Abstract
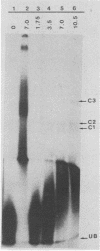
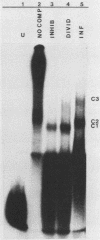
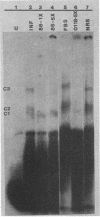
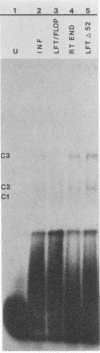
Replication of parvoviruses requires cis signals located in terminal palindromes that function as origins of replication in conjunction with trans-acting viral and cellular proteins. A gel retardation assay was used to identify proteins in crude nuclear extracts of bovine parvovirus (BPV)-infected bovine fetal lung cells that interact with the hairpinned left end (3' OH terminus of the viral minus strand in the flop conformation) of BPV. Three specific DNA-protein complexes formed. One complex was shown to involve a BPV structural protein(s) by inhibiting its formation when antiserum specific for these BPV proteins was used. By specific competition with serum containing antibodies against the BPV nonstructural proteins, a second complex was shown to involve a BPV nonstructural protein. A third complex contained protein of cellular origin and was also formed with extracts of uninfected bovine fetal lung cells. DNA competition assays suggest that the viral proteins do not bind to the right hairpin, which differs in sequence and secondary structure from the left terminus, or to a BPV terminus that lacks the first 52 nucleotides, preventing formation of the stem of the hairpin. The cellular protein is regulated in a cell cycle-dependent fashion, with its binding activity increased in uninfected, actively dividing cells compared with contact-inhibited cells. Since autonomous parvovirus replication requires an S-phase factor for progeny formation, the terminal binding protein demonstrated here is a candidate for this factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashktorab H., Srivastava A. Identification of nuclear proteins that specifically interact with adeno-associated virus type 2 inverted terminal repeat hairpin DNA. J Virol. 1989 Jul;63(7):3034–3039. doi: 10.1128/jvi.63.7.3034-3039.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Chow M. B., Ward D. C. Sequence analysis of the termini of virion and replicative forms of minute virus of mice DNA suggests a modified rolling hairpin model for autonomous parvovirus DNA replication. J Virol. 1985 Apr;54(1):171–177. doi: 10.1128/jvi.54.1.171-177.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalosse B. L., Barrijal S., Chen Y. Q., Cassiman J. J., Rommelaere J. Identification of a transformation-sensitive nuclear protein from normal human fibroblasts that specifically interacts with minute virus of mice DNA and correlates with cell resistance to the parvovirus. Mol Carcinog. 1989;2(5):245–251. doi: 10.1002/mc.2940020504. [DOI] [PubMed] [Google Scholar]
- Blundell M. C., Astell C. R. A GC-box motif upstream of the B19 parvovirus unique promoter is important for in vitro transcription. J Virol. 1989 Nov;63(11):4814–4823. doi: 10.1128/jvi.63.11.4814-4823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Tyson J. J., Lederman M., Stout E. R., Bates R. C. A kinetic hairpin transfer model for parvoviral DNA replication. J Mol Biol. 1989 Jul 20;208(2):283–296. doi: 10.1016/0022-2836(89)90389-6. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Cross F., Roberts J., Weintraub H. Simple and complex cell cycles. Annu Rev Cell Biol. 1989;5:341–396. doi: 10.1146/annurev.cb.05.110189.002013. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M., Prigent-Tiravy S., Wicker R. Kilham rat virus DNA replication in subcellular fractions. J Gen Virol. 1984 Nov;65(Pt 11):2021–2031. doi: 10.1099/0022-1317-65-11-2021. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. Factors that bind to adeno-associated virus terminal repeats. J Virol. 1989 Jul;63(7):3095–3104. doi: 10.1128/jvi.63.7.3095-3104.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kollek R., Tseng B. Y., Goulian M. DNA polymerase requirements for parvovirus H-1 DNA replication in vitro. J Virol. 1982 Mar;41(3):982–989. doi: 10.1128/jvi.41.3.982-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Cotmore S. F., Stout E. R., Bates R. C. Detection of bovine parvovirus proteins homologous to the nonstructural NS-1 proteins of other autonomous parvoviruses. J Virol. 1987 Nov;61(11):3612–3616. doi: 10.1128/jvi.61.11.3612-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Patton J. T., Stout E. R., Bates R. C. Virally coded noncapsid protein associated with bovine parvovirus infection. J Virol. 1984 Feb;49(2):315–318. doi: 10.1128/jvi.49.2.315-318.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Shull B. C., Stout E. R., Bates R. C. Bovine parvovirus DNA-binding proteins: identification by a combined DNA hybridization and immunodetection assay. J Gen Virol. 1987 Jan;68(Pt 1):147–157. doi: 10.1099/0022-1317-68-1-147. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., D'Urso G. An origin unwinding activity regulates initiation of DNA replication during mammalian cell cycle. Science. 1988 Sep 16;241(4872):1486–1489. doi: 10.1126/science.2843984. [DOI] [PubMed] [Google Scholar]
- Robertson A. T., Stout E. R., Bates R. C. Aphidicolin inhibition of the production of replicative-form DNA during bovine parvovirus infection. J Virol. 1984 Mar;49(3):652–657. doi: 10.1128/jvi.49.3.652-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Srivastava A., Berns K. I., Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983 May;33(1):135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Shull B. C., Chen K. C., Lederman M., Stout E. R., Bates R. C. Genomic clones of bovine parvovirus: construction and effect of deletions and terminal sequence inversions on infectivity. J Virol. 1988 Feb;62(2):417–426. doi: 10.1128/jvi.62.2.417-426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. O., Samulski R. J., Muzyczka N. In vitro resolution of covalently joined AAV chromosome ends. Cell. 1990 Jan 12;60(1):105–113. doi: 10.1016/0092-8674(90)90720-y. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Grafstrom R. H., Revie D., Oertel W., Goulian M. Studies on early intermediates in the synthesis of DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):263–270. doi: 10.1101/sqb.1979.043.01.032. [DOI] [PubMed] [Google Scholar]
- Tyson J. J., Chen K. C., Lederman M., Bates R. C. Analysis of the kinetic hairpin transfer model for parvoviral DNA replication. J Theor Biol. 1990 May 22;144(2):155–169. doi: 10.1016/s0022-5193(05)80316-9. [DOI] [PubMed] [Google Scholar]
- Willwand K., Kaaden O. R. Proteins of viral and cellular origin bind to the Aleutian disease virus (ADV) DNA 3'-terminal hairpin: presentation of a scheme for encapsidation of ADV DNA. J Virol. 1990 Apr;64(4):1598–1605. doi: 10.1128/jvi.64.4.1598-1605.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Callaham M. F., Huberman E. Perturbation of the cell cycle by adeno-associated virus. Virology. 1988 Dec;167(2):393–399. [PubMed] [Google Scholar]
- Wobbe C. R., Mitra S. Proteins tightly associated with the termini of replicative form DNA of Kilham rat virus, an autonomous parvovirus. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8335–8339. doi: 10.1073/pnas.82.24.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter S., Richards R., Armentrout R. W. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim Biophys Acta. 1980 May 30;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]