Abstract
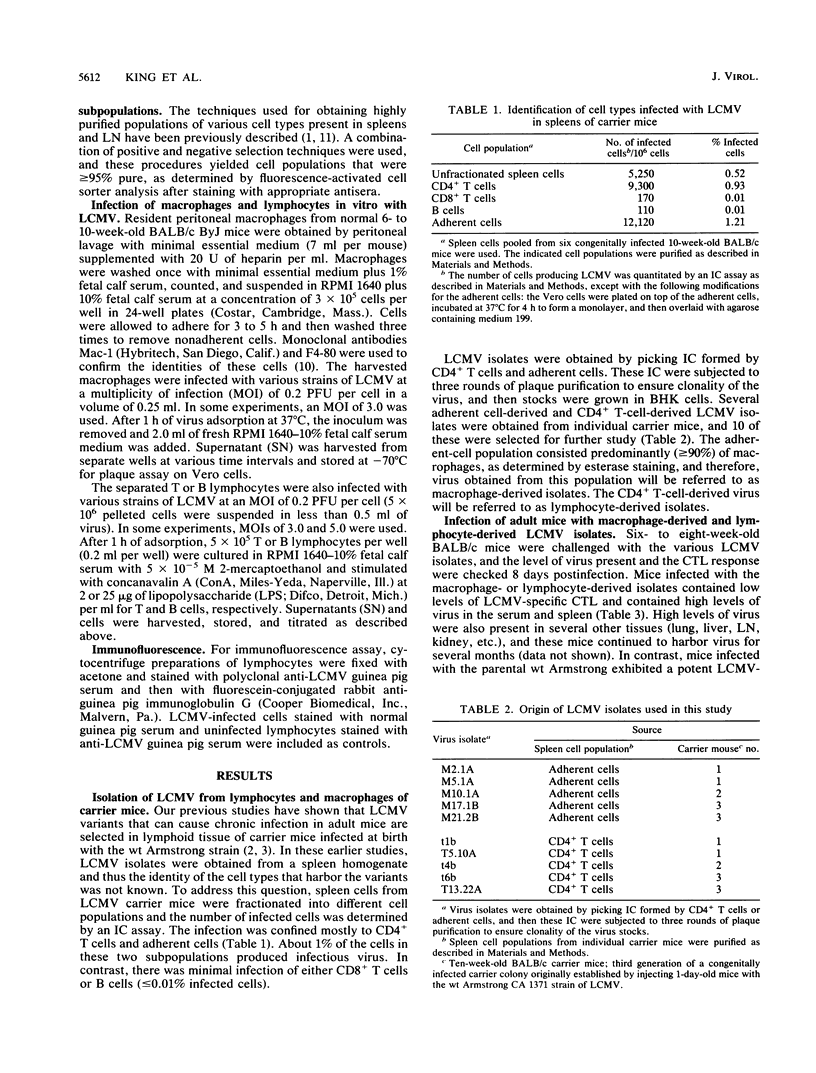
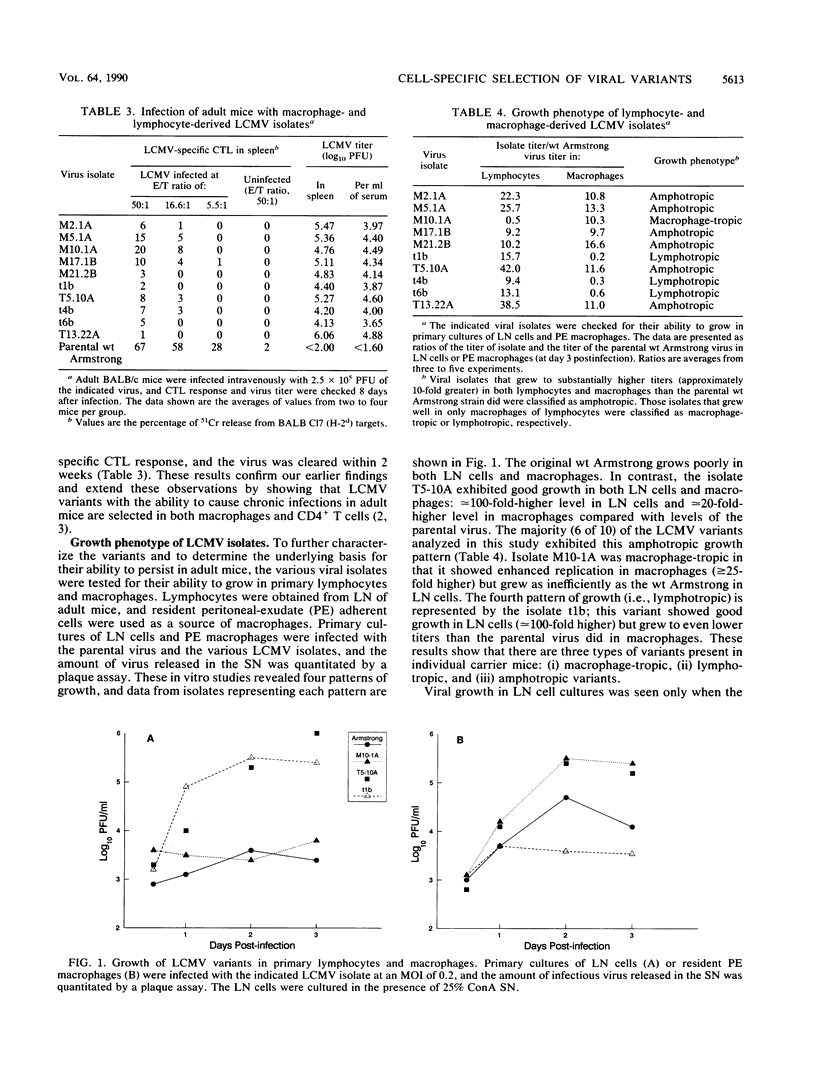
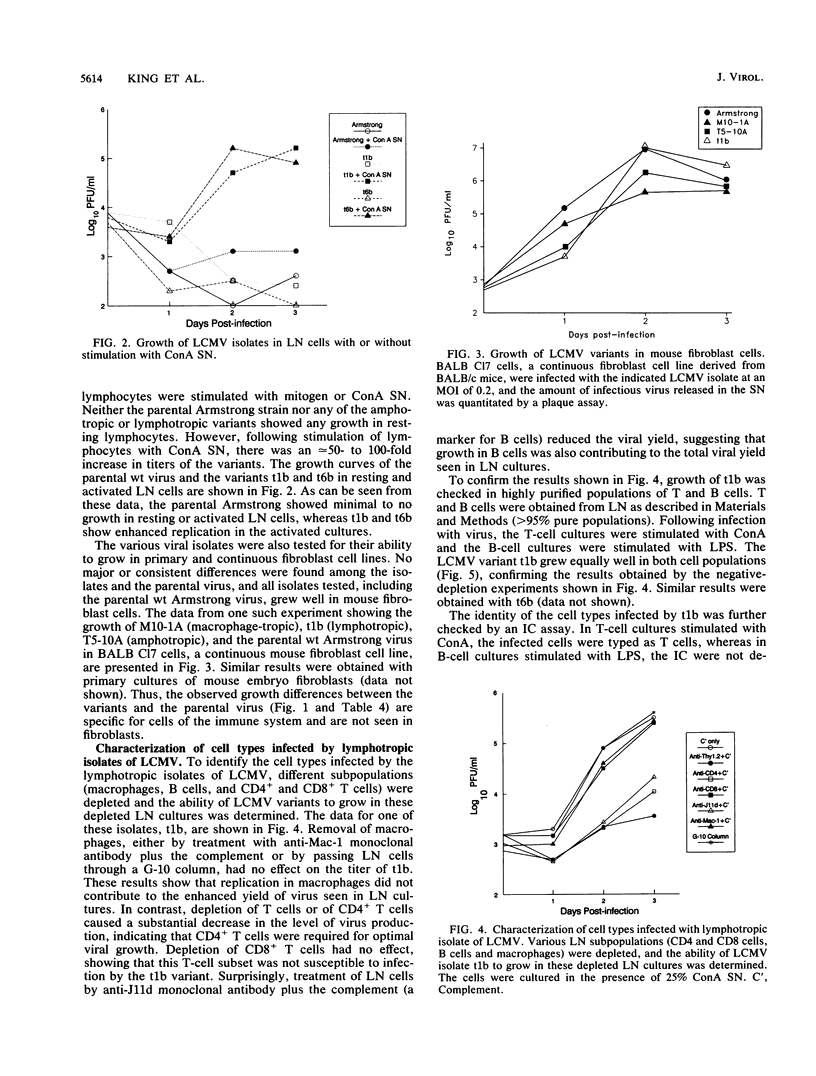
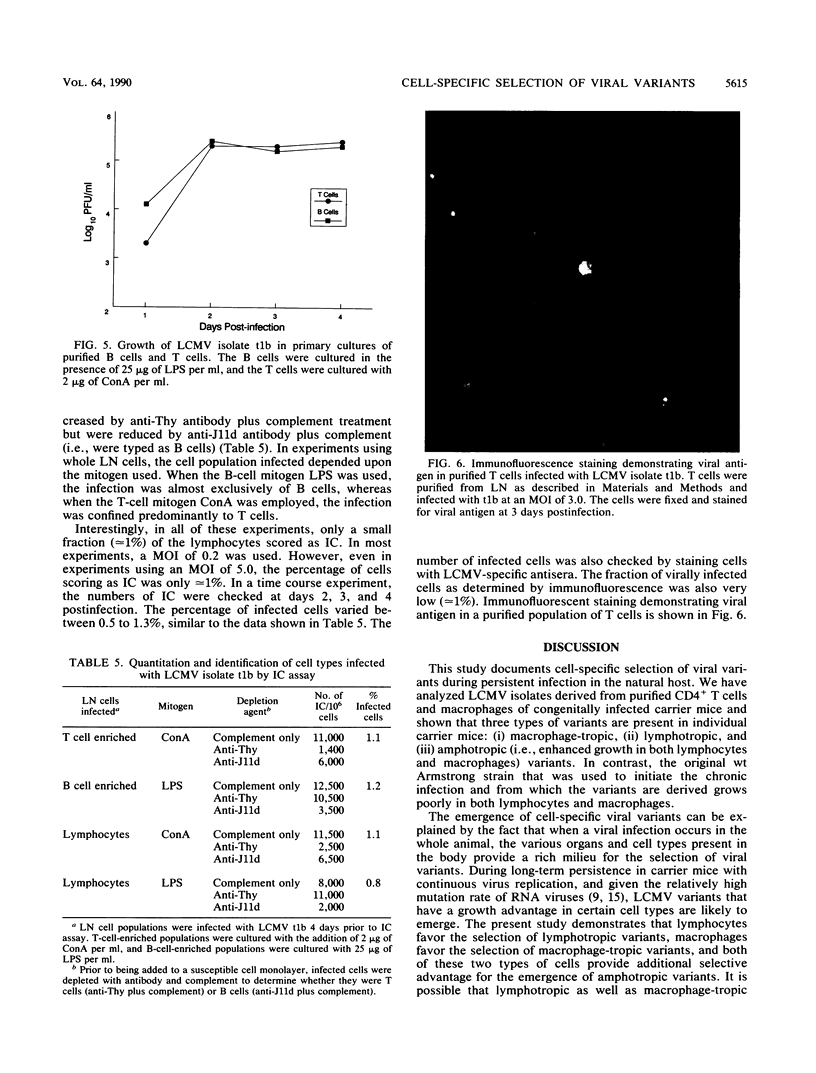
This study demonstrates cell-specific selection of viral variants during persistent lymphocytic choriomeningitis virus infection in its natural host. We have analyzed viral isolates obtained from CD4+ T cells and macrophages of congenitally infected carrier mice and found that three types of variants are present in individual carrier mice: (i) macrophage-tropic, (ii) lymphotropic, and (iii) amphotropic. The majority of the isolates were amphotropic and exhibited enhanced growth in both lymphocytes and macrophages. However, some of the lymphocyte-derived isolates grew well in lymphocytes but poorly in macrophages, and a macrophage-derived isolate replicated well in macrophages but not in lymphocytes. In striking contrast, the original wild-type (wt) Armstrong strain of lymphocytic choriomeningitis virus that was used to initiate the chronic infection and from which the variants are derived grew poorly in both lymphocytes and macrophages. These three types of variants also differed from the parental virus in their ability to establish a chronic infection in immunocompetent hosts. Adult mice infected with the wt Armstrong strain cleared the infection within 2 weeks, whereas adult mice infected with the variants harbored virus for several months. These results suggest that the ability of the variants to persist in adult mice is due to enhanced replication in macrophages and/or lymphocytes. This conclusion is further strengthened by the finding that the variants and the parental wt virus grew equally well in mouse fibroblasts and that the observed growth differences were specific for cells of the immune system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., King C. C., Oldstone M. B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987 May;61(5):1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Oldstone M. B. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988 May 1;167(5):1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn S., Rutledge R., Folks T., Gold J., Baker L., McCormick J., Feorino P., Piot P., Quinn T., Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985 Nov 22;230(4728):949–951. doi: 10.1126/science.2997922. [DOI] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Perry V. H., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec. 1984 Nov;210(3):503–512. doi: 10.1002/ar.1092100311. [DOI] [PubMed] [Google Scholar]
- Jamieson B. D., Butler L. D., Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987 Dec;61(12):3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Popescu M., Löhler J., Lehmann-Grube F. Infectious lymphocytes in lymphocytic choriomeningitis virus carrier mice. J Gen Virol. 1979 Mar;42(3):481–492. doi: 10.1099/0022-1317-42-3-481. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Tishon A., Southern P. J., Oldstone M. B. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J Immunol. 1988 Feb 15;140(4):1280–1284. [PubMed] [Google Scholar]



