Abstract
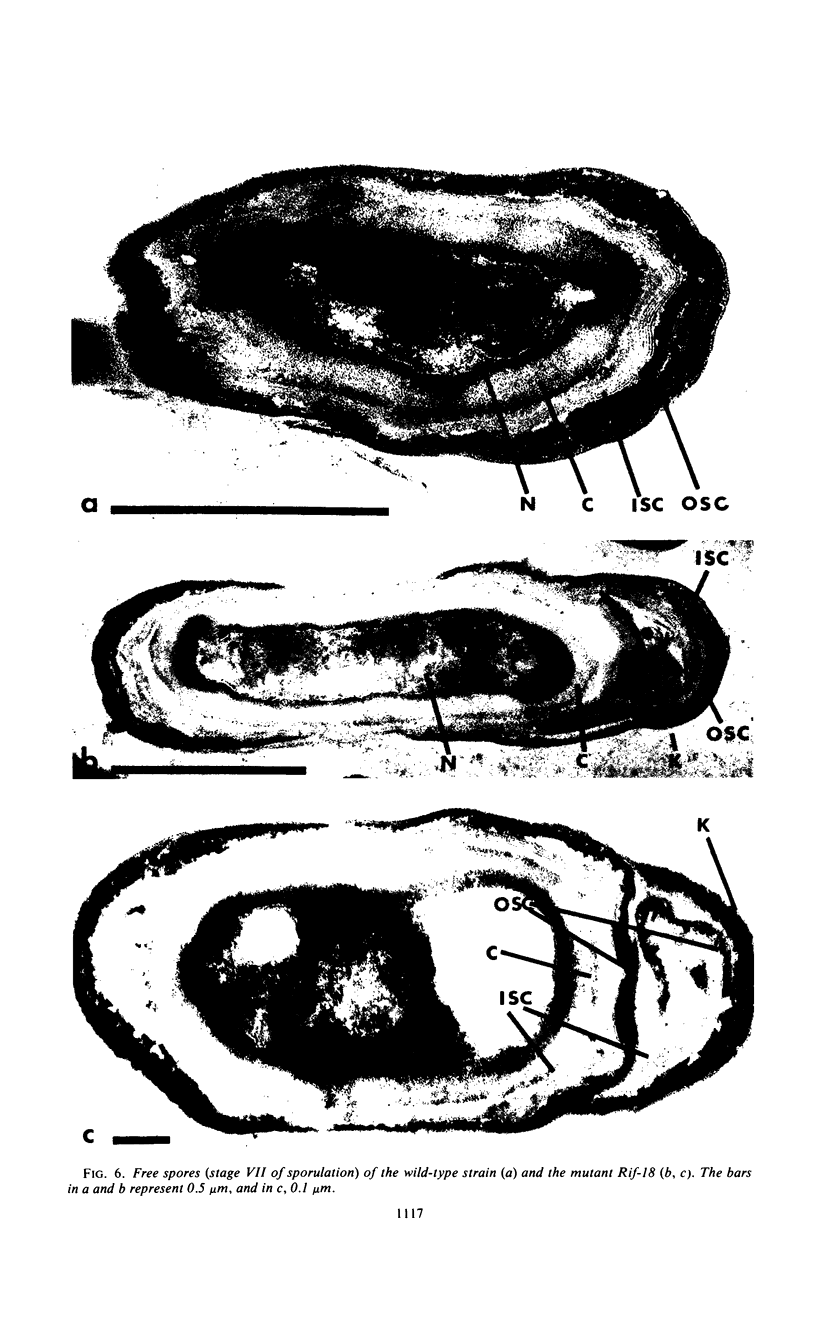
Electron microscopy was used to analyze sporulating cells and spores of Bacillus subtilis mutants (Rifr) which are resistant to rifampin, an inhibitor of ribonucleic acid polymerase. The spores of Rif-18 are pleomorphic and frequently exhibit terminal knobs. These knobs first occur during late stage IV and early stage V of sporulation and are extensions of the inner and outer spore coats. Since the rifampin resistance and altered spore morphology of Rif-18 are 100% cotransformable, these data suggest that the altered spore morphology is the result of an alteration in ribonucleic acid polymerase genes. The morphology and physical dimensions are also reported for spores from Rif-11, Rif-15, and Rif-21. Significant differences in size from the wild type were observed for these mutants.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doi R. H., Brown L. R., Rodgers G., Hsu Y. Bacillus subtilis mutant altered in spore morphology and in RNA polymerase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):404–410. doi: 10.1073/pnas.66.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Marr A. G. Measurement of size distributions of bacterial cells. J Bacteriol. 1966 Oct;92(4):805–811. doi: 10.1128/jb.92.4.805-811.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- SACKS L. E., ALDERTON G. Behavior of bacterial spores in aqueous polymer two-phase systems. J Bacteriol. 1961 Sep;82:331–341. doi: 10.1128/jb.82.3.331-341.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., JAMES P. C. Chemical and morphological studies of bacterial spore formation. IV. The development of spore refractility. J Cell Biol. 1962 Jan;12:115–133. doi: 10.1083/jcb.12.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]








