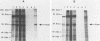Abstract
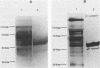
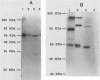
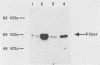
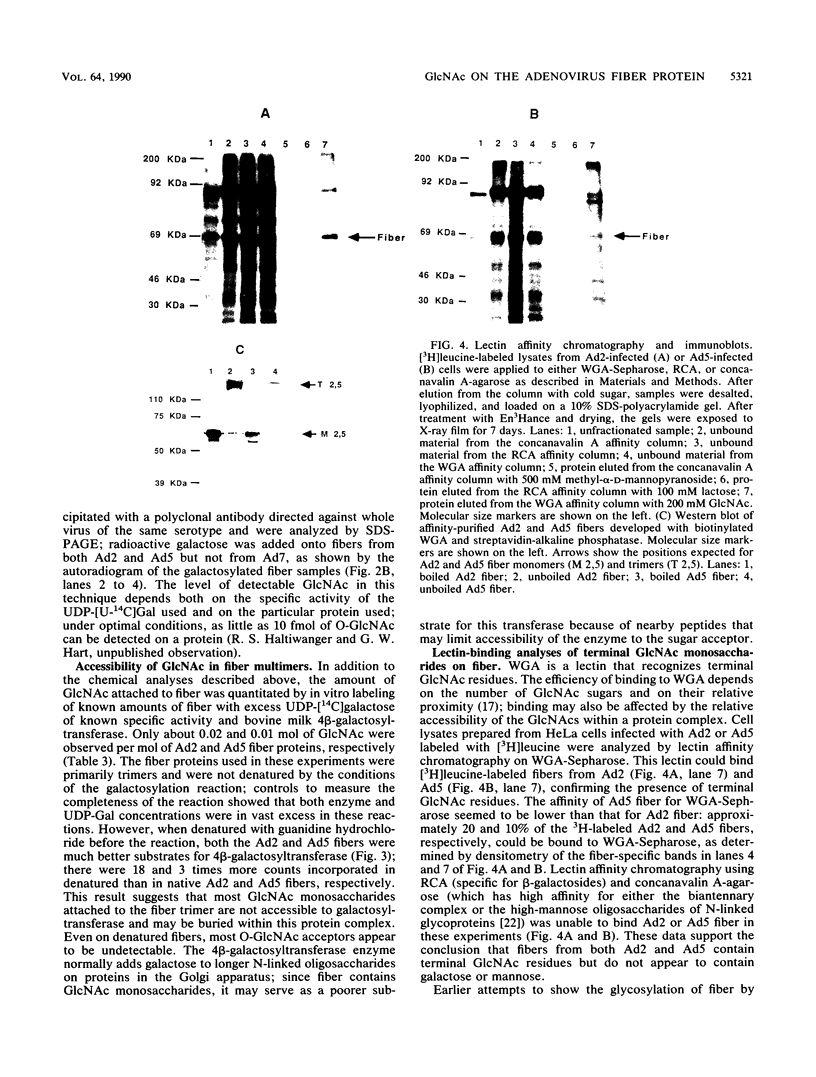
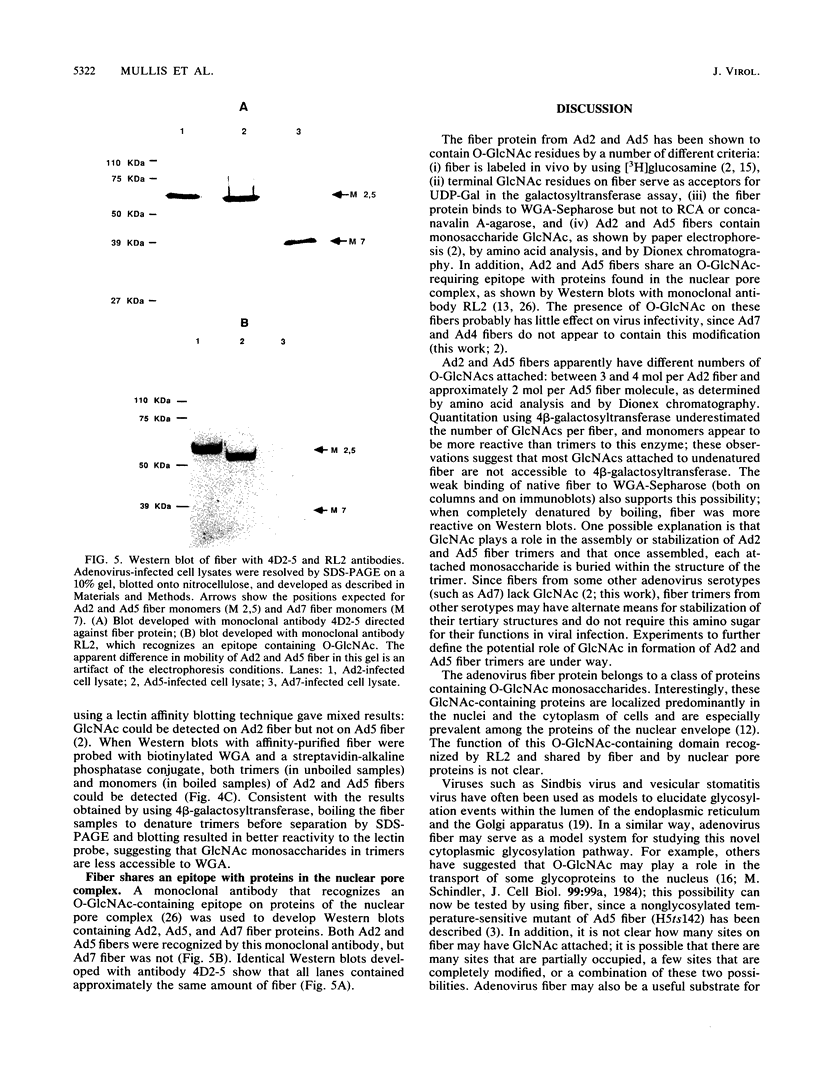
Fiber is an adenovirus capsid protein responsible for virus attachment to the cell surface and contains O-linked N-acetylglucosamine (GlcNAc). Results of both amino acid analysis and Dionex chromatography indicated that 3 to 4 and 1.7 to 2.5 mol of GlcNAc are attached per mol of affinity-purified adenovirus type 2 (Ad2) and Ad5 fibers, respectively. Fiber shares an epitope with nuclear pore proteins containing O-linked GlcNAc, as shown by reactivity to monoclonal antibody RL2 directed against these pore proteins. GlcNAc on fiber was found to serve as an acceptor for the transfer of galactose from UDP-galactose by 4 beta-galactosyl-transferase in Ad2 and Ad5 but not in Ad7; quantitation by labeling with UDP-[U-14C]galactose in this reaction gave a 100-fold-lower estimate of the GlcNAc content of fiber, suggesting that these monosaccharides are buried within fiber trimers and are not accessible to the transferase. Affinity chromatography on lectin-bound Sepharose beads showed that Ad2 and Ad5 fibers bound weakly to wheat germ agglutinin and did not bind to ricin or concanavalin A; weak binding to wheat germ agglutinin suggests either that GlcNAc is not easily accessible or that there are not sufficient GlcNAcs for efficient binding. These data suggest that O-linked GlcNAc might be important for Ad2 and Ad5 fiber assembly or stabilization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko D. M., Haltiwanger R. S., Hart G. W., Gibson W. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2573–2577. doi: 10.1073/pnas.85.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Boudin M. L., Strecker G., Michalski J. C. O-linked GlcNAc in serotype-2 adenovirus fibre. Eur J Biochem. 1989 Sep 1;184(1):205–211. doi: 10.1111/j.1432-1033.1989.tb15008.x. [DOI] [PubMed] [Google Scholar]
- Chee-Sheung C. C., Ginsberg H. S. Characterization of a temperature-sensitive fiber mutant of type 5 adenovirus and effect of the mutation on virion assembly. J Virol. 1982 Jun;42(3):932–950. doi: 10.1128/jvi.42.3.932-950.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroboczek J., Jacrot B. The sequence of adenovirus fiber: similarities and differences between serotypes 2 and 5. Virology. 1987 Dec;161(2):549–554. doi: 10.1016/0042-6822(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7552–7556. doi: 10.1073/pnas.84.21.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiwanger R. S., Holt G. D., Hart G. W. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990 Feb 15;265(5):2563–2568. [PubMed] [Google Scholar]
- Hanover J. A., Cohen C. K., Willingham M. C., Park M. K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987 Jul 15;262(20):9887–9894. [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Lee Y. C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988 Apr;170(1):54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Holt G. D., Haltiwanger R. S., Torres C. R., Hart G. W. Erythrocytes contain cytoplasmic glycoproteins. O-linked GlcNAc on Band 4.1. J Biol Chem. 1987 Nov 5;262(31):14847–14850. [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Scharff M. D., Maizel J. V., Jr Synthesis and assembly of adenovirus 2. I. Polypeptide synthesis, assembly of capsomeres, and morphogenesis of the virion. Virology. 1969 Dec;39(4):682–694. doi: 10.1016/0042-6822(69)90006-3. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. VI. Early and late glycopolypeptides. Virology. 1974 Apr;58(2):345–361. doi: 10.1016/0042-6822(74)90070-1. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1781–1785. doi: 10.1073/pnas.86.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Hart G. W. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989 Apr 21;57(2):243–251. doi: 10.1016/0092-8674(89)90962-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Merkle R. K., Cummings R. D. Lectin affinity chromatography of glycopeptides. Methods Enzymol. 1987;138:232–259. doi: 10.1016/0076-6879(87)38020-6. [DOI] [PubMed] [Google Scholar]
- Nyame K., Cummings R. D., Damian R. T. Schistosoma mansoni synthesizes glycoproteins containing terminal O-linked N-acetylglucosamine residues. J Biol Chem. 1987 Jun 15;262(17):7990–7995. [PubMed] [Google Scholar]
- Schindler M., Hogan M., Miller R., DeGaetano D. A nuclear specific glycoprotein representative of a unique pattern of glycosylation. J Biol Chem. 1987 Jan 25;262(3):1254–1260. [PubMed] [Google Scholar]
- Torres C. R., Hart G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984 Mar 10;259(5):3308–3317. [PubMed] [Google Scholar]