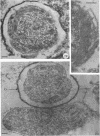
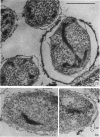
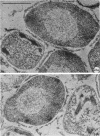
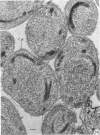
Abstract
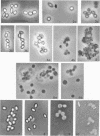
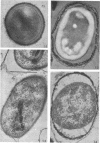
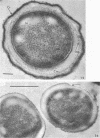
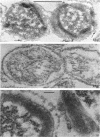
Coat-stripped spores suspended in hypertonic solutions and supplied with two essential cations can be converted into viable protoplasts by lysozyme digestion of both cortex and germ cell wall. Calcium ions are necessary to prevent membrane rupture, and magnesium ions are necessary for changes indicative of hydration of the core, particularily the nuclear mass. Since remnant spore coat covered such protoplasts of Bacillus subtilis and the germ cell wall of B. cereus spores is not lysozyme digestible, coatless spores of B. megaterium KM were more useful for these studies. Lysozyme digestion in cation-free environment produced a peculiar semi-refractile spore core free of a cortex but prone to rapid hydration and lytic changes on the addition of cations. Strontium could replace Ca2+ but Mn2+ could not replace Mg2+ in these digestions. When added to the spores, dipicolinic acid and other chelates appeared to compete with the membrane for the calcium needed for stabilization during lysozyme conversion to protoplasts. It is argued that calcium could function to stabilize the inner membrane anionic groups over the anhydrous dipicolinic acid-containing core of resting spores.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Cytological and chemical studies of the growth of protoplasts of Bacillus megaterium. J Biophys Biochem Cytol. 1958 May 25;4(3):257–266. doi: 10.1083/jcb.4.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C., Young I. E. CYTOLOGICAL COMPARISON OF SPORES OF DIFFERENT STRAINS OF BACILLUS MEGATERIUM. J Bacteriol. 1959 Dec;78(6):755–764. doi: 10.1128/jb.78.6.755-764.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster H. F., Foster J. W. Endotrophic calcium, strontium, and barium spores of Bacillus megaterium and Bacillus cereus. J Bacteriol. 1966 Mar;91(3):1333–1345. doi: 10.1128/jb.91.3.1333-1345.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD G. W., HITCHINS A. D. SENSITIZATION OF BACTERIAL SPORES TO LYSOZYME AND TO HYDROGEN PEROXIDE WITH AGENTS WHICH RUPTURE DISULPHIDE BONDS. J Gen Microbiol. 1963 Dec;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The binding of calcium at lipid-water interfaces. Eur J Biochem. 1967 Mar;1(1):61–69. doi: 10.1007/978-3-662-25813-2_11. [DOI] [PubMed] [Google Scholar]
- Hyatt M. T., Levinson H. S. Water vapor, aqueous ethyl alcohol, and heat activation of Bacillus megaterium spore germination. J Bacteriol. 1968 Jun;95(6):2090–2101. doi: 10.1128/jb.95.6.2090-2101.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos R. W., Carr C. W. The binding of calcium in mixtures of phospholipids. Proc Soc Exp Biol Med. 1967 Apr;124(4):1268–1272. doi: 10.3181/00379727-124-31984. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHROP J. H. Growth and phage production of lysogenic B. megatherium. J Gen Physiol. 1951 May;34(5):715–735. doi: 10.1085/jgp.34.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., HUNTER J. R. Adenosine deaminase and ribosidase in spores of Bacillus cereus. Biochem J. 1956 Mar;62(3):381–387. doi: 10.1042/bj0620381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanooka H., Terano H. Resistance of DNA against radiation-induced strand breakage in bacterial spores. Radiat Res. 1970 Sep;43(3):613–626. [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J., Swanson A., Halvorson H. O. Dipicolinic acid-less mutants of Bacillus cereus. J Bacteriol. 1967 Dec;94(6):2075–2076. doi: 10.1128/jb.94.6.2075-2076.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]