Abstract
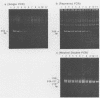
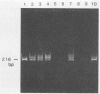
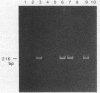
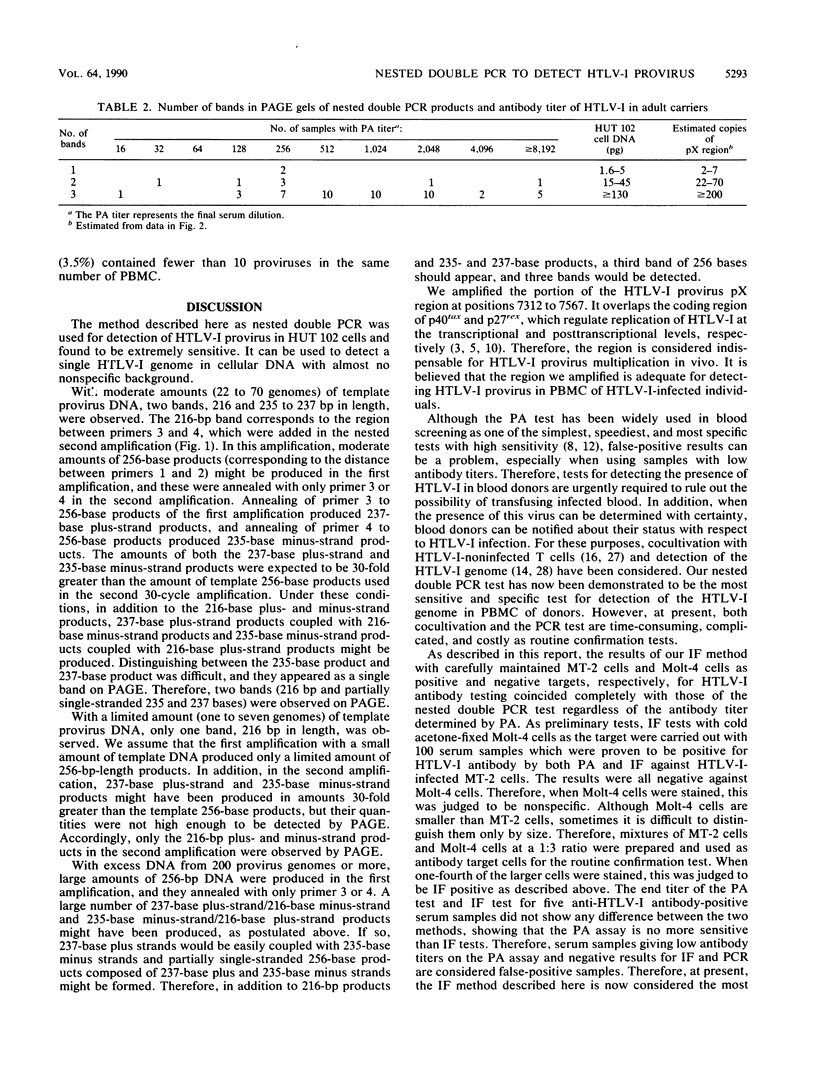
Human T-cell leukemia virus type I (HTLV-I) provirus DNA from the cultured cell line HUT 102 and from peripheral mononuclear cells (PBMC) of anti-HTLV-I antibody-positive Japanese blood donors was detected by the nested double polymerase chain reaction (PCR) method. This procedure consists of a first amplification and a second amplification with the products of the first amplification and primers interior to the first primers. Using this method, we demonstrated that it is possible to detect single-template DNA. Polyacrylamide gel electrophoresis of the nested double PCR products, with our primers, revealed three bands with excess amounts of template DNA, two bands with moderate amounts, and a single band with limited amounts. The amount of provirus in PBMC was roughly estimated from the results of the nested double PCR. Particle agglutination (PA) assays and indirect immunofluorescence testing (IF) with mixed MT-2 cells and Molt-4 cells as targets to detect anti-HTLV-I antibody were performed, and the results were compared with those of the nested double PCR of the pX region. None of the 101 PA-negative samples were positive in either the IF or PCR test. Of the 155 samples that were antibody positive by the PA assay, 57 were positive by both PCR and IF. Furthermore, the results of the IF and PCR tests coincided completely. It was therefore concluded that the IF method is most appropriate for confirmation of the PA assay currently used in most diagnostic laboratories and blood centers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagavati S., Ehrlich G., Kula R. W., Kwok S., Sninsky J., Udani V., Poiesz B. J. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988 May 5;318(18):1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Kan Y. W. Detection of specific DNA sequences by fluorescence amplification: a color complementation assay. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9178–9182. doi: 10.1073/pnas.86.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Slamon D. J., Rosenblatt J. D., Shah N. P., Quan S. G., Wachsman W. The x gene is essential for HTLV replication. Science. 1985 Jul 5;229(4708):54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- Duggan D. B., Ehrlich G. D., Davey F. P., Kwok S., Sninsky J., Goldberg J., Baltrucki L., Poiesz B. J. HTLV-I-induced lymphoma mimicking Hodgkin's disease. Diagnosis by polymerase chain reaction amplification of specific HTLV-I sequences in tumor DNA. Blood. 1988 Apr;71(4):1027–1032. [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia K., Spector D. H., Lawrie J., Spector S. A. Enzymatic amplification of human cytomegalovirus sequences by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1802–1809. doi: 10.1128/jcm.27.8.1802-1809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Fujino R., Matsui T., Yoshida T., Komoda H., Imai J. A new agglutination test for serum antibodies to adult T-cell leukemia virus. Gan. 1984 Oct;75(10):845–848. [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihira S., Nakasima S., Oyakawa Y., Moriuti Y., Ichimaru M., Okuda H., Kanamura M., Oota T. Transmission of human T cell lymphotropic virus type I by blood transfusion before and after mass screening of sera from seropositive donors. Vox Sang. 1987;52(1-2):43–44. doi: 10.1111/j.1423-0410.1987.tb02987.x. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Miller R. H., Feinstone S. M., Unoura M., Kobayashi K., Hattori N., Purcell R. H. Detection of serum hepatitis B virus DNA in patients with chronic hepatitis using the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1989 Jan;86(1):312–316. doi: 10.1073/pnas.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K., Amagasaki T., Ikeda S., Suzuyama J., Toriya K., Nishino K., Tagawa M., Ichimaru M., Kamihira S., Yamada Y. Preleukemic state of adult T cell leukemia: abnormal T lymphocytosis induced by human adult T cell leukemia-lymphoma virus. Blood. 1985 Jul;66(1):120–127. [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Tomita N., Ishii A., Nishizaki T., Kitajima K., Tanaka T., Nakamura T., Watanabe S., Oda T. Transformation of ATLA-negative leukocytes by blood components from anti-ATLA-positive donors in vitro. Int J Cancer. 1984 Jun 15;33(6):721–725. doi: 10.1002/ijc.2910330603. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Sandberg-Wollheim M., Mettus R. V., Ray P. E., DeFreitas E., Koprowski H. Amplification and molecular cloning of HTLV-I sequences from DNA of multiple sclerosis patients. Science. 1989 Jan 27;243(4890):529–533. doi: 10.1126/science.2536193. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Seiki M., Yoshida M. HTLV type I (U. S. isolate) and ATLV (Japanese isolate) are the same species of human retrovirus. Virology. 1984 Feb;133(1):238–241. doi: 10.1016/0042-6822(84)90446-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Seiki M., Yamaguchi K., Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]