Abstract
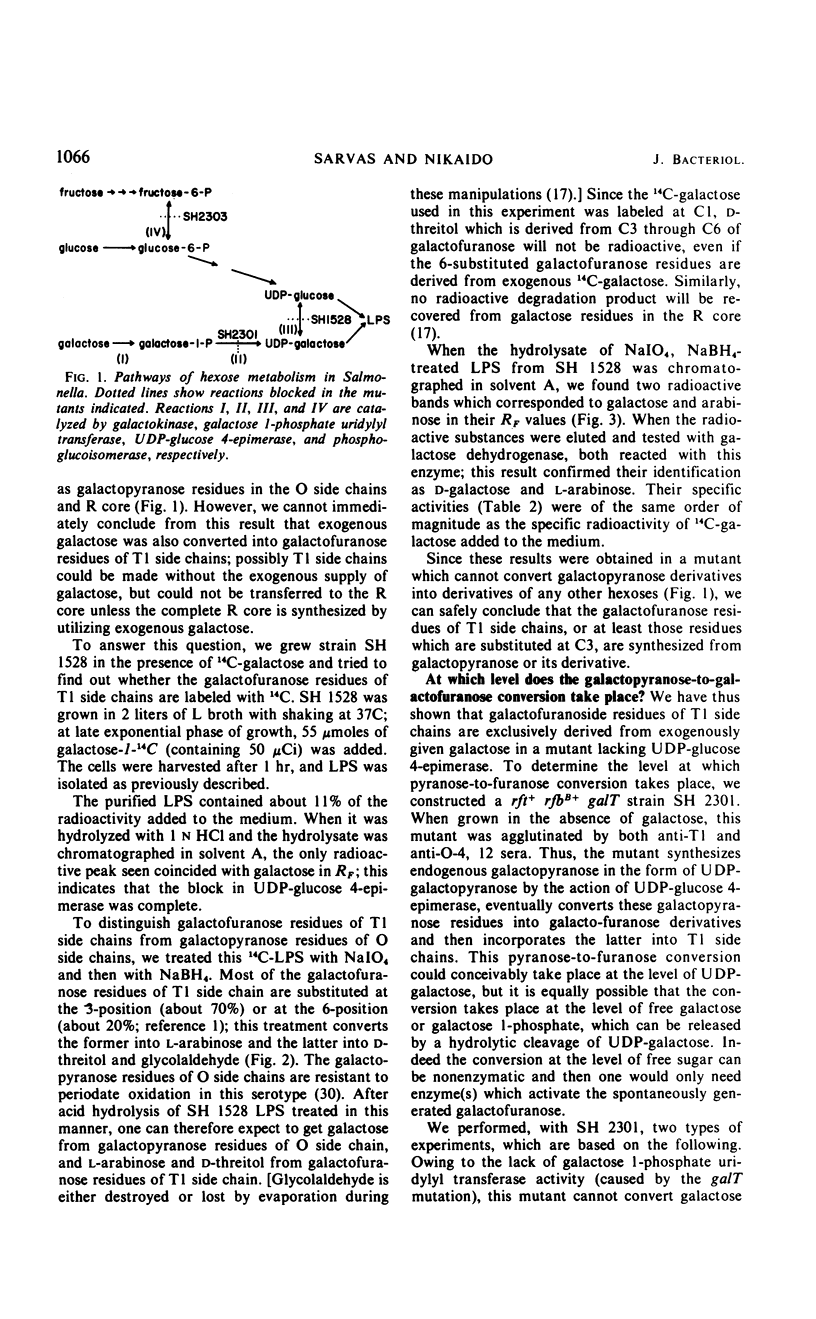
The “T1 side chain” portion of cell wall lipopolysaccharide from T1 strains of Salmonella contains d-galactofuranose and d-ribofuranose residues. Isotope labeling studies, using intact cells of mutants each blocked at either of the two different steps of d-galactose metabolism (uridine diphosphate-glucose 4-epimerase and galactose-1-P uridylyl transferase) or at phosphoglucoisomerase, led to the following conclusions. (i) d-Galactofuranose residues are synthesized from d-galactopyranose or its derivatives, rather than by a direct conversion from other hexopyranoses or their derivatives. (ii) The pyranose-to-furanose conversion does not appear to take place at the level of the free d-galactose or d-galactose 1-phosphate. This result suggests that the conversion may occur at the stage of uridine diphosphate-d-galactose. (iii) In a mutant lacking phosphoglucoisomerase, d-ribofuranose residues in T1 side chains contained 14C derived from exogenous d-fructose-U-14C, but little 3H from exogenous d-glucose-1-3H. Thus, no evidence was found for a direct pathway of aldohexose-to-ribose conversion involving a loss of one of the carbons in the C2-C6 moiety of aldohexoses. This suggests, but does not prove, that the T1 ribofuranose residues are synthesized by conventional mechanisms involving hexose monophosphate shunt and transketolase-transaldolase reactions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berst M., Hellerqvist C. G., Lindberg B., Lüderitz O., Svensson S., Westphal O. Structural investigations on T1 lipopolysaccharides. Eur J Biochem. 1969 Dec;11(2):353–359. doi: 10.1111/j.1432-1033.1969.tb00779.x. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. The 4-epimerization and decarboxylation of uridine diphosphate D-glucuronic acid by extracts from Phaseolus aureus seedlings. J Biol Chem. 1960 Apr;235:910–913. [PubMed] [Google Scholar]
- FRAENKEL D., OSBORN M. J., HORECKER B. L., SMITH S. M. Metabolism and cell wall structure of a mutant of Salmonella typhimurium deficient in phosphoglucose isomerase. Biochem Biophys Res Commun. 1963 Jun 20;11:423–428. doi: 10.1016/0006-291x(63)90086-x. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Formation of phage receptors induced by galactose in a galactose-sensitive mutant of Salmonella. Virology. 1960 Jun;11:508–510. doi: 10.1016/0042-6822(60)90093-3. [DOI] [PubMed] [Google Scholar]
- GANDER J. E. Biogenesis of galactocarolose by Penicillium charlesii. I. Incorporation of C14 from glucose-C14 and DL-tartrate-1,4-C14 into polyglactose. Arch Biochem Biophys. 1960 Dec;91:307–309. doi: 10.1016/0003-9861(60)90505-1. [DOI] [PubMed] [Google Scholar]
- Haworth W. N., Raistrick H., Stacey M. Polysaccharides synthesised by micro-organisms: The molecular structure of galactocarolose produced from glucose by Penicillium Charlesii G. Smith. Biochem J. 1937 Apr;31(4):640–644. doi: 10.1042/bj0310640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFFMANN F. A new antigen of Salmonella paratyphi B and Salmonella typhi murium. Acta Pathol Microbiol Scand. 1956;39(4):299–304. doi: 10.1111/j.1699-0463.1956.tb03405.x. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Kurahashi K., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, I. DETERMINATION OF ENZYME ACTIVITIES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1776–1786. doi: 10.1073/pnas.45.12.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Sarvas M., Mäkelä P. H. Bacteriophage attachment to the somatic antigen of salmonella: effect of o-specific structures in leaky R mutants and s, t1 hybrids. Infect Immun. 1970 Jan;1(1):88–97. doi: 10.1128/iai.1.1.88-97.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Pennie I. R., Ryley J. F. The reserve polysaccharides of Prototheca zopfli. Biochem J. 1967 Aug;104(2):32P–32P. [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Mäkelä P. H. Genetic determination of enzymes synthesizing O-specific sugars of Salmonella lipopolysaccharides. J Bacteriol. 1966 Mar;91(3):1126–1135. doi: 10.1128/jb.91.3.1126-1135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Sarvas M. Biosynthesis of T1 antigen in Salmonella: biosynthesis in a cell-free system. J Bacteriol. 1971 Mar;105(3):1073–1082. doi: 10.1128/jb.105.3.1073-1082.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. I. Linkage between o side chains and R core. J Biol Chem. 1969 Jun 10;244(11):2835–2845. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Plackett P., Buttery S. H. A galactofuranose disaccharide from the galactan of Mycoplasma mycoides. Biochem J. 1964 Jan;90(1):201–205. doi: 10.1042/bj0900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao E. V., Buchanan J. G., Baddiley J. The type-specific substance from Pneumococcus type 10A(34). Structure of the dephosphorylated repeating unit. Biochem J. 1966 Sep;100(3):801–810. doi: 10.1042/bj1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao E. V., Watson M. J., Buchanan J. G., Baddiley J. The type-specific substance from Pneumococcus type 29. Biochem J. 1969 Feb;111(4):547–556. doi: 10.1042/bj1110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Buchanan J. G., Baddiley J. The specific substance from Pneumococcus type 34 (41). The structure of a phosphorus-free repeating unit. Biochem J. 1963 Jul;88(1):1–7. doi: 10.1042/bj0880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable H. Z. Biosynthesis of ribose and deoxyribose. Adv Enzymol Relat Areas Mol Biol. 1966;28:391–460. doi: 10.1002/9780470122730.ch7. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas M. Inheritance of Salmonella T1 antigen. Ann Med Exp Biol Fenn. 1967;45(4):447–471. [PubMed] [Google Scholar]
- Stocker B. A., Wilkinson R. G., Mäkelä P. H. Genetic aspects of biosynthesis and structure of Salmonella somatic polysaccharide. Ann N Y Acad Sci. 1966 Jun 30;133(2):334–348. doi: 10.1111/j.1749-6632.1966.tb52375.x. [DOI] [PubMed] [Google Scholar]
- TINELLI R., STAUB A. M. [Chemical study of the somatic polyosides of Salmonella species. III. Periodic acid oxidation of the polyosides extracted from different Salmonella species]. Bull Soc Chim Biol (Paris) 1959;41:1221–1231. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Trejo A. G., Chittenden G. J., Buchanan J. G., Baddiley J. Uridine diphosphate alpha-D-galactofuranose, an intermediate in the biosynthesis of galactofuranosyl residues. Biochem J. 1970 Apr;117(3):637–639. doi: 10.1042/bj1170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat R. W., Berst M., Ruschmann E., Lüderitz O., Westphal O. Lipopolysaccharides of Salmonella T mutants. J Bacteriol. 1967 Nov;94(5):1366–1380. doi: 10.1128/jb.94.5.1366-1380.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Stocker B. A. Genetics and cultural properties of mutants of Salmonella typhimurium lacking glucosyl or galactosyl lipopolysaccharide transferases. Nature. 1968 Mar 9;217(5132):955–957. doi: 10.1038/217955a0. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Kalckar H. M. Endogenous induction of the galactose operon in Escherichia coli K12. Proc Natl Acad Sci U S A. 1966 Mar;55(3):622–629. doi: 10.1073/pnas.55.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa R., Levinthal M., Nikaido H. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequose. J Bacteriol. 1969 Oct;100(1):433–444. doi: 10.1128/jb.100.1.433-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


