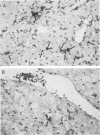
Abstract
Intracerebral inoculation of Theiler's murine encephalomyelitis virus into susceptible strains of mice produces chronic demyelinating disease in the central nervous system characterized by persistent viral infection. Immunogenetic data suggest that genes from both major histocompatibility complex (MHC) and non-MHC loci are important in determining susceptibility or resistance to demyelination. The role of the MHC in determining resistance or susceptibility to disease can be interpreted either as the presence of antigen-presenting molecules that confer resistance to viral infection or as the ability of MHC products to contribute to pathogenesis by acting as viral receptors or by mediating immune attack against virally infected cells. These alternatives can be distinguished by determining whether the contribution of the MHC to resistance is inherited as a recessive or dominant trait. Congenic mice with different MHC haplotypes on identical B10 backgrounds were crossed and quantitatively analyzed for demyelination, infectious virus, and local virus antigen production. F1 hybrid progeny derived from resistant B10 (H-2b), B10.D2 (H-2d), or B10.K (H-2k) and susceptible B10.R111 (H-2r), B10.M (H-2f), or B10.BR (H-2k) parental mice exhibited no or minimal demyelination, indicating that on a B10 background, resistance is inherited as a dominant trait. Although infectious virus, as measured by viral plaque assay, was cleared inefficiently from the central nervous systems of resistant F1 hybrid progeny mice, we found a direct correlation between local viral antigen production and demyelination. These data are consistent with our hypothesis that the immunological basis for resistance is determined by efficient presentation of the viral antigen to the immune system, resulting in local virus clearance and absence of subsequent demyelination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennink J. R., Yewdell J. W. Murine cytotoxic T lymphocyte recognition of individual influenza virus proteins. High frequency of nonresponder MHC class I alleles. J Exp Med. 1988 Nov 1;168(5):1935–1939. doi: 10.1084/jem.168.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore W. F., Welsh C. J., Tonks P., Nash A. A. Observations on demyelinating lesions induced by Theiler's virus in CBA mice. Acta Neuropathol. 1988;76(6):581–589. doi: 10.1007/BF00689596. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Chamorro M., Aubert C., Brahic M. Demyelinating lesions due to Theiler's virus are associated with ongoing central nervous system infection. J Virol. 1986 Mar;57(3):992–997. doi: 10.1128/jvi.57.3.992-997.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch R. J., Melvold R. W., Miller S. D., Lipton H. L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985 Aug;135(2):1408–1414. [PubMed] [Google Scholar]
- DANIELS J. B., PAPPENHEIMER A. M., RICHARDSON S. Observations on encephalomyelitis of mice (DA strain). J Exp Med. 1952 Dec;96(6):517–530. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Biddison W. E., Bennink J. R., Knowles B. B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J Exp Med. 1978 Aug 1;148(2):534–543. doi: 10.1084/jem.148.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Puthavathana P., Izui S., Lambert P. H. Induction of acute thrombocytopenia and infection of megakaryocytes by Rauscher murine leukemia virus reflect the genetic susceptibility to leukemogenesis. J Exp Med. 1983 Mar 1;157(3):1028–1039. doi: 10.1084/jem.157.3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F., Ribalta T., Campo E., Monforte R., Urbano A., Rozman C. Immunohistochemical analysis of the immune reaction in the nervous system in paraneoplastic encephalomyelitis. Neurology. 1990 Feb;40(2):219–222. doi: 10.1212/wnl.40.2.219. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Bologa L., Siegel L., Londe H. Theiler's virus in brain cell cultures: lysis of neurons and oligodendrocytes and persistence in astrocytes and macrophages. J Neurosci Res. 1986;15(4):491–501. doi: 10.1002/jnr.490150406. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Pan S., Knowles B. B., Tevethia S. S. Recognition of herpes simplex virus antigens on the surface of mouse cells of the H-2b haplotype by virus-specific cytotoxic T lymphocytes. J Immunol. 1984 Jan;132(1):475–481. [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Delayed-type hypersensitivity responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1987 Sep;68(Pt 9):2379–2388. doi: 10.1099/0022-1317-68-9-2379. [DOI] [PubMed] [Google Scholar]
- Lehrich J. R., Arnason B. G., Hochberg F. H. Demyelinative myelopathy in mice induced by the DA virus. J Neurol Sci. 1976 Oct;29(2-4):149–160. doi: 10.1016/0022-510x(76)90167-2. [DOI] [PubMed] [Google Scholar]
- Lindsley M. D., Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989 Apr 15;142(8):2677–2682. [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. The TO strains of Theiler's viruses cause "slow virus-like" infections in mice. Ann Neurol. 1979 Jul;6(1):25–28. doi: 10.1002/ana.410060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Melvold R. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J Immunol. 1984 Apr;132(4):1821–1825. [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvold R. W., Jokinen D. M., Knobler R. L., Lipton H. L. Variations in genetic control of susceptibility to Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. J Immunol. 1987 Mar 1;138(5):1429–1433. [PubMed] [Google Scholar]
- Melvold R. W., Jokinen D. M., Miller S. D., Dal Canto M. C., Lipton H. L. Identification of a locus on mouse chromosome 3 involved in differential susceptibility to Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1990 Feb;64(2):686–690. doi: 10.1128/jvi.64.2.686-690.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill H. C., Blanden R. V. Mechanisms determining innate resistance to ectromelia virus infection in C57BL mice. Infect Immun. 1983 Sep;41(3):1391–1394. doi: 10.1128/iai.41.3.1391-1394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paya C. V., Patick A. K., Leibson P. J., Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J Immunol. 1989 Jul 1;143(1):95–102. [PubMed] [Google Scholar]
- Penney J. B., Jr, Wolinsky J. S. Neuronal and oligodendroglial infection by the WW strain of Theiler's virus. Lab Invest. 1979 Mar;40(3):324–330. [PubMed] [Google Scholar]
- Rodriguez M., David C. S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985 Sep;135(3):2145–2148. [PubMed] [Google Scholar]
- Rodriguez M., Lafuse W. P., Leibowitz J., David C. S. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology. 1986 Jul;36(7):964–970. doi: 10.1212/wnl.36.7.964. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J. L., Powell H. C., Lampert P. W. Neonatal infection with the Daniels strain of Theiler's murine encephalomyelitis virus. Lab Invest. 1983 Dec;49(6):672–679. [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J., David C. S. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986 Mar 1;163(3):620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Patick A. K., Pease L. R. Abrogation of resistance to Theiler's virus-induced demyelination in C57BL mice by total body irradiation. J Neuroimmunol. 1990 Mar;26(3):189–199. doi: 10.1016/0165-5728(90)90001-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Rodriguez M. Virus-induced demyelination in mice: "dying back" of oligodendrocytes. Mayo Clin Proc. 1985 Jul;60(7):433–438. doi: 10.1016/s0025-6196(12)60865-9. [DOI] [PubMed] [Google Scholar]
- Thomsen A. R., Marker O. MHC and non-MHC genes regulate elimination of lymphocytic choriomeningitis virus and antiviral cytotoxic T lymphocyte and delayed-type hypersensitivity mediating T lymphocyte activity in parallel. J Immunol. 1989 Feb 15;142(4):1333–1341. [PubMed] [Google Scholar]
- Vidović D., Matzinger P. Unresponsiveness to a foreign antigen can be caused by self-tolerance. Nature. 1988 Nov 17;336(6196):222–225. doi: 10.1038/336222a0. [DOI] [PubMed] [Google Scholar]
- Zurbriggen A., Fujinami R. S. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989 Apr;63(4):1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]