Abstract
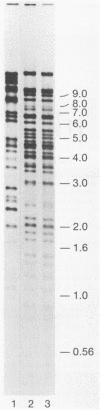
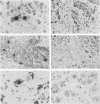
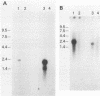
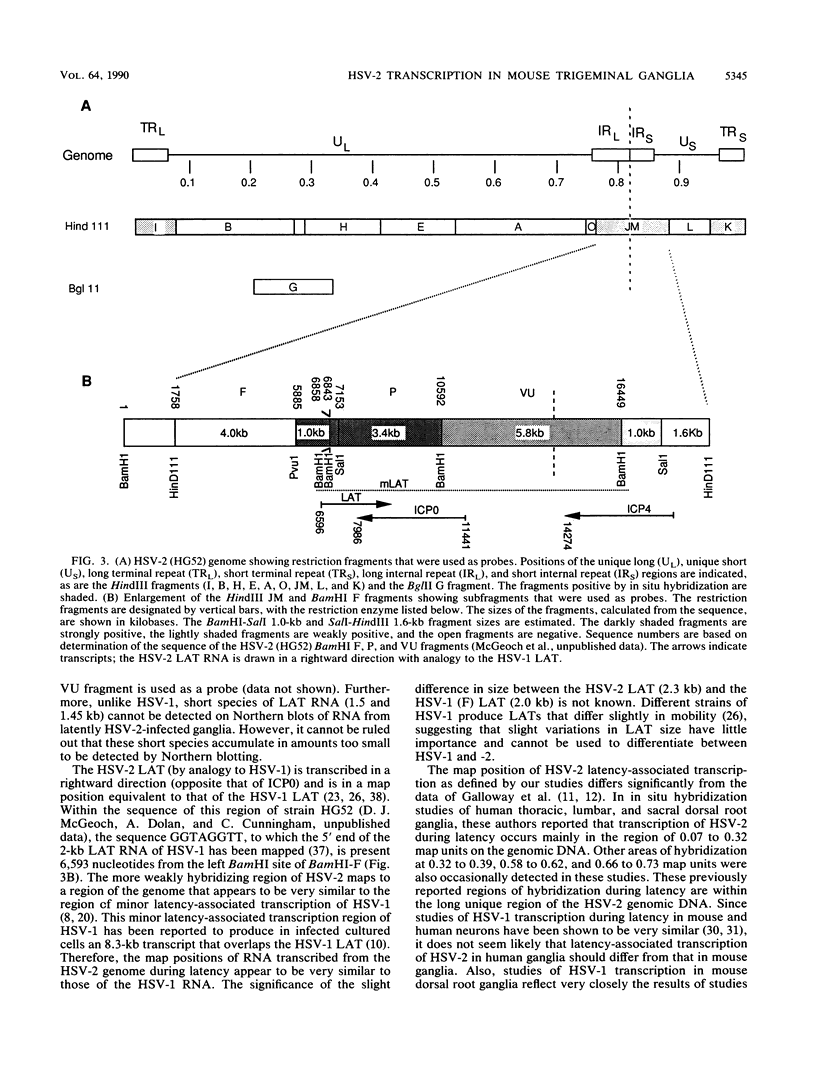
Using a cornea trigeminal ganglion model, we have investigated transcription by herpes simplex virus type 2 (HSV-2) during latency in mice. Latency was verified 2 months postinoculation by reactivation of HSV-2 after explant cocultivation of trigeminal ganglia from the majority of mice (83%). Transcription during latent HSV-2 infection was limited to the repeat regions of the viral genome as determined by in situ hybridization using restriction fragment probes representing 100% of the HSV-2 genome. Further mapping of the positively hybridizing region by using subfragments showed that transcription occurred from approximately 11.5 kb of contiguous DNA fragments. A 1.0-kb PvuI-BamHI fragment within the BamHI F fragment and a 0.3-kb BamHI-SalI fragment and a 3.4-kb SalI-BamHI fragment within the BamHI P fragment hybridized more strongly than other subfragments in in situ hybridization experiments. All positive signals were confined to the nucleus. The RNA that hybridized to the 3.4-kb SalI-BamHI DNA fragment probe by in situ hybridization corresponded to a 2.3-kb transcript on Northern (RNA) blots. Under our conditions for Northern blot hybridization, the 3.4-kb SalI-BamHI probe of HSV-2 hybridized to a limited degree with the latency-associated transcripts of HSV-1. Shorter spliced species of latency-associated transcript RNA, which are seen during HSV-1 latency, have not been detected in latent HSV-2 RNA. However, viral gene expression during HSV-2 latency appears to be very similar to that during HSV-1 latency.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Saadi S. A., Gross P., Wildy P. Herpes simplex virus type 2 latency in the footpad of mice: effect of acycloguanosine on the recovery of virus. J Gen Virol. 1988 Feb;69(Pt 2):433–438. doi: 10.1099/0022-1317-69-2-433. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Latency comes of age for herpesviruses. Cell. 1988 Mar 25;52(6):787–789. doi: 10.1016/0092-8674(88)90419-9. [DOI] [PubMed] [Google Scholar]
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Cheung A. K. Detection of pseudorabies virus transcripts in trigeminal ganglia of latently infected swine. J Virol. 1989 Jul;63(7):2908–2913. doi: 10.1128/jvi.63.7.2908-2913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements G. B., Subak-Sharpe J. H. Herpes simplex virus type 2 establishes latency in the mouse footpad. J Gen Virol. 1988 Feb;69(Pt 2):375–383. doi: 10.1099/0022-1317-69-2-375. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Straus S. E. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane S. L., Fraser N. W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989 Feb;63(2):943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C. M., McDougall J. K. Limited transcription of the herpes simplex virus genome when latent in human sensory ganglia. J Virol. 1982 Feb;41(2):686–691. doi: 10.1128/jvi.41.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Javier R. T., Stevens J. G., Dissette V. B., Wagner E. K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988 Sep;166(1):254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Krause P. R., Croen K. D., Straus S. E., Ostrove J. M. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J Virol. 1988 Dec;62(12):4819–4823. doi: 10.1128/jvi.62.12.4819-4823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynas C., Laycock K. A., Cook S. D., Hill T. J., Blyth W. A., Maitland N. J. Detection of herpes simplex virus type 1 gene expression in latently and productively infected mouse ganglia using the polymerase chain reaction. J Gen Virol. 1989 Sep;70(Pt 9):2345–2355. doi: 10.1099/0022-1317-70-9-2345. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Lirette R. P., Fraser N. W. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Gen Virol. 1990 Jan;71(Pt 1):125–132. doi: 10.1099/0022-1317-71-1-125. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Steiner I., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. A herpes simplex virus type 1 variant, deleted in the promoter region of the latency-associated transcripts, does not produce any detectable minor RNA species during latency in the mouse trigeminal ganglion. J Gen Virol. 1990 Apr;71(Pt 4):953–957. doi: 10.1099/0022-1317-71-4-953. [DOI] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E., Zaki S. A. Relation of cytohistopathology of genital herpesvirus infection to cervical anaplasia. Cancer Res. 1973 Jun;33(6):1452–1463. [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba M. Extraneural localisation of herpes simplex virus in latently infected guinea pigs. Nature. 1977 Jun 9;267(5611):529–531. doi: 10.1038/267529a0. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. R., Kern E. R., Richards J. T., Abbott T. M., Overall J. C., Jr Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982 Sep;146(3):397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Kit S., Myers M. G. Thymidine kinase-deficient herpes simplex virus type 2 genital infection in guinea pigs. J Virol. 1985 Aug;55(2):322–328. doi: 10.1128/jvi.55.2.322-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., O'Boyle D. R., 2nd, Lavi E., Fraser N. W. Latent herpes simplex virus type 1 transcription in human trigeminal ganglia. J Virol. 1988 Sep;62(9):3493–3496. doi: 10.1128/jvi.62.9.3493-3496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Haarr L., Porter D. D., Cook M. L., Wagner E. K. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988 Jul;158(1):117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Martin J. R. Herpes simplex virus type 2 transcripts in trigeminal ganglia during acute and latent infection in mice. J Neurol Sci. 1989 Nov;93(2-3):239–251. doi: 10.1016/0022-510x(89)90194-9. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dawson M., Ressel S. J., Dunstan M. E. Detection of herpes simplex virus mRNA in latently infected trigeminal ganglion neurons by in situ hybridization. Ann Neurol. 1982 Mar;11(3):285–291. doi: 10.1002/ana.410110309. [DOI] [PubMed] [Google Scholar]
- Timbury M. C. Temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1971 Nov;13(2):373–376. doi: 10.1099/0022-1317-13-2-373. [DOI] [PubMed] [Google Scholar]
- Vafai A., Murray R. S., Wellish M., Devlin M., Gilden D. H. Expression of varicella-zoster virus and herpes simplex virus in normal human trigeminal ganglia. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2362–2366. doi: 10.1073/pnas.85.7.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]