Abstract
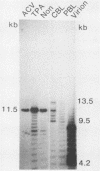
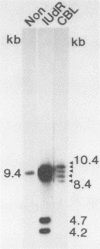
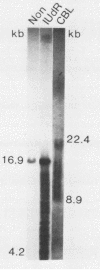
The linear form of Epstein-Barr virus (EBV) DNA has homologous direct tandem repeats of approximately 500 bp at each terminus (TR). After infection, EBV DNA circularizes via the TR to form the intracellular episomal DNA. To analyze the mechanism of the synthesis of linear DNA through possible replicative intermediates, the terminal fragments were identified in the total intracellular DNA and the covalently closed circular DNA from a productively infected cell line after induction of replication or after treatment with an inhibitor of viral DNA synthesis. These studies indicate that some of the fused terminal fragments detected in the total intracellular DNA are replication-dependent forms which are selectively excluded from the covalently closed circular fraction and are eliminated after treatment with acyclovir. The EBV terminal restriction enzyme fragments were identified in three producer cell lines, each with a characteristic number of TR in the intracellular episomal DNA. Identification of the termini in cell lines established with the three virus strains revealed that the newly transformed cell lines had a greater number of TR than did the template DNA in the producer cell line. The increase in the number of TR in progeny episomes indicates that linear DNA is produced from concatameric replicative intermediates rather than from amplified catenated circular intermediates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Tokazewski S. A. Replication of herpesvirus DNA. II. Sedimentation characteristics of newly synthesized DNA. Virology. 1977 Jun 15;79(2):292–301. doi: 10.1016/0042-6822(77)90356-7. [DOI] [PubMed] [Google Scholar]
- Brown N. A., Liu C. R., Wang Y. F., Garcia C. R. B-cell lymphoproliferation and lymphomagenesis are associated with clonotypic intracellular terminal regions of the Epstein-Barr virus. J Virol. 1988 Mar;62(3):962–969. doi: 10.1128/jvi.62.3.962-969.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. A., Liu C., Berenson J. R., Garcia C. R., Wang R., Calame K. L. Immunoglobulin JH, C mu, and C gamma gene rearrangements in human B lymphocytes clonally transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1985 Jan;82(2):556–560. doi: 10.1073/pnas.82.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Datta A. K., Pagano J. S. Replication of Epstein-Barr virus DNA in lymphoblastoid cells treated for extended periods with acyclovir. Am J Med. 1982 Jul 20;73(1A):77–81. doi: 10.1016/0002-9343(82)90068-7. [DOI] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Yee D., Griem K., Kieff E. DNA of Epstein-Barr virus. V. Direct repeats of the ends of Epstein-Barr virus DNA. J Virol. 1979 Jun;30(3):852–862. doi: 10.1128/jvi.30.3.852-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. R., Prezyna C., Benz W. C. Two Epstein-Barr virus-associated DNA polymerase activities. J Biol Chem. 1978 Dec 10;253(23):8617–8628. [PubMed] [Google Scholar]
- Griffin B. E., Björck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Bar virus DNA in transformed cells. J Virol. 1981 Oct;40(1):11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Jacob R. J., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979 Feb;29(2):448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Z., Raab-Traub N., Miller G. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and lymphoproliferative diseases. J Infect Dis. 1989 Oct;160(4):589–598. doi: 10.1093/infdis/160.4.589. [DOI] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. R., Sugden B. The structure of the termini of the DNA of Epstein-Barr virus. Cell. 1979 Jul;17(3):661–671. doi: 10.1016/0092-8674(79)90273-3. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M., Mocarski E. S. The cleavage recognition signal is contained within sequences surrounding an a-a junction in herpes simplex virus DNA. Virology. 1988 Nov;167(1):25–30. doi: 10.1016/0042-6822(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Dambaugh T., Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980 Nov;22(1 Pt 1):257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986 Dec 26;47(6):883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sato H., Takimoto T., Hatano M., Pagano J. S., Raab-Traub N. Epstein-Barr virus with transforming and early antigen-inducing ability originating from nasopharyngeal carcinoma: mapping of the viral genome. J Gen Virol. 1989 Mar;70(Pt 3):717–727. doi: 10.1099/0022-1317-70-3-717. [DOI] [PubMed] [Google Scholar]
- Sato H., Takimoto T., Ogura H., Tanaka J., Hatano M., Glaser R. Heterogeneity of Epstein-Barr virus derived from a nasopharyngeal carcinoma that has transforming and lytic properties. J Natl Cancer Inst. 1986 Jun;76(6):1019–1024. [PubMed] [Google Scholar]
- Sato H., Takimoto T., Pagano J. S., Raab-Traub N. Amplification of Epstein-Barr virus (EBV) DNA by superinfection with a strain of EBV derived from nasopharyngeal carcinoma. J Virol. 1988 Sep;62(9):3288–3294. doi: 10.1128/jvi.62.9.3288-3294.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E. The circular intracellular form of Epstein-Barr virus DNA is amplified by the virus-associated DNA polymerase. J Virol. 1985 Mar;53(3):1012–1015. doi: 10.1128/jvi.53.3.1012-1015.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T., Kamide M., Umeda R. Establishment of Epstein-Barr virus (EBV)-associated nuclear antigen (EBNA)-positive nasopharyngeal carcinoma hybrid cell line (NPC-KT). Arch Otorhinolaryngol. 1984;239(1):87–92. doi: 10.1007/BF00454266. [DOI] [PubMed] [Google Scholar]
- Takimoto T., Ogura H., Sato H., Umeda R., Hatano M. Isolation of transforming and early antigen-inducing Epstein-Barr virus from nasopharyngeal carcinoma hybrid cells (NPC-KT). J Natl Cancer Inst. 1985 Jan;74(1):57–60. [PubMed] [Google Scholar]
- Takimoto T., Sato H., Ogura H., Glaser R. Rescue of a biologically active Epstein-Barr virus from nonproducer cells. Cancer Res. 1986 Apr;46(4 Pt 2):2085–2087. [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Yao Q. Y., Rickinson A. B., Epstein M. A. A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int J Cancer. 1985 Jan 15;35(1):35–42. doi: 10.1002/ijc.2910350107. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]