performed in humans prospectively to determine the profiles of budesonide and its fatty acid conjugates in a variety of respiratory tissues and in plasma, and to compare these with distribution and retention data for fluticasone propionate. Because the study was performed in the clinical setting with tissues taken from living patients, the results have ‘real world’ relevance.
The present study builds logically on the findings of Van den Bosch and colleagues [15], who showed in the early 1990s that plasma concentrations of budesonide after inhalation were one-ninth of those in lung tissue when sampled between 1 and 6 h after inhalation. Similar to our study, Van den Bosch and coworkers [15] studied tissue taken from patients undergoing lobar or whole lung resection, but it should be noted that the process of esterification of budesonide had not been described at this time. In addition, the distinction between peripheral and central lung tissue was not made in the earlier study. Notwithstanding these differences, examination of our semilogarithmic plots for budesonide concentrations in lung tissue and comparing this with plasma data suggest differences in concentration in the present patients to be of a similar order over the first 6 h after inhalation to those observed by Van den Bosch and colleagues [15].
Fatty acid esters of budesonide were formed rapidly after inhalation of the drug (detectable in lung tissue from 1 h after administration) and were present in all samples (except one at 29 h) up to the latest, collected at 43 h after inhalation. The rapid formation of esters concurs with the findings of Miller-Larsson and colleagues [9], who showed 70–80% of bound radioactivity to be in an esterified form within 20 min of inhalation or intratracheal administration of radiolabelled budesonide in rats. Inspection of the present data also shows that concentrations of budesonide oleate generally exceeded those of parent budesonide in lung tissue. This observation is consistent with the results of Jendbro and coworkers [17], who showed accumulation of budesonide oleate in rat tracheal tissue, which gave rise to more persistent and higher concentrations of active budesonide than were seen in tissues in which esterification did not take place. Budesonide and budesonide oleate had respective concentrations two and 10–50 times greater in airway tissue than in muscle after intravenous administration in rats [17]. In contrast, a pilot study in humans has suggested similar concentrations of budesonide and budesonide oleate in lung tissue [18]. In that study, tissue samples were collected for other purposes, and it is likely that those results were affected by less stringent sample handling and preparation problems.
This study is also the first to compare concentrations of budesonide and fluticasone propionate in human lung tissue. Fluticasone propionate has a considerably longer overall plasma half-life than budesonide after inhalation, but a review of randomized, double-blind, controlled studies in patients with mild-to-moderate asthma concluded that the duration of action of fluticasone propionate was not sufficient to allow for once-daily administration [13]. This appears to conflict with what might be expected on the basis of the results of the present study, in which measurable concentrations of fluticasone propionate were present in the lungs for 22 h. In addition, Esmailpour and colleagues [16] have shown prolonged retention (for up to 21 h) of fluticasone propionate in the lungs of 17 patients undergoing lung resection. It is possible that the discrepancy between apparently prolonged airway retention and lack of 24-h efficacy for fluticasone propionate may be related to the high lipophilicity of fluticasone propionate, which may cause only relatively small proportions of drug to be dissolved and absorbed into the airway tissue and be available for interaction with intracellular glucocorticoid receptors. Under this hypothesis, the major fraction of drug would remain undissolved and would be eventually cleared by mucociliary action. This requires further investigation, as analytical methods used to date do not distinguish between undissolved drug in the airway lumen and dissolved drug in the epithelial lining fluid in the epithelial lining fluid from where it can get absorbed and gain access to intra-cellular glucocorticoid receptors. However, our data are clearly suggestive for the presence of undissolved fluticasone propionate, but not budesonide, drug powder particles in the airway lumen. The alternative explanation – that fluticasone propionate is present intracellularly in epithelial lining cells to a significantly greater extent than budesonide and its esters – would not be compatible with the much more rapid dissolution of budesonide [19] and prompt intracellular esterification and retention of budesonide esters [20].
Esmailpour and colleagues [16] found that two of three patients with viscous mucus in the upper airways had very high concentrations of fluticasone propionate (approximately 20 ng g−1) in central lung tissue. Although in the present study it was not possible to obtain samples of bronchial lining fluid because of airway collapse after resection, bronchial brushings provided detectable concentrations of fluticasone propionate in six patients for up to 18 h after inhalation, which is indicative of retention of undissolved drug powder in the airways. High variability of individual plasma concentration profiles of fluticasone propionate relative to those of budesonide in our study is indicative of variable rates of dissolution of fluticasone propionate in the airways. This is further supported by earlier findings showing that when dissolved fluticasone propionate was administered into the airways (human nose or rat trachea), its airway concentration declined much more rapidly than that of budesonide [9, 21].
Intracellular esterification is not observed with fluticasone propionate, but is specific for budesonide and ciclesonide [11, 14, 22]. Fatty acid conjugates of budesonide appear to form an intracellular depot of budesonide from which the drug is released over an extended period through the action of intracellular lipases. The presence of esterified budesonide in lung tissue over a prolonged period (up to 43 h) in our study is in accordance with this hypothesis and would explain the prolongation of duration of action of budesonide over that expected from this drug's general pharmacokinetics and moderate lipophilicity. The higher LOQ of parent budesonide in the tissue (1.25 pmol g−1) than that of budesonide esters (0.625 pmol g−1) did not allow the concentration of parent budesonide to be followed beyond 10 h after inhalation. However, the concentration of 1.25 pmol g−1 is more than double the value of in vitro affinity of budesonide for the glucocorticoid receptor (0.5 nmol l−1[23]), indicating that budesonide concentrations were high enough for 50% saturation of the glucocorticoid receptor in lung tissue for >10 h. In vitro studies suggest that fatty acid conjugates of budesonide have very low affinity for glucocorticoid receptors [24].
There were differences between the present study and previous findings in results pertaining to regional deposition of fluticasone propionate. Earlier results have shown concentrations of drug in central lung tissue to be three to four times higher than those in peripheral lung samples [16]. This was not observed in the present cohort of patients, with individual data showing wide variation between patients and inhalation times. Only three of 12 patients with detectable fluticasone propionate concentrations in both central and peripheral lung tissue has a central:peripheral ratio of >1.5. Factors playing a role here may have included differences in formulation (Esmailpour and colleagues [16] used delivery via pressurized metered dose inhaler and spacer), differing definitions of central and peripheral tissue, and differences in quality of inhaler technique between studies.
The detection of budesonide, but not budesonide oleate or fluticasone propionate, in intercostal muscle tissue up to approximately 10 h after inhalation is most likely to reflect redistribution from plasma. Higher initial plasma concentrations of budesonide and more rapid elimination relative to that of fluticasone propionate reflect the difference between the two drugs in terms of general pharmacokinetics, such as a smaller volume of distribution and shorter elimination half-life for budesonide [10].
In this study, as in the previous lung tissue concentration studies with fluticasone propionate and budesonide [15, 16], only single doses were given of each steroid. It is not known to what extent repeated dosing would affect lung concentrations. Plasma levels of fluticasone propionate double after 1 week's treatment compared with a single dose [10]. For budesonide, accumulation is much more limited [10], which further underscores the difference in pharmacokinetics between the two steroids. Whether the accumulation of fluticasone propionate in plasma after repeated dosing is a result of lung retention is not known, but it is unlikely to be an important explanation, since plasma half-life is virtually identical after inhalation as after intravenous dosing of the drug [10].
The clinical relevance of these findings is underlined by the apparent effect of esterification on the duration of action of budesonide after inhalation. Accumulating data indicate that the esterification of budesonide investigated in the present study is responsible for the extended duration of action of the drug in the airways that permits once-daily administration in mild asthma, as summarized by Selroos and colleagues [25].
In conclusion, budesonide oleate is formed rapidly in vivo in human airways after inhalation of parent drug and is detectable in lung tissue for almost 2 days after a single inhalation. Esterification takes place intracellularly within the lungs, and the sustained action of budesonide is probably explained by this fatty acid conjugation. The presence of fluticasone propionate in lung tissue for up to 22 h after inhalation appears to be the result of undissolved drug in the airway lumen not accessible to intracellular glucocorticoid receptors. Future research that might shed further light on these issues would include studies of extracellular and intracellular distribution of inhaled corticosteroids and their metabolites or conjugates, together with further study of central and peripheral lung deposition.
We thank Ian Wright (Wright Medical Communications Ltd) who provided editorial assistance on behalf of Astra Zeneca. This study was funded by AstraZeneca R&D, Lund, Sweden.
REFERENCES
- 1.National Asthma Education and Prevention Program (NAEPP). Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. [Google Scholar]
- 2.National Asthma Education Prevention Program (NAEPP). Expert Panel Report: guidelines for the diagnosis and management of asthma – update on selected topics. J Allergy Clin Immunol. 2002;110:S141–219. [PubMed] [Google Scholar]
- 3.Pauwels R. Use of corticosteroids in asthma. In: D'Arcy PF, McElnay JL, editors. Pharmacy and Pharmacotherapy of Asthma. Chichester: Ellis Harwood; 1989. pp. 104–18. [Google Scholar]
- 4.Edsbäcker S. Pharmacological factors that influence the choice of inhaled corticosteroids. Drugs. 1999;58(Suppl. 4):7–16. doi: 10.2165/00003495-199958004-00002. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157:S1–S53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- 6.Brogden RN, McTavish D. Budesonide: an updated review of its pharmacological properties, and therapeutic efficacy in asthma and rhinitis. Drugs. 1992;44:375–407. doi: 10.2165/00003495-199244030-00007. [DOI] [PubMed] [Google Scholar]
- 7.Campbell LM, Watson DG, Venables TL, Taylor MD, Richardson PDL. Once daily budesonide Turbohaler compared with placebo as initial prophylactic therapy for asthma. Br J Clin Res. 1991;2:111–22. [Google Scholar]
- 8.Jones AH, Langdon CG, Lee PS, Lingham SA, Nankani JP, Follows RM, Tollemar U, Richardson PD. Pulmicort Turbohaler once daily as initial prophylactic therapy for asthma. Respir Med. 1994;88:293–9. doi: 10.1016/0954-6111(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 9.Miller-Larsson A, Mattsson H, Hjertberg E, Dahlbäck M, Tunek A, Brattsand R. Reversible fatty acid conjugation of budesonide: novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos. 1998;26:623–30. [PubMed] [Google Scholar]
- 10.Thorsson L, Edsbäcker S, Källén A, Löfdahl CG. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbuhaler. Br J Clin Pharmacol. 2001;52:529–38. doi: 10.1046/j.0306-5251.2001.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tunek A, Sjödin K, Hallström G. Reversible formation of fatty acid esters of budesonide, an antiasthma glucocorticoid, in human lung and liver microsomes. Drug Metab Dispos. 1997;11:1311–7. [PubMed] [Google Scholar]
- 12.Wieslander E, Delander E-L, Sjödin K, Tunek A, Brattsand R. Fatty acid conjugation in normal human bronchial epithelial cells. Am J Respir Crit Care Med. 1998;157:A402. [Google Scholar]
- 13.Purucker ME, Rosebraugh CJ, Zhou F, Meyer RJ. Inhaled fluticasone propionate by Diskus in the treatment of asthma: a comparison of the efficacy and safety of the same nominal dose given either once or twice a day. Chest. 2003;124:1584–93. doi: 10.1378/chest.124.4.1584. [DOI] [PubMed] [Google Scholar]
- 14.Nave R, Meyer W, Fuhst R, Zech K. Formation of fatty acid conjugates of ciclesonide active metabolite in the rat lung after 4-week inhalation of ciclesonide. Pulm Pharmacol Ther. 2005;18:390–6. doi: 10.1016/j.pupt.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Van den Bosch JM, Westermann CJ, Aumann J, Edsbäcker S, Tonnesson M, Selroos O. Relationship between lung tissue and blood plasma concentrations of inhaled budesonide. Biopharm Drug Dispos. 1993;14:455–9. doi: 10.1002/bdd.2510140511. [DOI] [PubMed] [Google Scholar]
- 16.Esmailpour N, Högger P, Rabe KF, Heitmann U, Nakashima M, Rohdewald P. Distribution of inhaled fluticasone propionate between human lung tissue and serum in vivo. Eur Respir J. 1997;10:1496–9. doi: 10.1183/09031936.97.10071496. [DOI] [PubMed] [Google Scholar]
- 17.Jendbro M, Johansson J-M, Strandberg P, Falk-Nilsson H, Edsbäcker S. Pharmacokinetics of budesonide and its major ester metabolite after inhalation and intravenous administration of budesonide in the rat. Drug Metab Dispos. 2001;29:769–76. [PubMed] [Google Scholar]
- 18.Thorsson L, Thunnisen FBJM, Korn S, Carlshaf A, Edsbäcker S, Wouters EFM. Formation of fatty acid conjugates of budesonide in human lung tissue in vivo. Am J Respir Crit Care Med. 1998;157:A404. [Google Scholar]
- 19.Davies NM, Feddah MIR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharmaceut. 2003;255:175–87. doi: 10.1016/s0378-5173(03)00091-7. [DOI] [PubMed] [Google Scholar]
- 20.Edsbäcker S, Brattsand R. Budesonide fatty-acid esterification: a novel mechanism prolonging binding to airway tissue. Review of. Ann Allergy Asthma Immunol. 2002;88:609–16. doi: 10.1016/S1081-1206(10)61893-5. available data. [DOI] [PubMed] [Google Scholar]
- 21.Petersen H, Kullberg A, Edsbäcker S, Greiff L. Nasal retention of budesonide and fluticasone in man: formation of airway mucosal budesonide-esters in vivo. Br J Clin Pharmacol. 2001;51:159–63. doi: 10.1111/j.1365-2125.2001.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drollmann A, Watz H, Nave R, Boss H, Magnussen H, Hoffmann H. In vivo metabolism of ciclesonide in the human lung. Proc Am Tnorac Soc. 2006;3(Suppl. 3):A75. [Google Scholar]
- 23.Dahlberg E, Thalén A, Brattsand R, Gustafsson J-Å, Johansson U, Roempke K, Saartok T. Correlation between chemical structure, receptor binding and biological activity of some novel, highly active 16α, 17α-acetal substituted glucocorticoids. Mol Pharmacol. 1984;25:70–6. [PubMed] [Google Scholar]
- 24.Wieslander E, Delander E-L, Järkelid L, Hjertberg E, Tunek A, Brattsand R. Pharmacologic importance of the reversible fatty acid conjugation of budesonide studies in a rat cell line in vitro. Am J Respir Cell Mol Biol. 1998;19:477–84. doi: 10.1165/ajrcmb.19.3.3195. [DOI] [PubMed] [Google Scholar]
- 25.Selroos O, Edsbäcker S, Hultquist C. Once-daily inhaled budesonide for the treatment of asthma: clinical evidence and pharmacokinetic explanation. J Asthma. 2004;41:771–90. doi: 10.1081/jas-200038344. [DOI] [PubMed] [Google Scholar]


