Abstract
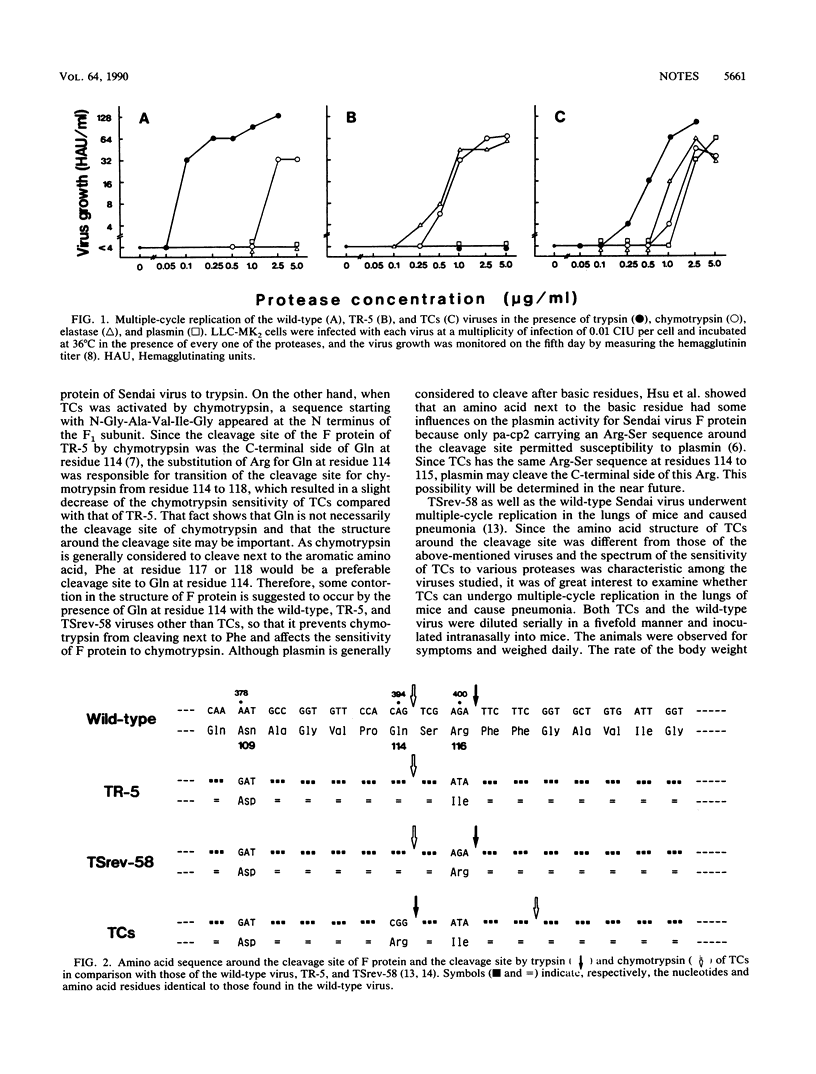
A protease-activation mutant of Sendai virus, TCs, was isolated from a trypsin-resistant mutant, TR-5. TCs was activated in vitro by both trypsin and chymotrypsin. TCs was, however, less sensitive to trypsin and chymotrypsin than were the wild-type virus and TR-5, respectively. F protein of TCs had a single amino acid substitution at residue 114 from glutamine to arginine, resulting in the appearance of the new cleavage site for trypsin and the shift of the cleavage site for chymotrypsin. Activation of TCs in the lungs of mice occurred less efficiently than that of the wild type, and TCs caused a less severe pneumopathogenicity than did the wild-type virus, which supports our previous view that the in vitro trypsin sensitivity of Sendai virus can be a good indication of pneumopathogenicity in mice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Molla A., Ando M., Araki S., Maeda H. Molecular mechanism of complex infection by bacteria and virus analyzed by a model using serratial protease and influenza virus in mice. J Virol. 1989 May;63(5):2252–2259. doi: 10.1128/jvi.63.5.2252-2259.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F. X., Garten W., Klenk H. D., Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981 Sep;113(2):725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- Glickman R. L., Syddall R. J., Iorio R. M., Sheehan J. P., Bratt M. A. Quantitative basic residue requirements in the cleavage-activation site of the fusion glycoprotein as a determinant of virulence for Newcastle disease virus. J Virol. 1988 Jan;62(1):354–356. doi: 10.1128/jvi.62.1.354-356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Protease activation mutants of Sendai virus: sequence analysis of the mRNA of the fusion protein (F) gene and direct identification of the cleavage-activation site. Virology. 1987 Jan;156(1):84–90. doi: 10.1016/0042-6822(87)90438-7. [DOI] [PubMed] [Google Scholar]
- Itoh M., Homma M. Single amino acid change at the cleavage site of the fusion protein is responsible for both enhanced chymotrypsin sensitivity and trypsin resistance of a Sendai virus mutant, TR-5. J Gen Virol. 1988 Nov;69(Pt 11):2907–2911. doi: 10.1099/0022-1317-69-11-2907. [DOI] [PubMed] [Google Scholar]
- Itoh M., Shibuta H., Homma M. Single amino acid substitution of Sendai virus at the cleavage site of the fusion protein confers trypsin resistance. J Gen Virol. 1987 Nov;68(Pt 11):2939–2944. doi: 10.1099/0022-1317-68-11-2939. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Goldberg A. R., Choppin P. W. Proteolytic cleavage by plasmin of the HA polypeptide of influenza virus: host cell activation of serum plasminogen. Virology. 1973 Nov;56(1):172–180. doi: 10.1016/0042-6822(73)90296-1. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y., Tashiro M., Homma M. Pneumopathogenicity in mice of a Sendai virus mutant, TSrev-58, is accompanied by in vitro activation with trypsin. J Virol. 1988 Aug;62(8):3040–3042. doi: 10.1128/jvi.62.8.3040-3042.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol Immunol. 1980;24(2):113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- NAGATA I., MAENO K., YOSHII S., MATSUMOTO T. PLAQUE FORMATION BY HVJ IN CALF KIDNEY CELLS. (BRIEF REPORT). Arch Gesamte Virusforsch. 1965;15:257–259. doi: 10.1007/BF01257738. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Shaughnessy M. A., Lamb R. A. Analysis of the relationship between cleavability of a paramyxovirus fusion protein and length of the connecting peptide. J Virol. 1989 Mar;63(3):1293–1301. doi: 10.1128/jvi.63.3.1293-1301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIGETA S. PLAQUE FORMATION AND GROWTH CHARACTERISTICS OF SENDAI VIRUS IN CHICK KIDNEY CELL CULTURES. Tohoku J Exp Med. 1964 Jul 25;83:114–120. doi: 10.1620/tjem.83.114. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Akami M., Matumoto M. Plaque formation by sendai virus of parainfluenza virus group, type 1 on monkey, calf kidney and chick embryo cell monolayers. Jpn J Microbiol. 1971 Mar;15(2):175–183. doi: 10.1111/j.1348-0421.1971.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Silver S. M., Scheid A., Choppin P. W. Loss on serial passage of rhesus monkey kidney cells of proteolytic activity required for Sendai virus activation. Infect Immun. 1978 Apr;20(1):235–241. doi: 10.1128/iai.20.1.235-241.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Evidence of proteolytic activation of Sendai virus in mouse lung. Arch Virol. 1983;77(2-4):127–137. doi: 10.1007/BF01309262. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect Immun. 1983 Feb;39(2):879–888. doi: 10.1128/iai.39.2.879-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Protection of mice from wild-type Sendai virus infection by a trypsin-resistant mutant, TR-2. J Virol. 1985 Jan;53(1):228–234. doi: 10.1128/jvi.53.1.228-234.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Pritzer E., Khoshnan M. A., Yamakawa M., Kuroda K., Klenk H. D., Rott R., Seto J. T. Characterization of a pantropic variant of Sendai virus derived from a host range mutant. Virology. 1988 Aug;165(2):577–583. doi: 10.1016/0042-6822(88)90601-0. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P., Ovcharenko A. V., Bukrinskaya A. G. Proteolytic activation of influenza WSN virus in cultured cells is performed by homologous plasma enzymes. J Gen Virol. 1982 Dec;63(2):469–474. doi: 10.1099/0022-1317-63-2-469. [DOI] [PubMed] [Google Scholar]


