Abstract
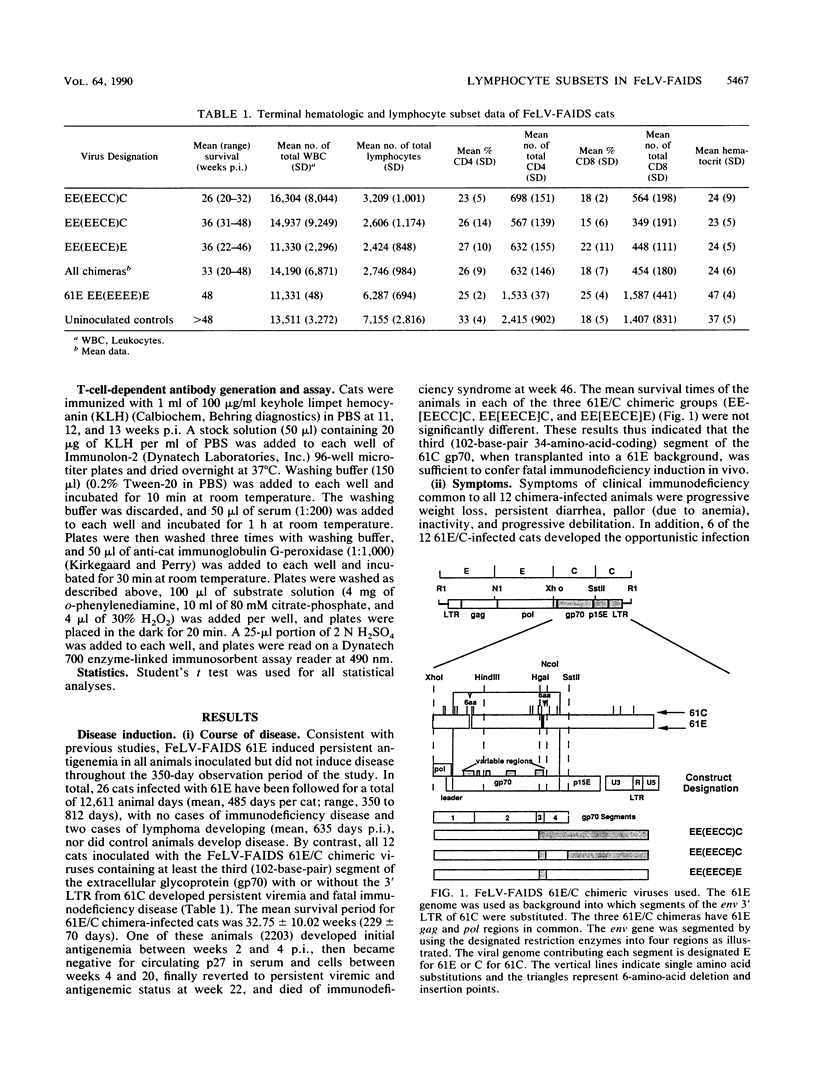
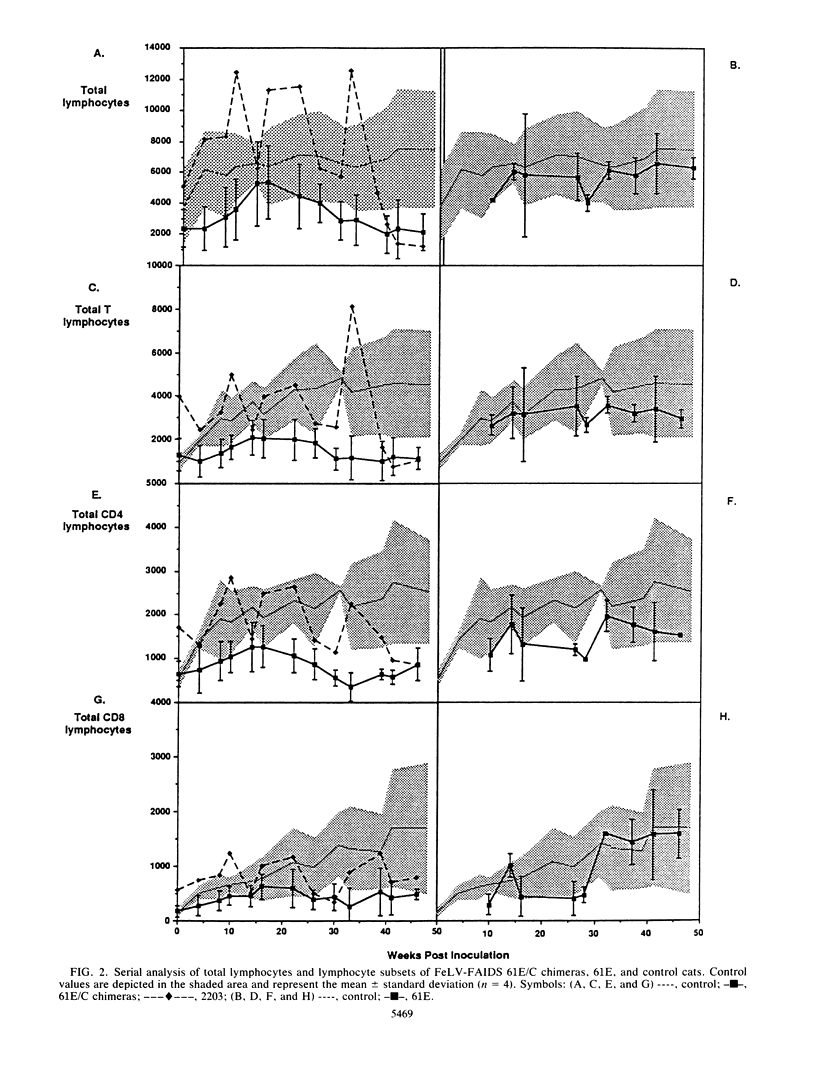
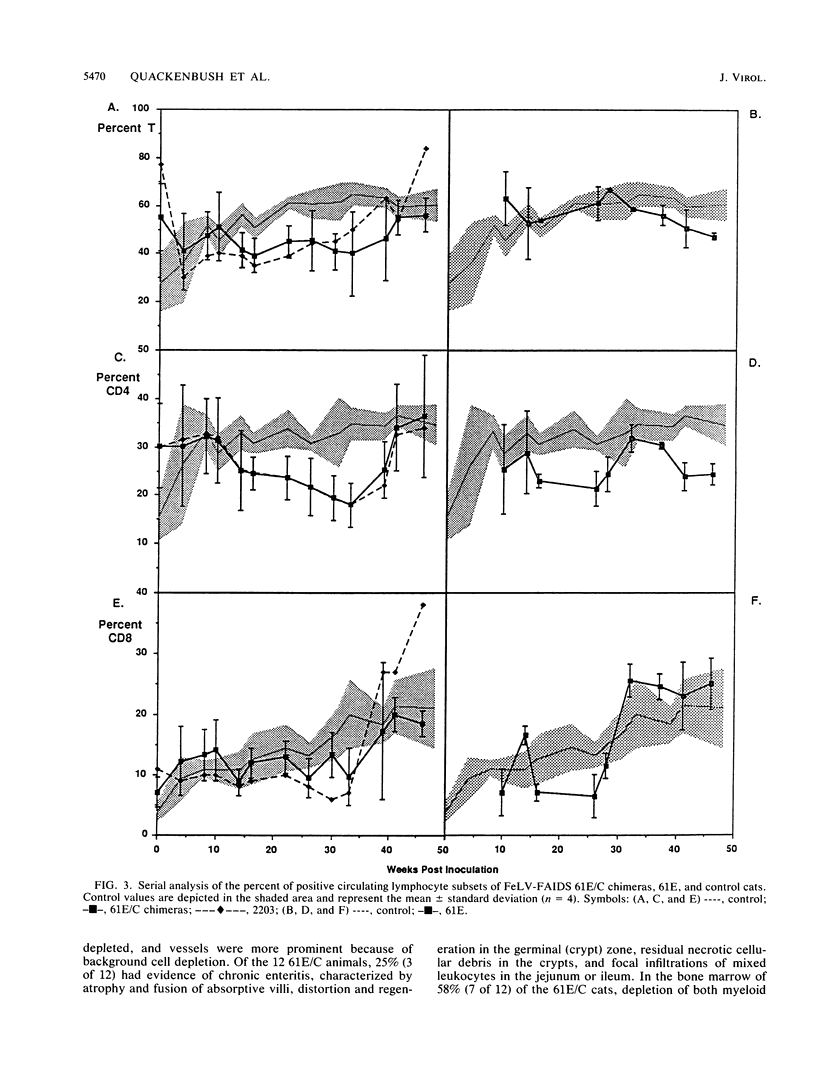
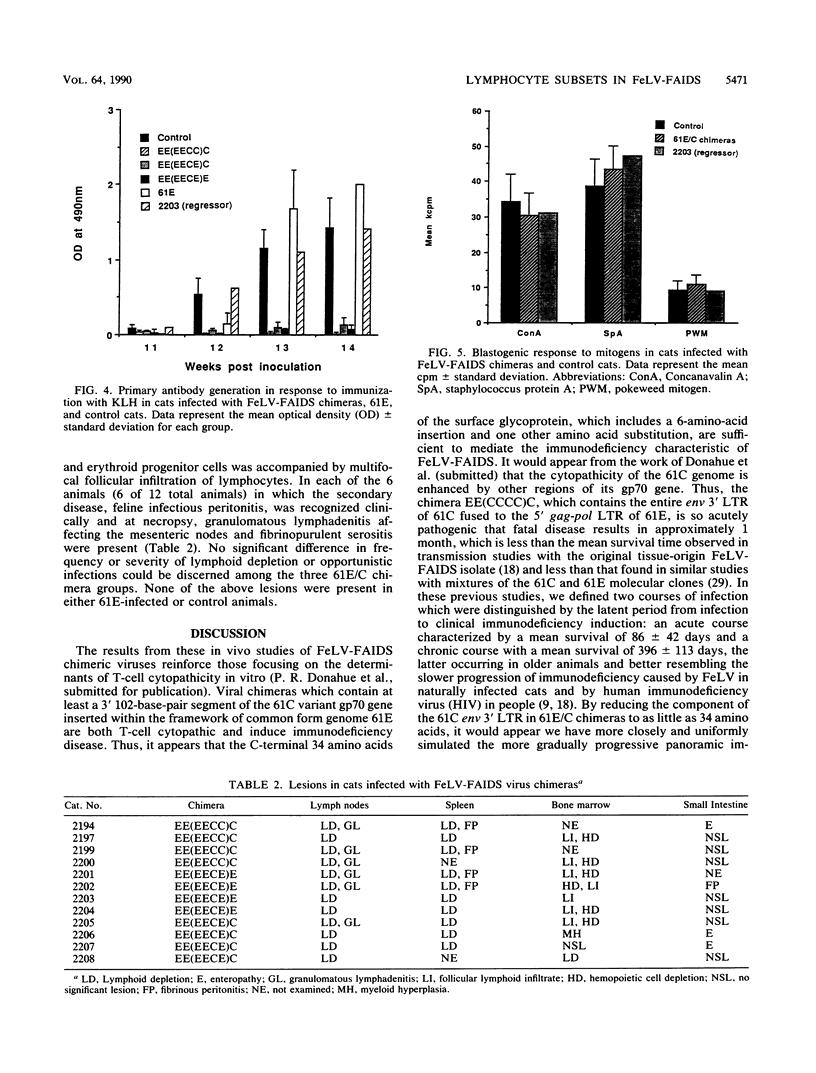
The FeLV-FAIDS strain of feline leukemia virus consistently induces fatal immunodeficiency. To investigate the immunopathogenesis and viral genetic determinants responsible for the induction of immunodeficiency disease in vivo, we have generated chimeras between the two major viral genomes in the original virus isolate, designated common form clone 61E and major variant clone 61C, which were molecularly cloned directly from DNA of the same animal and tissue. Each of three 61E/C chimeras, containing at minimum a 34-amino-acid segment (including a 6-amino-acid insertion and one amino acid substitution) near the C terminus of the 61C surface glycoprotein (gp70), induced fatal immunodeficiency disease in all (12 of 12) infected animals over a course of 33 +/- 10 weeks. By contrast, animals infected with virus 61E, although persistently antigenemic, remained asymptomatic throughout a 48-week observation period. Beginning 14 weeks after infection, a significant decrease (8 to 10%) in the percent of circulating CD4+ T lymphocytes developed in the 61E/C chimera-infected cats, compared with either 61E-infected or control animals. At this time, no significant changes were seen in CD8 cells, B cells, or mitogen-induced blastogenesis. Prior to this initial decline in CD4 cells, the ability of all antigenemic 61E/C-infected cats to generate a primary antibody response to the T-cell-dependent antigen keyhole limpet hemocyanin was markedly impaired, whereas all 61E-infected cats, one 61E/C-infected but nonviremic cat, and all uninfected control cats produced normal antibody responses. The results reported here demonstrate that a major determinant of in vivo immunodeficiency induction by FeLV-FAIDS is contained within a 34-amino-acid C-terminal segment of its surface glycoprotein and that this gp70 alteration determines the early and persistent deficits in CD4+ T lymphocytes and T-cell-dependent antibody responses. We hypothesize that these early immunologic alterations could result from early deletion of a CD4+ helper T-cell subset.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Hoover E. A., Cooper M. D. Identification of a CD4 homologue in the cat. Tissue Antigens. 1990 Feb;35(2):92–98. doi: 10.1111/j.1399-0039.1990.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Anderson L. J., Jarrett W. F., Jarrett O., Laird H. M. Feline leukemia-virus infection of kittens: mortality associated with atrophy of the thymus and lymphoid depletion. J Natl Cancer Inst. 1971 Oct;47(4):807–817. [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Bernstein D. C., Mann D. L., Shearer G. M., Berzofsky J. A. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature. 1989 Jun 1;339(6223):383–385. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Hoover E. A. Inhibition of normal lymphocyte mitogenic reactivity by serum from feline leukemia virus-infected cats. Cancer Res. 1977 Nov;37(11):3985–3989. [PubMed] [Google Scholar]
- Cockerell G. L., Hoover E. A., Krakowka S., Olsen R. G., Yohn D. S. Lymphocyte mitogen reactivity and enumeration of circulating B- and T-cells during feline leukemia virus infection in the cat. J Natl Cancer Inst. 1976 Nov;57(5):1095–1099. doi: 10.1093/jnci/57.5.1095. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Krakowka S., Hoover E. A., Olsen R. G., Yohn D. S. Characterization of feline T-and B-lymphocytes and identification of an experimentally induced T-cell neoplasm in the cat. J Natl Cancer Inst. 1976 Oct;57(4):907–913. doi: 10.1093/jnci/57.4.907. [DOI] [PubMed] [Google Scholar]
- Copelan E. A., Rinehart J. J., Lewis M., Mathes L., Olsen R., Sagone A. The mechanism of retrovirus suppression of human T cell proliferation in vitro. J Immunol. 1983 Oct;131(4):2017–2020. [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda L. A., Butler J. J., Mansell P., Hersh E. M., Reuben J., Newell G. R. Lymphadenopathy in homosexual men. Morbid anatomy with clinical and immunologic correlations. Am J Clin Pathol. 1983 May;79(5):559–568. doi: 10.1093/ajcp/79.5.559. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Hirshaut Y., Hess P. Detection of the feline leukemia virus and other mammalian oncornaviruses by immunofluorescence. Bibl Haematol. 1973;39:778–799. doi: 10.1159/000427906. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr Immunopathology induced by the feline leukemia virus. Springer Semin Immunopathol. 1982;5(1):75–106. doi: 10.1007/BF00201958. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Mathes L. E., Rojko J. L., Schaller J. P., Olsen R. G. Modifications of the immunofluorescence assay for feline leukemia virus group-specific antigens. Am J Vet Res. 1978 Dec;39(12):1877–1880. [PubMed] [Google Scholar]
- Hoover E. A., McCullough C. B., Griesemer R. A. Intranasal transmission of feline leukemia. J Natl Cancer Inst. 1972 Apr;48(4):973–983. [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I., Quackenbush S. L., Gasper P. W. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987 Dec;70(6):1880–1892. [PubMed] [Google Scholar]
- Hoover E. A., Perryman L. E., Kociba G. J. Early lesions in cats inoculated with feline leukemia virus. Cancer Res. 1973 Jan;33(1):145–152. [PubMed] [Google Scholar]
- Klotz F. W., Cooper M. D. A feline thymocyte antigen defined by a monoclonal antibody (FT2) identifies a subpopulation of non-helper cells capable of specific cytotoxicity. J Immunol. 1986 Apr 1;136(7):2510–2514. [PubMed] [Google Scholar]
- Klotz F. W., Gathings W. E., Cooper M. D. Development and distribution of B lineage cells in the domestic cat: analysis with monoclonal antibodies to cat mu-, gamma-, kappa-, and lambda-chains and heterologous anti-alpha antibodies. J Immunol. 1985 Jan;134(1):95–100. [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lutz H., Pedersen N. C., Durbin R., Theilen G. H. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods. 1983 Jan 28;56(2):209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P. Abrogation of lymphocyte blastogenesis by a feline leukaemia virus protein. Nature. 1978 Aug 17;274(5672):687–689. doi: 10.1038/274687a0. [DOI] [PubMed] [Google Scholar]
- Miedema F., Petit A. J., Terpstra F. G., Schattenkerk J. K., de Wolf F., Al B. J., Roos M., Lange J. M., Danner S. A., Goudsmit J. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988 Dec;82(6):1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Chen C. S., Hoover E. A. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986 Jan 23;319(6051):333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- Orosz C. G., Zinn N. E., Olsen R. G., Mathes L. E. Retrovirus-mediated immunosuppression. I. FeLV-UV and specific FeLV proteins alter T lymphocyte behavior by inducing hyporesponsiveness to lymphokines. J Immunol. 1985 May;134(5):3396–3403. [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Boyle J. F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am J Vet Res. 1981 Mar;42(3):368–377. [PubMed] [Google Scholar]
- Pedersen N. C., Boyle J. F., Floyd K. Infection studies in kittens, using feline infectious peritonitis virus propagated in cell culture. Am J Vet Res. 1981 Mar;42(3):363–367. [PubMed] [Google Scholar]
- Perryman L. E., Hoover E. A., Yohn D. S. Immunologic reactivity of the cat: immunosuppression in experimental feline leukemia. J Natl Cancer Inst. 1972 Nov;49(5):1357–1365. [PubMed] [Google Scholar]
- Poss M. L., Mullins J. I., Hoover E. A. Posttranslational modifications distinguish the envelope glycoprotein of the immunodeficiency disease-inducing feline leukemia virus retrovirus. J Virol. 1989 Jan;63(1):189–195. doi: 10.1128/jvi.63.1.189-195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Finn B. L., Olsen R. G. Characterization and mitogenesis of feline lymphocyte populations. Int Arch Allergy Appl Immunol. 1982;68(3):226–232. doi: 10.1159/000233103. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Mathes L. E., Olsen R. G., Schaller J. P. Pathogenesis of experimental feline leukemia virus infection. J Natl Cancer Inst. 1979 Sep;63(3):759–768. doi: 10.1093/jnci/63.3.759. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Quackenbush S. L., Olsen R. G. Reactivation of latent feline leukaemia virus infection. Nature. 1982 Jul 22;298(5872):385–388. doi: 10.1038/298385a0. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Bernstein D. C., Tung K. S., Via C. S., Redfield R., Salahuddin S. Z., Gallo R. C. A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS). J Immunol. 1986 Oct 15;137(8):2514–2521. [PubMed] [Google Scholar]
- Snyder H. W., Jr, Hardy W. D., Jr, Zuckerman E. E., Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature. 1978 Oct 19;275(5681):656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- Teeuwsen V. J., Logtenberg T., Siebelink K. H., Lange J. M., Goudsmit J., Uytdehaag F. G., Osterhaus A. D. Analysis of the antigen- and mitogen-induced differentiation of B lymphocytes from asymptomatic human immunodeficiency virus-seropositive male homosexuals. Discrepancy between T cell-dependent and T cell-independent activation. J Immunol. 1987 Nov 1;139(9):2929–2935. [PubMed] [Google Scholar]
- Tompkins M. B., Ogilvie G. K., Gast A. M., Franklin R., Weigel R., Tompkins W. A. Interleukin-2 suppression in cats naturally infected with feline leukemia virus. J Biol Response Mod. 1989 Feb;8(1):86–96. [PubMed] [Google Scholar]
- Trainin Z., Wernicke D., Ungar-Waron H., Essex M. Suppression of the humoral antibody response in natural retrovirus infections. Science. 1983 May 20;220(4599):858–859. doi: 10.1126/science.6302837. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Temin H. M. Cell killing by avian leukosis viruses. J Virol. 1981 Sep;39(3):713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke D., Trainin Z., Ungar-Waron H., Essex M. Humoral immune response of asymptomatic cats naturally infected with feline leukemia virus. J Virol. 1986 Nov;60(2):669–673. doi: 10.1128/jvi.60.2.669-673.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. G., Griesemer R. A. Feline infectious peritonitis. Pathol Vet. 1966;3(3):255–270. doi: 10.1177/030098586600300309. [DOI] [PubMed] [Google Scholar]



