Abstract
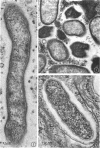
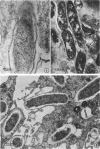
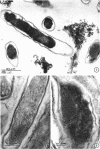
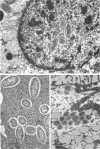
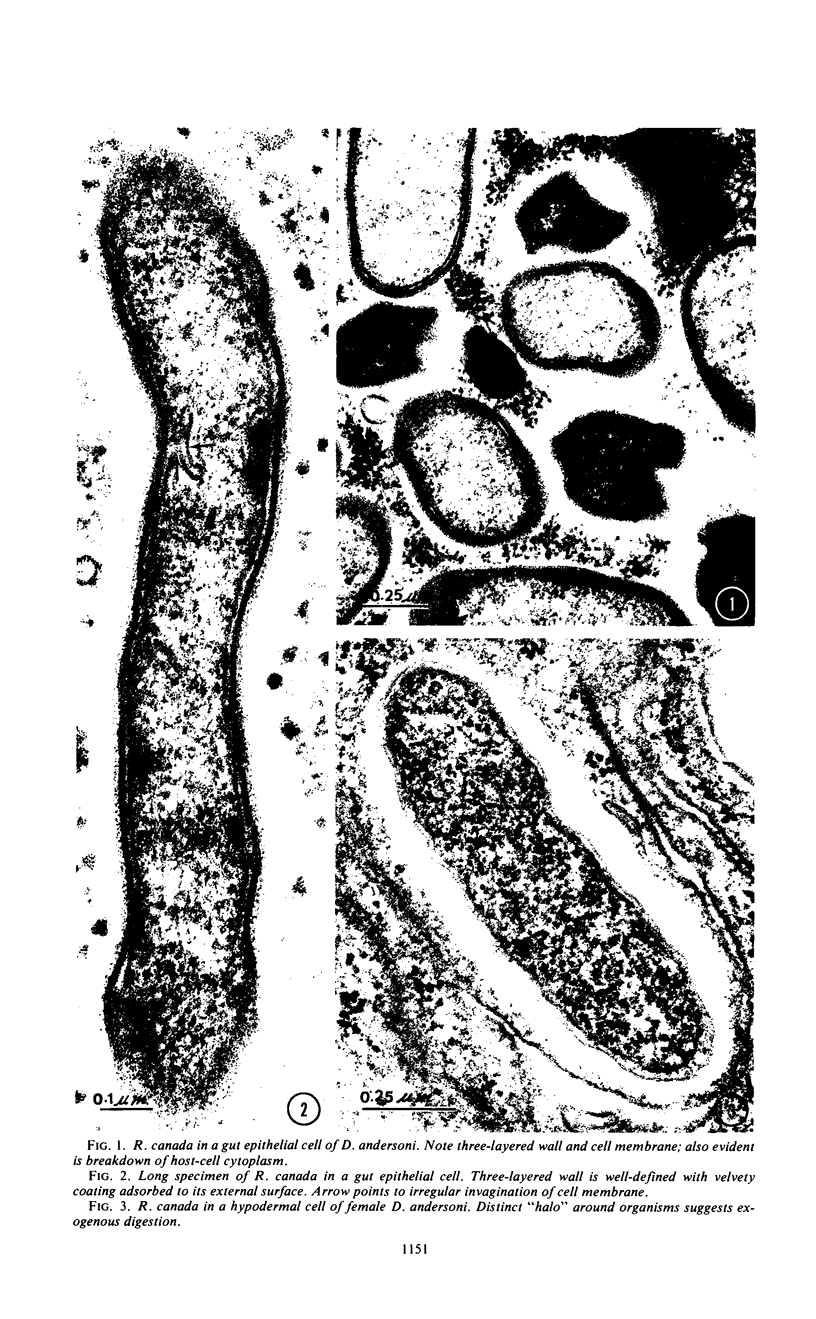
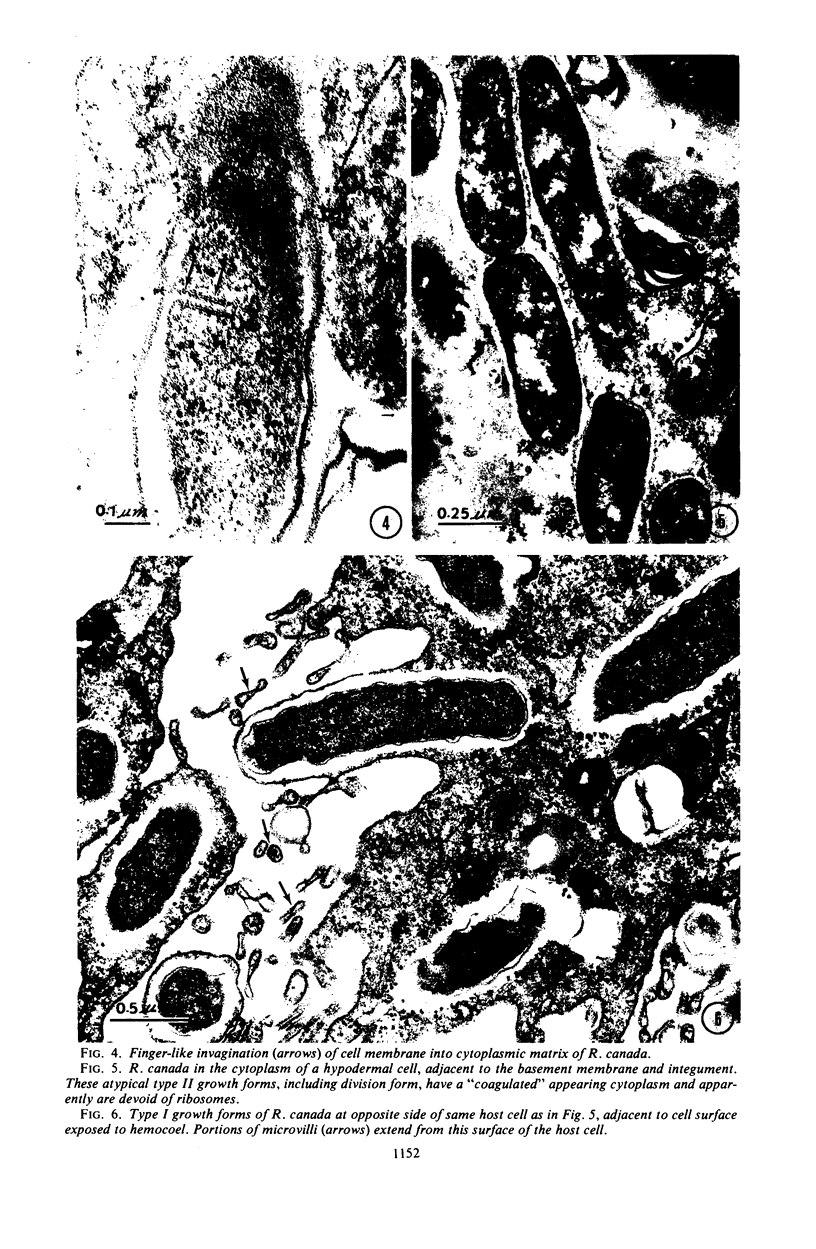
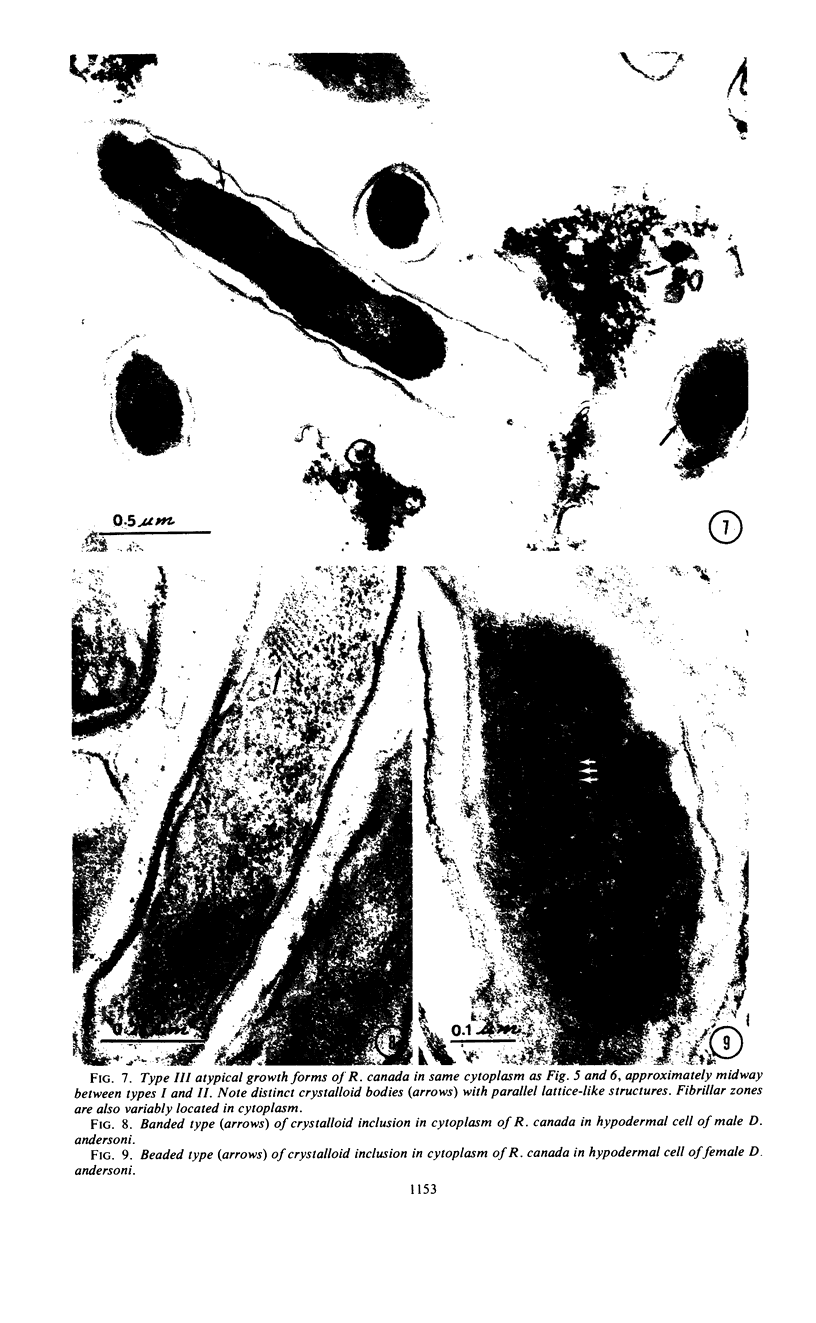
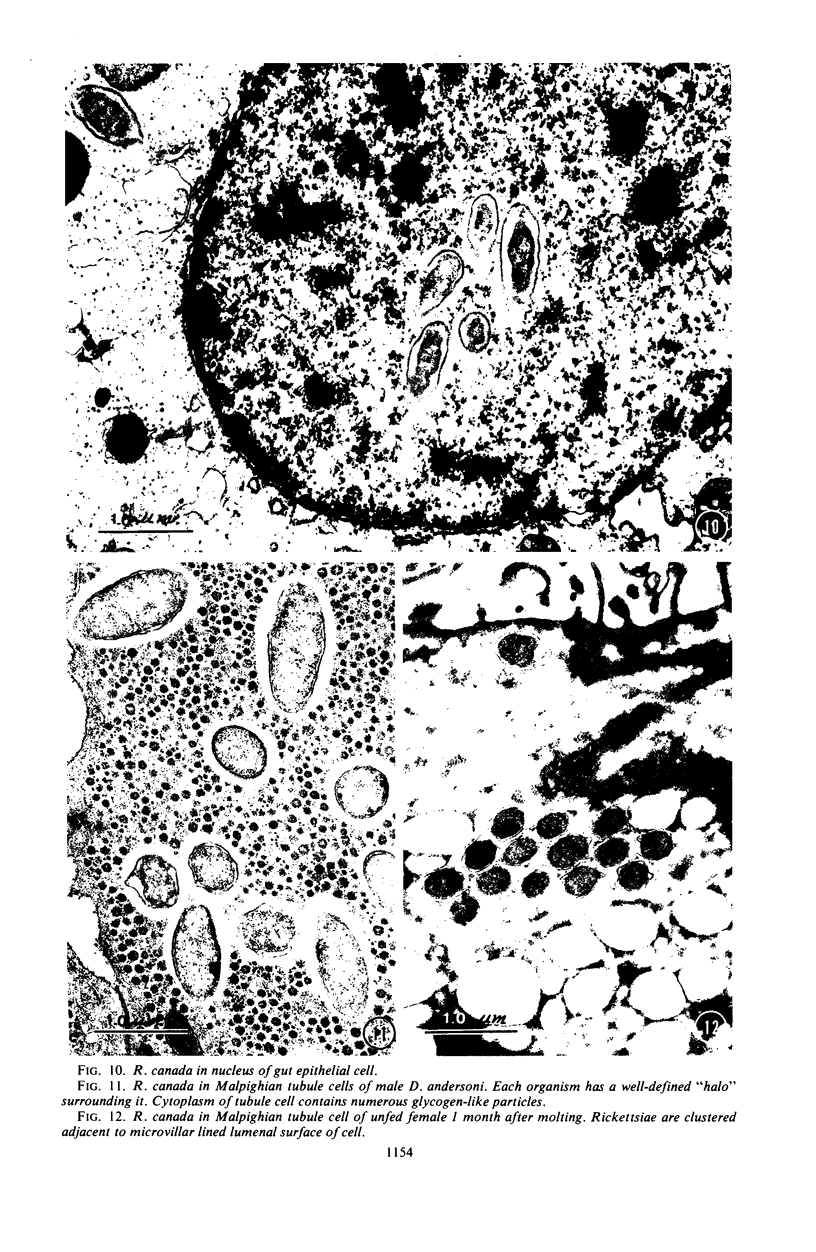
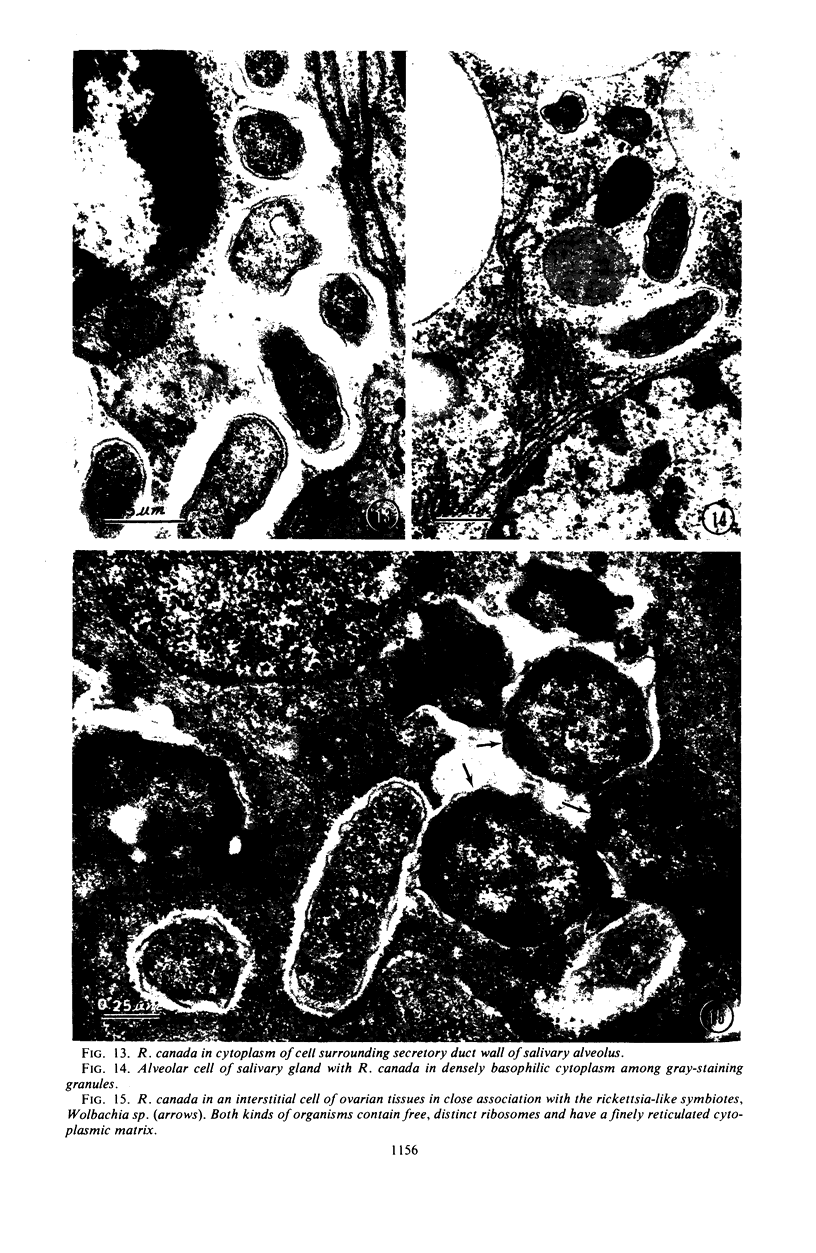
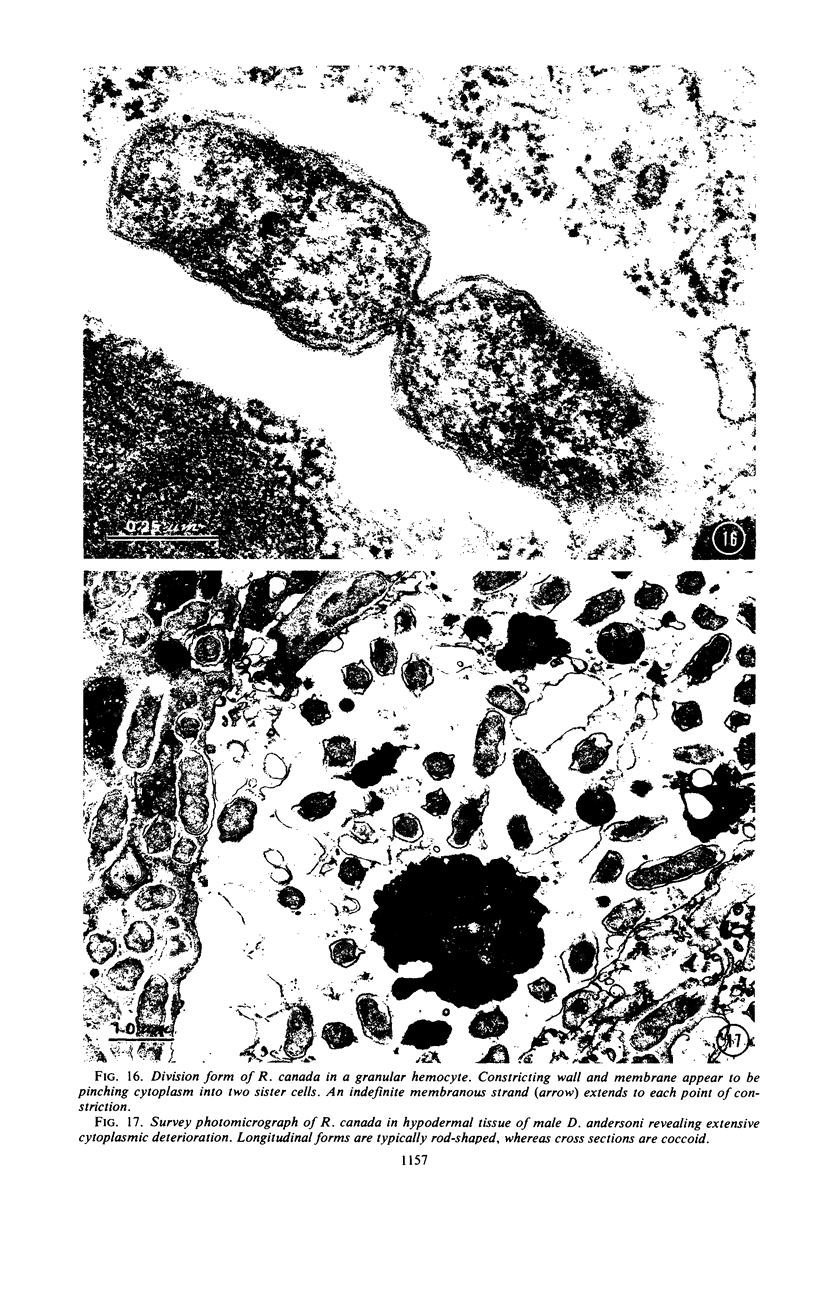
Preliminary observations on growth and developmental fine structure of Rickettsia canada in various organs and tissues of the hard tick, Dermacentor andersoni Stiles, are reported. R. canada is typically rod-shaped, being delimited with a three-layered wall having a velvety coating adsorbed to its exterior surface. A finely reticulated cytoplasmic matrix containing prominent ribosomes is delimited with a three-layered unit membrane. Average length and width of these organisms are 1.6 by 0.4 μm. Although R. canada produces a generalized infection in D. andersoni, hypodermal and muscle tissues experience heaviest growth. Three morphologically distinct rickettsial forms were observed in individual hypodermal cells: (i) typical growth forms with a finely reticulated cytoplasmic matrix and distinct ribosomes; (ii) atypical forms with lightly to densely staining cytoplasm and a coagulated appearance in which ribosomes cannot be distinguished from the matrix; and (iii) forms with crystalline bodies that have a striated to beaded lattice structure and, at times, a fibrillar body in the cytoplasm as well. Occasional finger-like to irregular invaginations of the plasma membrane are noted. Intranuclear growth was demonstrated by electron microscopy in gut epithelial cells only. Growth and development of R. canada were manifest in all tissues examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., FUKUSHI K., PICKENS E. G., LACKMAN D. B. ELECTRON MICROSCOPIC OBSERVATIONS OF THE DEVELOPMENT OF COXIELLA BURNETII IN THE CHICK YOLK SAC. J Bacteriol. 1964 Oct;88:1130–1138. doi: 10.1128/jb.88.4.1130-1138.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Pickens E. G., Lackman D. B. Details of the ultrastructure of Rickettsia prowazekii grown in the chick yolk sac. J Bacteriol. 1967 Jul;94(1):260–262. doi: 10.1128/jb.94.1.260-262.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. R., Hopps H. E., Barile M. F., Bernheim B. C. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J Bacteriol. 1965 Nov;90(5):1387–1404. doi: 10.1128/jb.90.5.1387-1404.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. G., Kordová N., Rehácek J. Fine structure of Rickettsia prowazeki in the haemocytes of ticks Hyalomma dromedarii. Acta Virol. 1967 Jan;11(1):60–62. [PubMed] [Google Scholar]
- Burgdorfer W., Anacker R. L., Bird R. G., Bertram D. S. Intranuclear growth of Rickettsia rickettsii. J Bacteriol. 1968 Oct;96(4):1415–1418. doi: 10.1128/jb.96.4.1415-1418.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Brinton L. P. Intranuclear growth of rickettsia Canada, a member of the typhus group. Infect Immun. 1970 Jul;2(1):112–114. doi: 10.1128/iai.2.1.112-114.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W. Observations on Rickettsia canada a recently described member of the typhus group rickettsiae. J Hyg Epidemiol Microbiol Immunol. 1968;12(1):26–31. [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Granados R. R., Ward L. S., Maramorosch K. Insect viremia caused by a plant-pathogenic virus: electron microscopy of vector hemocytes. Virology. 1968 Apr;34(4):790–796. doi: 10.1016/0042-6822(68)90100-1. [DOI] [PubMed] [Google Scholar]
- Higashi N. Recent advances in electron microscope studies on ultrastructure of rickettsiae. Zentralbl Bakteriol Orig. 1968 Apr;206(3):277–283. [PubMed] [Google Scholar]
- ITO S., VINSON J. W. FINE STRUCTURE OF RICKETTSIA QUINTANA CULTIVATED IN VITRO AND IN THE LOUSE. J Bacteriol. 1965 Feb;89:481–495. doi: 10.1128/jb.89.2.481-495.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadin J., Creemers J., Jadin J. M., Giroud P. Ultrastructure of Rickettsia prowazeki. Acta Virol. 1968 Jan;12(1):7–10. [PubMed] [Google Scholar]
- KORDOVA N., REHACEK J. MICROSCOPIC EXAMINATION OF THE ORGANS OF TICKS INFECTED WITH RICKETTSIA PROWAZEKI. Acta Virol. 1964 Sep;8:465–469. [PubMed] [Google Scholar]
- Kordová N., Rosenberg M., Mrena E. Die Vermehrung der Rickettsia prowazeki in L-Zellen. II. Elektronenmikroskopische Untersuchungen an infizierten Gewebezellen in Dünnschnitten. Arch Gesamte Virusforsch. 1965;15(5):707–720. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linss W., Stelzner A., Urbach H. Elektronenmikroskopische Untersuchungen über die Entwicklung von Coxiella burneti im Hodengewebe des Meerschweinshens. Arch Gesamte Virusforsch. 1969;26(1):86–96. [PubMed] [Google Scholar]
- McKiel J. A., Bell E. J., Lackman D. B. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Can J Microbiol. 1967 May;13(5):503–510. doi: 10.1139/m67-065. [DOI] [PubMed] [Google Scholar]
- Paretsky D. Biochemistry of rickettsiae and their infected hosts, with special reference to Coxiella burneti. Zentralbl Bakteriol Orig. 1968 Apr;206(3):283–291. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG M., KORDOVA N. Study of intracellular forms of Coxiella burneti in the electron microscope. Acta Virol. 1960 Jan;4:52–55. [PubMed] [Google Scholar]
- Shands J. W., Jr Embedding free-floating cells and microscopic particles; serum albumin coagulum-epoxy resin. Stain Technol. 1968 Jan;43(1):15–17. doi: 10.3109/10520296809115036. [DOI] [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]