SUMMARY
Here we report an unexpected role for the secreted Frizzled-related protein (sFRP) Sizzled/Ogon as an inhibitor of the extracellular proteolytic reaction that controls BMP signaling during Xenopus gastrulation. Microinjection experiments suggest that the Frizzled domain of Sizzled regulates the activity of Xolloid-related (Xlr), a metalloproteinase that degrades Chordin, through the following molecular pathway: Szl ┤ Xlr ┤ Chd ┤ BMP → P-Smad1 → Szl. In biochemical assays, the Xlr proteinase has similar affinities for its endogenous substrate Chordin and for its competitive inhibitor Sizzled, which is resistant to enzyme digestion. Extracellular levels of Sizzled and Chordin in the gastrula embryo and enzyme reaction constants were all in the 10−8 M range, consistent with a physiological role in the regulation of dorsal-ventral patterning. Sizzled is also a natural inhibitor of BMP1, a Tolloid metalloproteinase of medical interest. Furthermore, mouse sFRP2 inhibited Xlr, suggesting a wider role for this molecular mechanism.
INTRODUCTION
Cell differentiation in vertebrate embryos occurs in a stereotypical manner. For example, the mesoderm differentiates from dorsal to ventral into notochord, somite, kidney, lateral plate, and blood tissues. The Spemann organizer, a group of cells on the dorsal side of the gastrula embryo, has proven a fertile ground for discovering novel molecules mediating these dorsal-ventral (DV) cell differentiations (reviewed in De Robertis and Kuroda, 2004). This dorsal center expresses many secreted antagonists of Bone Morphogenetic Protein (BMP)—Chordin (Chd), Noggin and Follistatin—as well as Wnt antagonists of the secreted Frizzled-related protein (sFRP) family (Rattner et al., 1997; Pera and De Robertis, 2000), such as Frzb-1, Crescent, and sFRP-2. On the opposite side of the embryo, a ventral center marked by the sFRP Sizzled (Szl) is formed (Salic et al., 1997; De Robertis and Kuroda, 2004). DV patterning is thought to result from a conversation between the dorsal and ventral centers mediated by diffusible proteins regulating BMP signaling levels (Reversade and De Robertis, 2005). A gradient of BMP activity is set by the dorsal BMP antagonist Chordin, which is in turn inactivated by cleavage at specific sites by Xolloid-related (Xlr), a ventrally produced zinc metalloproteinase (Piccolo et al., 1997; Dale et al., 2002). This biochemical pathway is an ancient one since Drosophila DV patterning is also mediated by interactions between Dpp/BMP, Sog/Chordin, and Tolloid/Xolloid (De Robertis and Kuroda, 2004).
Extensive genetic screens in zebrafish have identified seven zygotic mutations affecting DV patterning (Hammerschmidt and Mullins, 2002; Schier and Talbot, 2005). Mutants with exaggerated dorsal structures, such as notochord and neural tissue, were defective in components required for BMP signaling, such as BMP2b, BMP7, the BMP receptor Alk8, Smad5, and Tolloid. Only two ventralizing mutations were found: chordino and ogon/mercedes. The chordino phenotype was caused by mutations in Chordin (Schulte-Merker et al., 1997) and ogon/mercedes by mutations in the zebrafish homolog of Xenopus Sizzled (Yabe et al., 2003; Martyn and Schulte-Merker, 2003). Mutant ogon embryos had ventralized loss-of-function phenotypes similar to those of the BMP antagonist chordino. In addition, zebrafish studies had shown that the ogon phenotype, like chordino, could be rescued by inhibition of BMP signaling (Hammerschmidt et al., 1996; Miller-Bertoglio et al., 1999; Wagner and Mullins, 2002). Therefore, the identification of ogon as Sizzled, an sFRP presumed to have Wnt antagonist activities, rather than the BMP inhibitor that was expected, came as a big surprise.
Although Sizzled was initially described as an Xwnt8 antagonist (Salic et al., 1997), subsequent studies suggested that this was not the case (Bradley et al., 2000; Collavin and Kirschner, 2003; Yabe et al., 2003). In Xenopus, loss of function using a Szl antisense morpholino oligo (MO) showed that Sizzled functions as a feedback inhibitor of ventral-most mesoderm, causing an expansion of ventral blood islands (Collavin and Kirschner, 2003). In zebrafish, Szl/ogon transcript levels were greatly increased by point mutations in Szl/ogon itself (Martyn and Schulte-Merker, 2003; Yabe et al., 2003). Microinjection of zebrafish Szl/ogon mRNA caused strong dorsalization of the embryo. This anti-BMP effect appeared to be mediated by a transcriptional increase in chordin and a decrease in BMP2b expression (Martyn and Schulte-Merker, 2003), and the effects of injected Szl/ogon mRNA were lost in chordino−/− mutants (Yabe et al., 2003). These zebrafish studies concluded that the sFRP molecule Sizzled/ogon functions through an unknown molecular mechanism in a negative feedback loop involving either BMP signaling or an as yet to be identified ventralizing Wnt (Martyn and Schulte-Merker, 2003; Yabe et al., 2003).
In the present paper, we show that the Sizzled/ogon sFRP protein regulates DV patterning in Xenopus through a novel molecular mechanism: it is a competitive inhibitor of the proteolytic activity of the Xolloid-related enzyme. The protease inhibitory activity maps to the frizzled (Fz) cysteine-rich domain (CRD) of Sizzled. Previously, Fz domains were known to function only as Wnt binding modules (Bhanot et al., 1996; Leyns et al., 1997; Rattner et al., 1997; Povelones and Nusse, 2005). There are three Tolloid-like genes in Xenopus: Xolloid-related (designated Tolloid-like 1 in mouse and Tolloid in zebrafish), Xolloid (designated Tolloid-like 2 in mouse), and BMP-1 or Procollagen C-Peptidase (PCP). The three enzymes can cleave Chordin, yet Xlr is the most effective. BMP1/PCP and Xolloid are expressed uniformly in the early embryo, whereas Xlr is produced in the ventral region (Goodman et al., 1998; Dale et al., 2002). In addition to Chordin, BMP1/PCP has other extracellular substrates, such as the Procollagen carboxy-terminal peptide that must be removed before collagen fibers can form (Kessler et al., 1996; Greenspan, 2005). We found that Sizzled is a very effective inhibitor of BMP1/PCP. We also report that a sFRP from mouse, sFRP2, functions as zinc metalloproteinase inhibitor. Thus, we may have uncovered a more general property of Fz domains. In sum, this work reveals an unexpected role for sFRPs in the regulation of extracellular proteases of the Tolloid family.
RESULTS
Sizzled Regulates Spemann Organizer Signals
In the course of studies on the loss-of-function phenotype of Chordin in Xenopus (Oelgeschläger et al., 2003), we noted that microinjection of antisense Chd MO caused a marked increase in Szl transcripts in early tadpoles (Figures 1A and 1B). To our surprise, this phenotype was very similar to the one caused by Szl MO (Collavin and Kirschner, 2003), as shown in Figures 1B and 1C. This remarkable increase of Szl transcripts accrued mainly in ventral and posterior ectoderm (Figure S1). Intrigued by how two secreted molecules that at gastrula are expressed at a considerable distance from each other (Figure 1D) produced identical loss-of-function phenotypes, we undertook an investigation of how this works at a molecular level. In Xenopus, a number of secreted proteins are transcribed on the ventral or dorsal centers (Figure 1E). Sizzled and Chordin presumably function as components of a wider network of secreted proteins that together constitute the molecular machinery of DV patterning.
Figure 1. Sizzled Is Expressed Ventrally Yet Requires a Dorsal Component to Function.
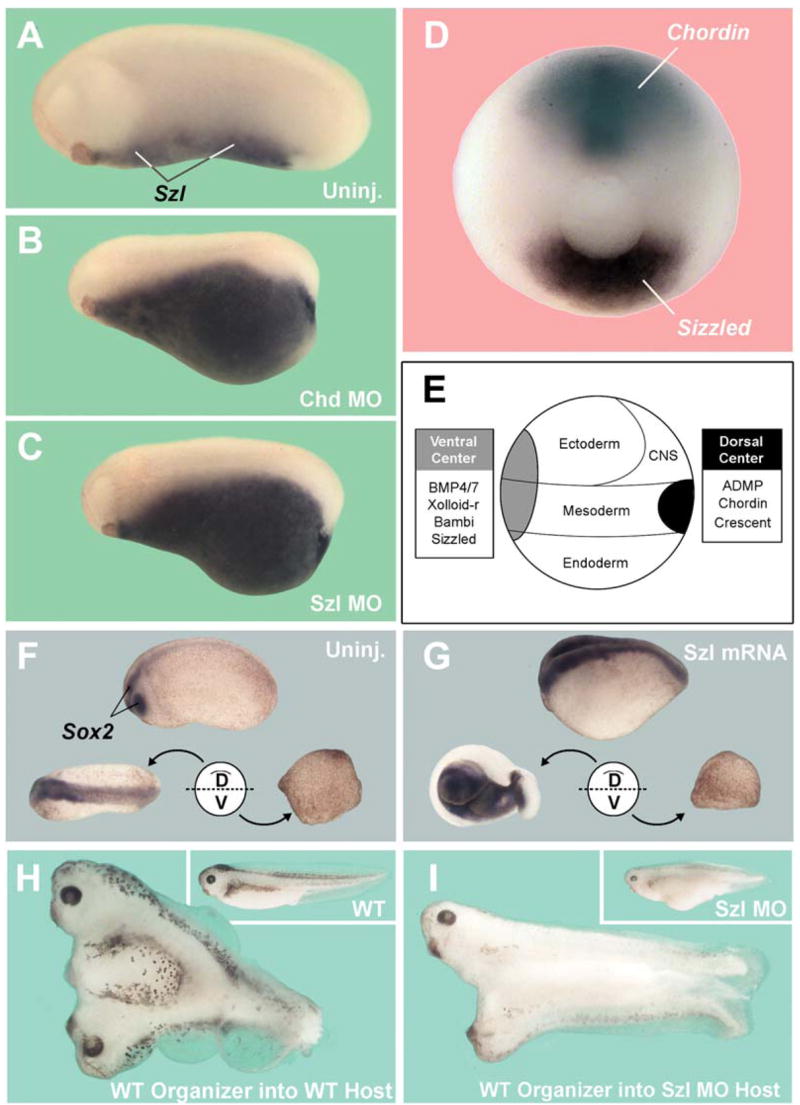
(A–C) Szl-depleted embryo is ventralized, as marked by the accumulation of Szl ventro-posterior transcripts and phenocopies knockdown of Chd.
(D) Chd and Szl are expressed at opposite poles of the Xenopus gastrula.
(E) DV patterning is regulated via proteins secreted by the dorsal and ventral signaling centers. (F) Embryo bisected at blastula across the DV axis can self-regulate to form a well-proportioned dorsal-half embryo, while the ventral half forms a belly-piece. Sox2 marks the CNS tissue. Uninjected wild-type embryo shown on top.
(G) Injection of Szl mRNA increases Sox2 staining in intact (top) and dorsal-half embryos but is without phenotypic effect in the ventral-half embryo (n = 40).
(H) A Spemann organizer transplant on the ventral side of a wild-type embryo forms a Y-shaped Siamese embryo with two heads and a single tailbud (n = 10).
(I) Wild-type Spemann organizer grafted into a Szl-depleted host forms an H-shaped embryo with CNS induction and two distinct tailbuds (n = 11).
The activities of Sizzled and of the Spemann organizer are intertwined. In overexpression experiments, Szl mRNA had dorsalizing effects that can be visualized by an expansion of the pan-neural marker Sox2 (Figures 1F and 1G). In bisection experiments (Reversade and De Robertis, 2005), this neural-promoting (anti-BMP) activity of Szl mRNA was observed in dorsal half-embryos but was without any effect in the ventral half (Figure 1G). This indicated that Sizzled does not affect ventral tissues directly and that it requires a functional Spemann organizer in order to be active. Reciprocally, the inductive activity of the Spemann organizer required Sizzled in the responding tissue. When wild-type organizers were transplanted to the ventral side of embryos depleted of Sizzled, the secondary axes developed with reduced amounts of somites and neural tissue, in particular brain and eyes (Figures 1H and 1I and data not shown). Siamese twins induced by organizer grafts almost invariably formed Y-shaped embryos with two rostral ends and a single tail lacking a ventral fin (Figure 1H). In Szl-depleted hosts, the amount of induced central nervous system (CNS) was reduced and the twins adopted an H shape with two well-separated tailbuds, each one with its own ventral fin (Figure 1I). The development of the ventral fin requires high BMP signaling levels in zebrafish and Xenopus (Hammerschmidt and Mullins, 2002; Reversade et al., 2005). These embryological results suggested that the Spemann organizer requires Sizzled to exert its full activity.
Sizzled Regulates Chordin Degradation
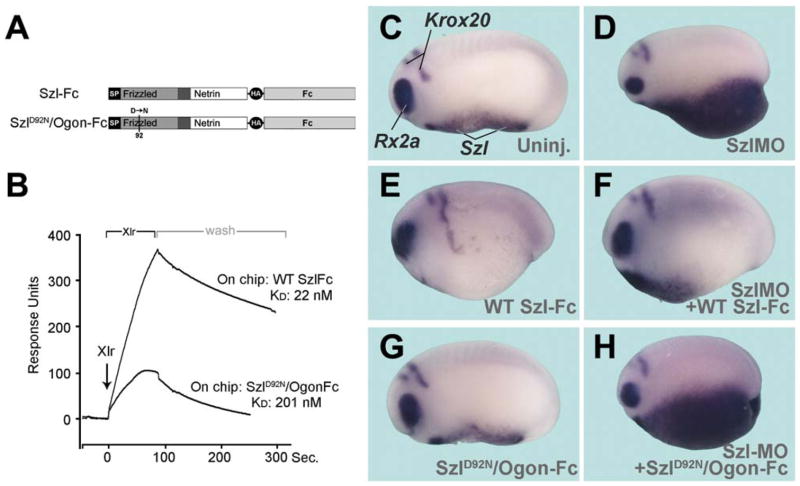
Is Sizzled a BMP or a Wnt inhibitor? Western blots of total extracts of microinjected embryos showed that the levels of phospho-Smad1 were markedly increased at the early gastrula stage by Szl knockdown (Figure 2A, compare lanes 1 and 4), reflecting an increase in BMP signaling (Persson et al., 1998). The ventral accumulation of Szl transcripts caused by Szl MO is mediated by BMP4 signals (Figures 2B and 2C). At gastrula stage, the ventral center was greatly expanded by Szl knockdown, and DV pattern markers (BMP4, Myf-5, Vent-2) indicated increased BMP signals comparable to those caused by knockdown of the BMP antagonist Chordin (Figure S2). On the other hand, Wnt signaling, measured by the levels of β-catenin protein, did not appear to be significantly affected by Szl knockdown or over-expression (Figure 2A, lanes 4 and 5). Consistent with this, the ventral Sizzled feedback loop was not inhibited by overex-pression of the Wnt antagonist Dickkopf-1 or by Xwnt8 MO, as would be expected if Szl functioned principally as an anti-Wnt (Figure S3). These results showed that endogenous Sizzled functions as a BMP antagonist in Xenopus embryos and failed to reveal any effect on canonical Wnt signaling.
Figure 2. Sizzled Is a Feedback Inhibitor of BMP Signaling that Requires Xlr Activity.
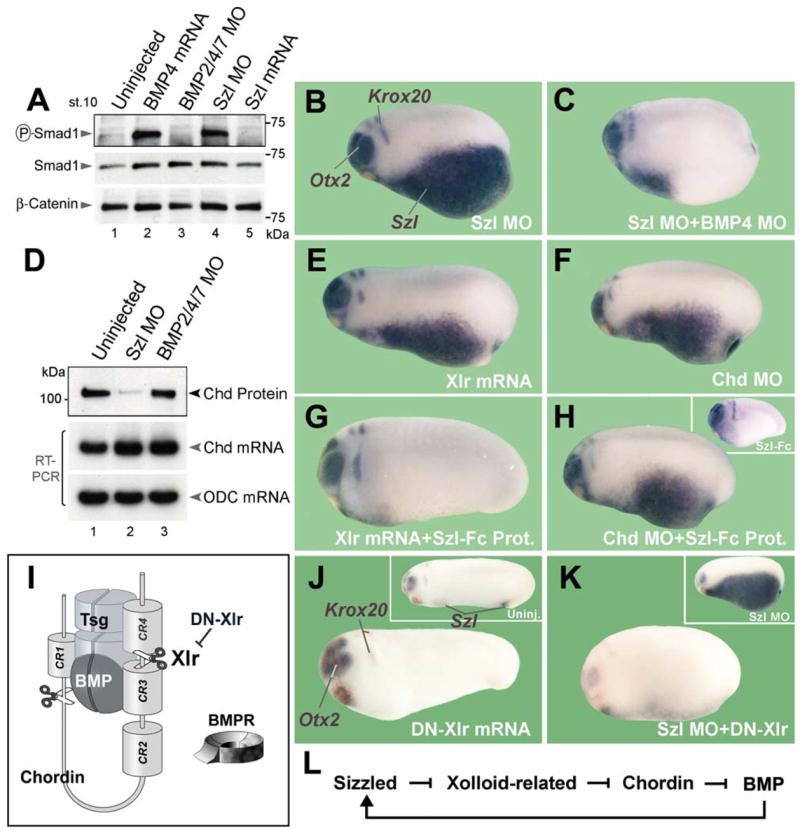
(A) Endogenous Smad1 phosphorylation at early gastrula is increased by Szl MO.
(B) An autoregulatory negative feedback loop is interrupted in Szl MO embryos, resulting in ventro-posterior accumulation of Szl transcripts.
(C) Szl upregulation is mediated by BMP4 signaling.
(D) Chd protein, but not Chd mRNA levels, are decreased by Szl knockdown (compare lanes 1 and 2). BMP2/4/7 MO injection serves as negative control.
(E and F) Xlr mRNA overexpression or Chd knockdown increase ventral Szl transcripts, indicating increased BMP signaling.
(G) Szl-Fc protein injection into the blastula cavity suppresses the pro-BMP effects of Xlr mRNA.
(H) Szl-Fc protein has no effect in Chd-depleted embryos. Inset shows phenotype of Szl-Fc injection.
(I) The role of Xlr in the regulation of Chordin anti-BMP activity. Tsg (Twisted gastrulation) is a cofactor of Chordin.
(J) Microinjection of DN-Xlr mRNA expands Otx2 expression, a sign of dorsalization.
(K) Szl MO feedback loop requires endogenous Xlr function since it is inhibited by DN-Xlr mRNA; compare with inset showing sibling embryo injected with Szl MO alone.
All microinjection results were performed at least three times and minimum of 15 embryos were analyzed by hybridization in each sample.
(L) Schematic diagram of the proposed BMP negative feedback loop via Sizzled, in which Szl inhibits Xlr activity.
To investigate why BMP signaling is increased in Szl knockdowns, we examined the effects of Szl MO on Chordin levels. Uninjected, Szl MO-, or control BMP2/4/7 MO-injected embryos were dissociated and allowed to secrete Chordin into the medium between gastrula stages 10 and 12 (Figure 2D). We found that endogenous Chordin protein levels were decreased in the supernatant of Szl-depleted embryos, while the cell pellets showed abundant chordin mRNA transcripts. This suggested that the stability of Chordin protein might be affected, prompting us to study the effect of Sizzled on the Xlr chordinase.
In vivo, Chordin is under the proteolytic control of Xlr (Figure 2, panel I), which can release BMPs from a ternary complex of Chd/Tsg/BMP, allowing renewed signaling through cell-surface receptors (Piccolo et al., 1997; Dale et al., 2002; Reversade and De Robertis, 2005). Overexpression of Xlr mRNA phenocopied the loss of Chordin (upregulation of ventral Szl expression), indicating high BMP activity (Figures 2E and 2F). The effects of Sizzled protein were tested using a protein fused to the immunoglobulin constant region (Szl-Fc), which can be purified on protein A columns to yield samples sufficiently concentrated (2.5 × 10−5 M) for biological and biochemical studies. Xenopus Szl-Fc protein dorsalized the embryo and synergized with Chordin when injected into the blastula cavity (Figure 2H, inset and Figure S4). Importantly, the pro-BMP effects of Xlr mRNA were eliminated by Szl-Fc protein microinjection (compare Figures 2E and 2G). The same amount of Szl-Fc was without phenotypic effect in Chordin-depleted embryos (Figures 2F and 2H), confirming the observations of Yabe et al. (2003).
We then used a dominant-negative form of Xlr (DN-Xlr) to determine whether Xlr function was required for the feedback regulation of Sizzled expression. DN-Xlr mRNA dorsalizes wild-type embryos (Figure 2J; Dale et al., 2002). Remarkably, microinjection of DN-Xlr mRNA was able to block the ventral upregulation of Szl transcripts in Szl MO embryos (Figure 2K; compare with sibling Szl MO embryo shown in the inset). Taken together, these in vivo experiments suggest the molecular pathway for DV patterning depicted in Figure 2L, in which Sizzled works as an inhibitor of Xlr.
Sizzled Binds to Xolloid-Related
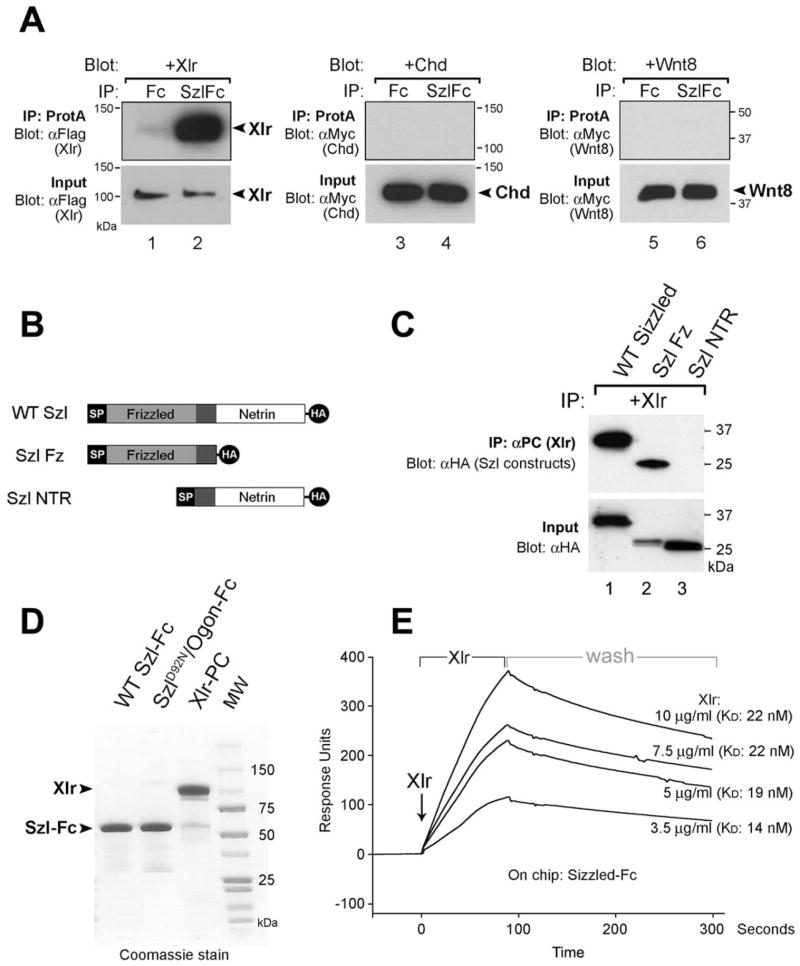
We next took a direct biochemical approach to determine whether the Sizzled and Xlr proteins interacted with each other. Szl-Fc protein was prebound to protein A beads, incubated with conditioned medium from transfected 293T cells containing Flag-tagged Xlr (Dale et al., 2002) and washed. It was found that Xlr bound to Szl-Fc beads but not to control beads containing secreted Fc fragment alone (Figure 3A, lanes 1 and 2). In similar conditions, Szl-Fc was unable to bind Chordin, Xwnt8, or the ternary complex of Chd/Tsg/BMP4 proteins (Figure 3A, lanes 3–6 and data not shown).
Figure 3. Sizzled Binds to Xolloid-Related.
(A) Xlr protein, but not Chd or Xwnt8, binds to Szl-Fc in immunoprecipitation assays. Secreted Fc fragment alone does not bind.
(B) Sizzled constructs encoding the frizzled (Szl-Fz) or netrin (Szl-NTR) domains.
(C) Szl is pulled down by Xlr specifically via the Fz domain.
(D) Coomassie Blue stain of affinity-purified Szl-Fc, SzlD92N/Ogon-Fc, and Xlr-PC reagents.
(E) Xlr protein binds to Sizzled-Fc on the BIAcore sensor chip with KD = 19 ± 3.7 nM.
The sFRP protein family has a conserved primary structure with an amino-terminal Fz domain and a carboxy-terminal NTR domain related to the axon guidance molecule Netrin (Leyns et al., 1997; Banyai and Patthy, 1999). To determine which domain of Sizzled interacted with Xlr, the molecule was HA-tagged and subdivided into its two domains, Szl-Fz and Szl-NTR (Figure 3B). We then used the reciprocal pull-down, in which Xlr tagged with protein C (PC) was bound to Ca2+-dependent anti-PC antibody beads (Roche), incubated with full-length or individual domain Sizzled proteins, washed, and then eluted with EDTA (a procedure that circumvented nonspecific binding of Szl to agarose beads). It was found that the Xlr binding region mapped to the Fz domain and not to the netrin domain (Figure 3C, lanes 2 and 3).
To determine whether the binding between Sizzled and Xlr was direct and in the physiological range, Szl-Fc and Xlr-PC were affinity-purified (Figure 3D). Binding affinity was determined by surface plasmon resonance (BIAcore) analysis. Szl-Fc was prebound to protein A crosslinked to the surface of a sensor chip, and changes in the refractive index caused by association of Xlr protein at constant flow (and by its dissociation when washing with buffer alone) were recorded at different Xlr concentrations (Figure 3E). From the kinetic rates of association and dissociation, the equilibrium dissociation constant (KD) was calculated and found to be about 20 nM (19 ± 3.7 nM) Xlr. We conclude that Sizzled binds specifically to Xlr, through the frizzled domain, and within physiological range (2 × 10−8 M).
Sizzled Mimicking an ogon Mutation Shows Decreased Binding to Xlr
Zebrafish genetics identified an ogon point mutation in the frizzled domain, in which an aspartate changed into asparagine results in a functionally null Szl/ogon protein (Martyn and Schulte-Merker, 2003; Yabe et al., 2003). We mimicked this point mutation in Xenopus Sizzled (Figure 4A), designated it SzlD92N, and tested its ability to bind to Xlr. As can be seen in Figure 4B, SzlD92N/Ogon-Fc associated with Xlr at a reduced rate when compared to its wild-type counterpart. The KD was calculated at 201 nM, about ten times lower than the affinity of the wild-type Szl protein for Xlr. Importantly, the D92N point mutation completely eliminated the dorsalizing activity of Szl-Fc protein microinjected into the blastula cavity of Xenopus embryos (Figure 4, compare E to G). In Szl MO embryos, in which the ventral Sizzled autoregulatory loop is blocked, wild-type Szl-Fc was able to rescue the ventral accumulation of Szl transcripts, but microinjection of the same amount of SzlD92N/Ogon-Fc was without any effect (compare Figure 4F to 4H). We conclude from these experiments that a point mutation in Sizzled that severely decreases its binding affinity for the Xlr metalloproteinase also eliminates its biological activity in embryos.
Figure 4. The SzlD92N Point Mutation Mimicking Zebrafish ogon Inhibits Xlr Binding and Biological Activity.
(A) Diagram of Szl-Fc and SzlD92N/Ogon-Fc constructs.
(B) BIAcore measurements of binding of 10 μg/ml Xlr in the flow to wild-type Szl-Fc or SzlD92N/Ogon-Fc immobilized on the chip. Note the ten-fold decrease in binding affinity to Xlr.
(C–H) Microinjection of 40 nl of 25 μM wild-type Szl-Fc protein into the blastula cavity dorsalizes the Xenopus embryo (E) and rescues the Szl knockdown phenotype (F). Injection of same concentration of SzlD92N/Ogon-Fc protein had no obvious phenotypic effect on wild-type (G) or Szl MO (H) embryos.
Sizzled Inhibits Xlr via Its Frizzled Domain
We next investigated whether Sizzled could inhibit the proteolytic activity of Xolloid-related. Proteases of the Tolloid family cleave the Chordin substrate at two conserved sites (Figure 5A) (Piccolo et al., 1997; Dale et al., 2002). Our biochemical reaction used affinity-purified Xlr-Flag as the enzyme, Xenopus Chordin produced in baculovirus vector as the substrate, and purified Xenopus Szl-Fc as the inhibitor. Proteolytic digestions were carried out at 25°C in the conditions described by Piccolo et al. (1997), and the resulting products analyzed in Western blots using an N-terminal antibody (α-NChd) or a C-terminal Myc tag (Figures 5A and 5B). Xlr efficiently cleaved full-length Chordin (Figure 5B, lanes 1 and 2). Addition of Szl-Fc inhibited proteolytic cleavage in a dose-dependent way, resulting in the stabilization of full-length Chordin (Figure 5B, lanes 3 to 8). Remarkably, all inhibitory activity was lost when the SzlD92N point mutant was used in the same proteolytic reaction (Figure 5C).
Figure 5. Sizzled, but not SzlD92N Mutant, Inhibits the Proteolytic Activity of Xlr on Its Natural Substrate Chordin.
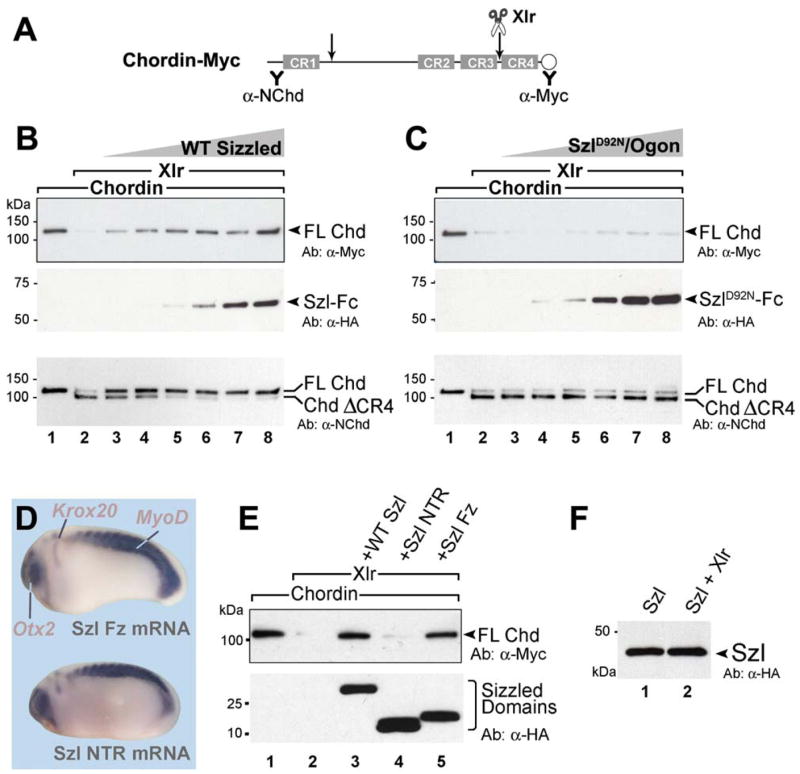
(A) Diagram of Chordin protein structure and antibody recognition sites.
(B) Specific proteolysis of Chd (20 nM) by Xlr enzyme can by inhibited by increasing concentrations of Szl-Fc (lanes 3–8, 4–100 nM).
(C) Increasing concentrations of SzlD92N/Ogon-Fc do not inhibit Xlr activity.
(D) Dorsalizing biological activity of Szl is caused by its frizzled domain, not the netrin domain.
(E) Sizzled inhibits Xlr proteolysis of Chordin via its frizzled domain.
(F) Sizzled is not digested by Xlr after 2 hr digestion with 2 nM Xlr enzyme.
We also investigated which domain of Sizzled contained the Xlr inhibitory activity. Microinjection of mRNAs encoding each domain of Szl showed that the dorsalizing (anti-BMP) activity resided in the Fz, and not in the NTR, domain (Figure 5D). In enzyme assays, the NTR domain of Szl did not affect proteolysis of Chordin, while the Szl Fz domain was sufficient to inhibit Xlr activity (Figure 5E, compare lanes 4 and 5). We conclude that the in vivo phenotypes of Szl can be entirely explained by the metalloproteinase inhibitory activity of its Fz domain.
Sizzled Is a Competitive Inhibitor of Xlr
We next took a more quantitative approach in which digestion products were measured using a chemifluorescent substrate on a Typhoon blot imager. First, we analyzed the kinetics of the enzyme reaction at increasing substrate (Chd) concentrations (Figure 6A). From double reciprocal Lineweaver-Burk plots, we determined that the Km (Michaelis constant, the concentration at which 50% of the enzyme is complexed with its substrate) was approximately 25 nM Chd (Figure 6A). This compares well to the Km of BMP1/Procollagen C-peptidase on its endogenous substrate Procollagen type I (96 nM; Hojima et al., 1985).
Figure 6. Sizzled, Xlr, and Chordin Interact at the Physiological 10−8 M Range.
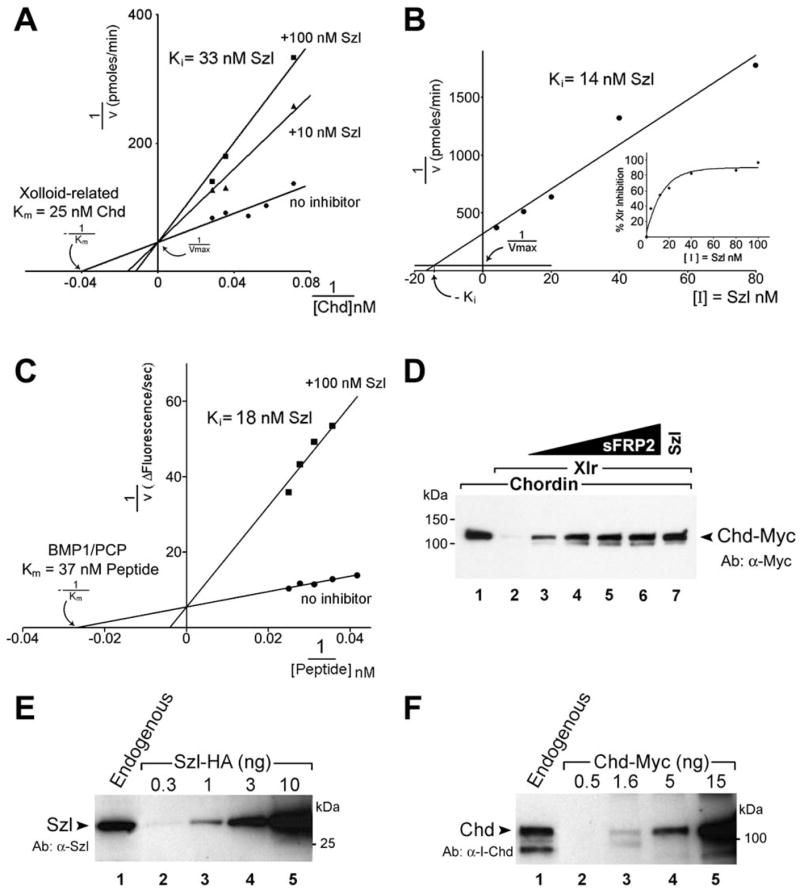
(A) Lineweaver-Burk double reciprocal plot showing chordinase activity of Xlr as a function of [substrate]. Michaelis constant Km was 25 nM. Szl (10 nM or100 nM) behaves as a competitive inhibitor of substrate digestion. The inhibition constant Ki was calculated at 33 nM.
(B) Dixon plot of data obtained by varying [inhibitor]. At Km=25 nM, −Ki corresponds to the [Inhibitor] that intersects at a height of 1/Vmax. Thus, Ki = 14 nM by this method.
(C) Lineweaver-Burk plot showing that Szl-Fc competitively inhibits the cleavage of fluorogenic heptapeptide by BMP1/PCP.
(D) The proteolytic activity of Xlr on Chordin substrate (lanes 1 and 2) can be inhibited by increasing concentrations of pure murine sFRP2 (lanes 3–6). Concentrations used were 3, 10, 25, and 50 nM sFRP2. Note in lane 7 that 40 nM Xenopus Szl-Fc had comparable activity.
(E) Endogenous Sizzled proteins secreted by seven dissociated embryos (lane 1) compared to a dilution curve of recombinant Szl-HA stained with affinity-purified Xenopus anti-Szl antibody.
(F) Endogenous Chordin protein secreted by seven embryos during gastrulation (lane 1) compared to a Chordin-Myc dilution curve stained with α-I-Chd antibody.
Second, we determined that Szl is a competitive inhibitor of the proteolytic reaction. In the presence of a constant concentration of Szl-Fc, the apparent Km for the substrate changed while the maximal velocity (Vmax) did not, indicating a competitive inhibitory kinetics (Figure 6A). The Ki (inhibitor constant, the concentration of inhibitor at which half of the enzyme is complexed to the inhibitor) was calculated from the slope in the presence of 10 or 100 nM Szl (Dixon and Webb, 1979) and corresponded to approximately 33 nM Szl. Finally, we quantified the effects of increasing inhibitor concentration at a single concentration of substrate (Figure 6B). Use of the Dixon plot (1/v versus [inhibitor]) allows one to determine the Ki provided the Km is known (Dixon and Webb, 1979). The Ki calculated in this way was 14 nM. While this value may appear quite different than the Ki of 33 nM calculated from Figure 6A, we note that both measurements are congruent with the 20 nM equilibrium dissociation constant (KD) determined for Xlr binding to Sizzled immobilized to a BIAcore chip. It is remarkable that independent measurements of the affinities of Chordin (Km), Sizzled (Ki), and Xlr (KD) in this reaction all were in the low 10−8 M range.
Since the inhibition by Sizzled is competitive, i.e., it can be counteracted by increasing substrate concentrations, in formal enzyme kinetic terms this means that the inhibitor binds to the substrate binding site in the enzyme. Sizzled behaves as a competitive inhibitor of the enzymatic activity of Xolloid-related because it can bind to the enzyme with a similar affinity and to the same site as its physiological substrate Chordin but is not cleaved by the Xlr protease, as shown in Figure 5F.
Sizzled Inhibits BMP1/PCP
Does Sizzled inhibit other Tolloid-like metalloproteinases? There are three closely related Tolloid genes in Xenopus, of which only Xlr exhibits localized expression during early development (Goodman et al., 1998; Dale et al., 2002). We found that BMP1/PCP (Figure 6C) and Xolloid (data not shown) were also targets of Szl inhibition. A short artificial fluorogenic substrate for BMP1/PCP exists, which greatly simplifies enzyme kinetic measurements. This heptapeptide was originally developed to study caspases (Enari et al., 1996) but fortuitously has similarities to the cleavage recognition sites present in Procollagen-C peptides (Greenspan, 2005). Cleavage of the fluorogenic peptide by BMP1/PCP was efficient, with a Km of 37 nM, and Szl inhibited competitively this digestion, with a Ki of 18 nM (Figure 6C). Xlr also showed proteolytic activity on the heptapeptide substrate but with lower affinity (Km=114 nM), and Szl was able to competitively inhibit this reaction as well (data not shown). Since an artificial heptapeptide can be competed by Szl, this strongly supports the view that Sizzled acts on the active site of BMP1/PCP and Xlr proteases, as also suggested by the competitive enzyme kinetics. We conclude that Szl is a natural inhibitor of Tolloid-like proteases.
sFRP2 Inhibits Xlr
We next investigated whether other sFRPs had biochemical activity similar to that of Sizzled/Ogon. It was found that mouse sFRP2, a commercially available protein (R&D Systems), caused a dose-dependent inhibition of Chordin cleavage by Xlr proteinase (Figure 6D, lanes 3–6). The inhibitory activity of sFRP2 was comparable to that of Sizzled at similar concentration (Figure 6D, lane 7). The amino acid sequence of mouse sFRP2 is 30% identical and 45% similar to Xenopus Sizzled. Most of the similarities are found in the Fz CRD, whereas the netrin domain is not significantly conserved (data not shown). In Xenopus, the sFRP2 gene is expressed in the prospective neural plate and the Spemann organizer (Pera and De Robertis, 2000) and shares 33% identity and 48% similarity to Xenopus Sizzled. We conclude that another member of the sFRP gene family serves as an inhibitor of zinc metalloproteinases.
Endogenous Sizzled and Chordin Concentrations
Finally, we determined the amounts of Sizzled and Chordin proteins present in the Xenopus gastrula (Figures 6E and 6F). Embryos were dissociated and proteins secreted during gastrulation (between stages 10.5 to 12) were collected in 5 μl/embryo of Ca2+- and Mg2+-free medium. Proteins originating from seven embryos (two independent experiments) were analyzed in Western blots using affinity-purified polyclonal antibodies directed against Szl and Chd (see Experimental Procedures). Dilutions of purified Szl-HA or recombinant Chd-Myc of known concentrations (determined by Coomassie Blue staining) were used as standards (Figures 6E and 6F, lanes 2–5). It was estimated that each embryo secreted 0.45 ng of Szl and 1.5 ng of Chd protein (Figures 6E and 6F, lane 1). Assuming that the gastrula has a total volume of 1.4 μl (Piccolo et al., 1996) and an extracellular space of about 30%, the concentration of Szl was estimated at 30 nM and that of Chordin at 33 nM, if they were distributed uniformly in the extracellular space. In the ventral and dorsal extracellular space these concentrations would be expected to be significantly higher as they originate from localized sources. The concentration of Xlr enzyme was not determined but is expected to be much lower. We find it remarkable that the substrate and the inhibitor, for which the active site of the enzyme has similar affinities, are present in vivo at comparable concentrations, which are consistent with their proposed patterning activities.
DISCUSSION
This research was initiated in an attempt to understand why knockdown of Chordin and Sizzled in Xenopus embryos produced identical phenotypes, in particular a striking accumulation of Szl transcripts in ventral ectoderm. We discovered that this phenomenon could be explained by a novel molecular mechanism mediating regulation of DV patterning. Sizzled has the sequence of an sFRP yet was found here to inhibit BMP signaling by an indirect mechanism such that:
This finding introduces a new layer of regulation to the Chordin/BMP DV patterning pathway, in which Sizzled acts as a proteolytic inhibitor of Xolloid-related, an enzyme that degrades the BMP inhibitor Chordin, releasing BMPs complexed with it. When Szl translation is blocked by MO (Collavin and Kirschner, 2003), a negative autoregulatory feedback loop is interrupted, such that Szl transcripts accumulate in ventral ectoderm. Several lines of evidence (Figure 2) support the BMP regulatory loop outlined above: First, Szl knockdown causes an increase in phospho-Smad1 without significantly affecting the level of Wnt signaling. Second, Szl knockdown causes a reduction in the stability of Chordin protein secreted at the gastrula stage. Third, the ventralizing effects of Xlr mRNA can be blocked by microinjected Szl protein. Fourth, inhibiting Chordin proteolysis with DN-Xlr interrupts the BMP4 feedback loop. These biological experiments suggest that Sizzled controls DV patterning by regulating the levels of Chordin in the embryo. Supporting this view, Szl/ogon is devoid of anti-BMP effects in ventral half-embryos lacking the Spemann organizer.
Sizzled Is a Competitive Enzyme Inhibitor
To demonstrate that Sizzled is indeed an inhibitor of the Xlr metalloproteinase, we used a direct biochemical approach. Chordin was digested with affinity-purified Xlr enzyme. This could be inhibited by Sizzled in a concentration-dependent manner. A measure of the affinity of Xlr for its substrate Chordin is provided by the Michaelis constant, Km, which was found to be about 25 nM Chd at 25ºC. This value is within physiological range (Hojima et al., 1985; Greenspan, 2005). The inhibition constant, Ki, which represents the concentration of Sizzled at which 50% of the enzyme is bound to the inhibitor, was calculated between 13 and 33 nM. The kinetics of inhibition indicate that Sizzled is a competitive inhibitor, in which Chordin and Sizzled compete with similar affinities for the substrate binding site in the enzyme. Sizzled functions as an inhibitor, rather than a substrate, because it is not cleaved by Xlr. The affinities determined by enzyme kinetics were in the same range as the equilibrium dissociation constant, KD, calculated at 20 nM for the direct binding of purified Xolloid-related to Sizzled immobilized on a BIAcore sensor chip. We were able to estimate the endogenous levels of Sizzled and Chordin secreted into the extracellular space during gastrulation: if uniformly distributed, each would correspond to about 30 nM. In the ventral and dorsal centers, their concentrations are expected to be much higher, depending on diffusion rates. These endogenous levels are congruent with the affinity constants available for Szl, Xlr, and Chd. They are all in the low 10−8 M range and are consistent with the view that competitive inhibition of protease activity by Sizzled may indeed play a role in the physiological regulation of DV patterning.
There is much genetic evidence from zebrafish supporting the molecular mechanism described here. Extensive screens identified only two ventralizing (high BMP) zygotic loci, which mapped to the sizzled and chordin genes and had very similar overall mutant phenotypes (Hammerschmidt and Mullins, 2002; Schier and Talbot, 2005). The present work provides an explanation for why these phenotypes are similar: Chordin is the substrate and Sizzled the inhibitor of a Tolloid-like metalloproteinase. Seldom does one have such strong genetic support for the view that a particular protein (Chordin) is the endogenous substrate of a protease/inhibitor (Tolloid/Sizzled) pair.
Zebrafish genetic studies identified a point mutation in the Fz domain that causes a complete loss of function of szl/ogon (Yabe et al., 2003; Martyn and Schulte-Merker, 2003). Recreating this mutation in Xenopus Sizzled greatly helped our investigations. The binding affinity of SzlD92N to Xlr was ten times lower than that of wild-type Szl in binding assays, and the mutation caused a complete loss of biological activity of the mutant protein microinjected into the blastula embryo (Figure 4). SzlD92N was unable to inhibit cleavage of Chordin by Xlr, strongly supporting our proposal that Sizzled acts as a metalloproteinase inhibitor. We conclude that the wild-type function of Szl/ogon is to regulate the enzymatic activity of the Xlr metalloproteinase.
An Extracellular Network of DV Patterning Molecules
Figure 7A shows a model of how a network of extracellular proteins may regulate DV patterning. In the ventral center, high levels of BMP4/7 signaling via Smad1 activate the expression of the BMP target genes Bambi, Crossveinless-2, Xlr, and Sizzled. Bambi (BMP and Activin Membrane Bound Inhibitor) encodes a natural dominant-negative BMP receptor lacking serine/threonine kinase activity (Onichtchouk et al., 1999) that strongly synergizes with Szl in knockdown experiments (Reversade and De Robertis, 2005). Crossveinless-2 (CV-2) is a secreted protein containing five BMP binding modules similar to those of Chordin (De Robertis and Kuroda, 2004). Xolloid-related is a ventrally expressed metalloproteinase that appears to play a critical role in DV patterning (Dale et al., 2002). Tolloid proteolysis increases BMP signaling by degrading the BMP antagonist Chordin and releasing BMPs bound to Chordin (Piccolo et al., 1997; Goodman et al., 1998; Reversade and De Robertis, 2005). High BMP signals inhibit the expression of dorsal center genes such as Chordin and ADMP (Anti-Dorsalizing Morphogenetic Protein). ADMP is a BMP expressed dorsally that signals only at a distance from the dorsal organizer because it is inhibited locally by Chordin and released ventrally by Xlr (Reversade and De Robertis, 2005). Sizzled is a key regulator of Xlr activity and, interestingly, both proteins are secreted by ventral center cells.
Figure 7. Model of the Regulation of DV Patterning in Xenopus Embryos by a Network of Extracellular Proteins.
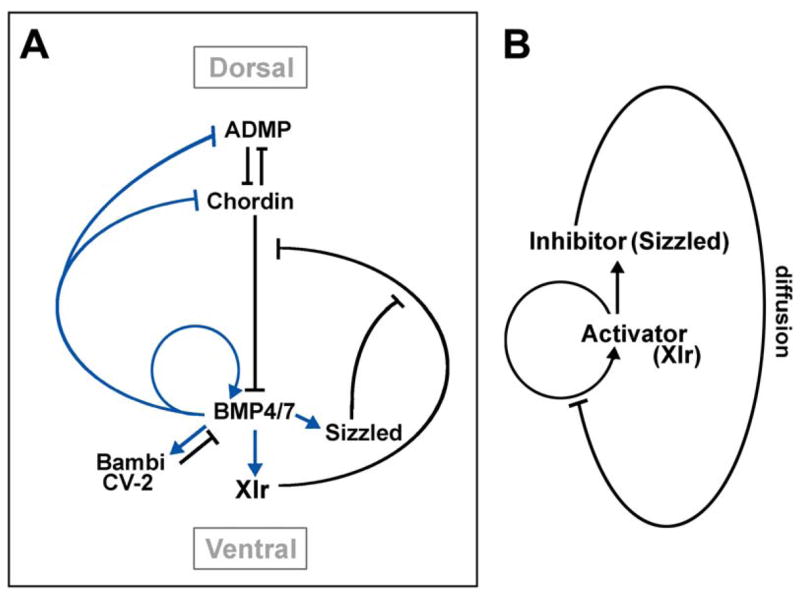
(A) Diagram summarizing the observations reported here and in Reversade and De Robertis (2005). Transcriptional regulation is shown in blue, direct protein-protein interactions in black. The regulatory loops are shown at equilibrium and do not describe how the pattern arises through the activity of maternal signals present in the egg.
(B) Diagram of how an activator (Xlr) and an inhibitor (Sizzled) would generate pattern, according to the model of Meinhardt and Gierer (2000).
Turing (1952) proposed that an activator and an inhibitor diffusing from a single source can generate a stable pattern provided that they diffuse at different rates. Our understanding of pattern-generating systems has been further enriched by the realization that an activator that induces its own expression and that of a more diffusible inhibitor will produce stable morphogen patterns at equilibrium (Meinhardt and Gierer, 2000); a diagram of how this would be achieved in the case of Xolloid-related (an activator of BMP signaling) and its inhibitor Sizzled is shown in Figure 7B. On the dorsal side, a similar function has been proposed recently for the ADMP-Chordin pair (Reversade and De Robertis, 2005). Double diffusion-reaction systems emanating from both the dorsal and ventral sides may play an important role in assuring the robustness of DV patterning by BMP signals. A critical link in this self-regulating communication between the dorsal and ventral sides of the embryo appears to be provided by the activity of the Xolloid-related chordinase (Figure 7A).
In the present study, we have made efforts to quantify the enzyme kinetic and binding constants of the reactions between Xlr, Sizzled, and Chordin. We think that determining the affinities of all the interactions shown in Figure 7A will be an important undertaking that will allow one to model how a network of extracellular proteins works. We find it remarkable that many of these interactions are mediated by direct protein-protein interactions in the extracellular space. The affinities of these bindings can be quantified, and for each component, gain- and loss-of-function reagents are available in the Xenopus system. This offers an excellent opportunity for investigating how protein networks regulate intercellular signaling in the extracellular space.
A Natural Inhibitor of BMP1 and Xlr
The novel protease inhibitory activity of Sizzled described here mapped to the Frizzled domain. Many proteins contain Fz domains, such as Frizzled Wnt receptors, sFRP Wnt antagonists, Collagen XVIII (a basement membrane component), Smoothened (Hedgehog co-receptor), MuSK (muscle-specific tyrosine kinase receptor), Ror (orphan RTK-like neurotrophic receptors), and zinc Carboxypeptidase Z (Xu and Nusse, 1998; Rehn et al., 1998). The sole activity previously demonstrated for Fz domains was to bind Wnts (Bhanot et al., 1996; Leyns et al., 1997; Hsieh et al., 1999; Povelones and Nusse, 2005), but it is possible that this module might have additional functions. Here we have provided evidence that in addition to Sizzled/Ogon, sFRP2 is an inhibitor of Xolloid-related. Other experiments indicate that mammalian sFRP3, known by the name Frzb-1 in Xenopus, and Crescent may also be enzyme inhibitors of Tolloids (A.L.A., H.X.L., and E.M.D.R, unpublished data). With at least seven sFRPs in vertebrates (sFRPs 1 to 5, Crescent, and Sizzled; Rattner et al., 1997; Pera and De Robertis, 2000), three Tolloid-like genes (Dale et al., 2002), and many more extracellular members of the zinc metalloprotease superfamily (Stöcker et al., 1995), a great potential for additional signaling regulation exists. A question for the future is whether other Fz domain proteins, such as Frizzled receptors, regulate extracellular metalloproteinases as well.
Sizzled inhibited both the cleavage of Chordin and of an artificial heptapeptide substrate by Xlr and by BMP1/PCP. The observation that Sizzled is a competitive inhibitor of the digestion of a short peptide substrate indicates that Szl inhibits binding of substrate to the active site of Tolloid-like enzymes. BMP1/PCP is an enzyme of considerable interest since it is required for the carboxy-propeptide cleavage of fibrillar procollagens. Procollagens are major constituents of the extracellular matrix, with Procollagen I being the most abundant protein in the human body (Byers, 2001). Because the Procollagen-C peptide must be removed before collagen triple helical fibers can be formed, it has been proposed that inhibitors of BMP1/PCP might serve to prevent fibrosis or excessive scar tissue formation after surgery (Greenspan, 2005). Tolloid-related enzymes are part of the astacin family of proteases, for which no known endogenous inhibitors exist so far (Bond and Beynon, 1995; Baker et al., 2002). Astacins are part of a much larger superfamily of zinc metalloproteinases, the Metzincins, that degrade the extracellular matrix (Stöcker et al., 1995; Baker et al., 2002). Identifying the peptide sequences in Sizzled that block the BMP1/PCP active site could help in the rational design of specific inhibitors of extracellular metalloproteinases with potential therapeutic value.
EXPERIMENTAL PROCEDURES
Morpholino Oligos and Embryonic Manipulations
Antisense MOs (Gene Tools LLC) were as described: Chd MO (Oelgeschläger et al., 2003), Szl MO (Collavin and Kirschner, 2003), and BMP2, BMP4, BMP7 MOs (Reversade et al., 2005). Each MO was microinjected four times radially into 2- or 4-cell embryos (34 ng total). Bisection experiments were performed at stage 9 in 0.3 × Barth solution (Reversade and De Robertis, 2005). Spemann organizer transplantations were as described (Oelgeschläger et al., 2003). For mRNA microinjection, 200 pg of Xenopus Sizzled (Pera and De Robertis, 2000), 400 pg Szl-Fz, 400 pg Szl-NTR, 400 pg BMP4, and 1 ng of Xlr or of its dominant-negative Y286N missense mutant (DN-Xlr) form (Dale et al., 2002) were used. Affinity-purified Szl-Fc or SzlD92N-Fc proteins were microinjected (25 μM, 40 nl) into the blastocoele at mid-blastula (stage 8). Procedures for mRNA synthesis, whole-mount in situ hybridization and RT-PCR are available at http://www.hhmi.ucla.edu/derobertis/index.html.
Biochemical Methods
Xenopus Sizzled-HA, Xlr-PC, and Xlr-Flag were tagged at the C termini by PCR. For purification at high concentration, Szl-Fc was generated by fusion of a PCR fragment containing the heavy chain constant domain of human immunoglobulin (from EST gi: 61216116) to the C terminus of Sizzled. Szl-Fz encompassed the first 155 residues up to Ser155. Szl-NTR was generated by internal deletion of the frizzled module from Cys27 to Cys136 with a Glu and a Leu insertion resulting from the restriction enzyme linker. For SzlD92N-Fc, site-directed mutagenesis Quik-change kit (Stratagene) was used. Proteins were produced by transient transfection (Fugene, Roche) of HEK 293T cells. Secreted proteins in conditioned media were then affinity-purified using PC (Roche), Protein A (Sigma), or HA beads (Covance) according to manufacturer’s instructions. Endogenous Chordin was detected with blot-purified α-I-Chd antibody as described in Oelgeschläger et al. (2003) and endogenous Szl was detected using 1:5000 dilution of anti-Szl antibody. A synthetic peptide encompassing the final 50 residues of Xenopus Sizzled was used to immunize rabbits (Covance) and affinity-purified using the same antigen. Phospho-Smad1 was detected in stage 10.5 whole embryos using α-phospho-hSmad1 antibody (Persson et al., 1998). Immunoprecipitation of Szl-interacting proteins was performed with conditioned media containing Xlr-Flag, S2-produced Xwnt8-Myc (Hsieh et al., 1999), or baculovirus Chd-Myc (Piccolo et al., 1996) and pulled down with Szl-Fc or Fc alone following methods described in Reversade and De Robertis (2005). The reciprocal pulldown was performed with Xlr-PC on anti-PC agarose beads followed by Szl binding and elution with EDTA as described by the manufacturer (Roche).
Surface Plasmon Resonance Analysis
Surface plasmon resonance (SPR) measurements were performed in a BIAcore 3000 system. Protein A (Sigma) was dissolved at 10 μg/ml in 10 mM sodium acetate (pH 5.0) and immobilized on a CM5 sensor chip using the amine coupling method to a level of about 2500 response units. Binding and washes were performed in Xld Buffer (Piccolo et al., 1997) using affinity-purified proteins dialyzed in the same buffer. Each experimental cycle consisted of an initial flow of 300 μg/ml Szl-Fc or SzlD92N-Fc into the respective flow cells to pre-bind Sizzled prior to the flow of Xlr-PC at various concentrations. After each cycle, chip surfaces were regenerated by removing noncrosslinked proteins with 10 mM HCl. Data were analyzed with BIAevaluation 4.1 software and curve-fitting was done with the assumption of one-to-one binding (Wang et al., 2003).
Xlr and BMP1 Enzymatic Digestion Assays
For in vitro digestion, 30 nM Chd-Myc produced in baculovirus (Piccolo et al., 1996) was incubated in Xld Buffer with affinity-purified Xlr-Flag containing the indicated concentrations of Szl-Fc, SzlD92N-Fc, or sFRP2 (R&D Systems, carrier-free) at 25° for 2 hr. Western blots were visualized with Pico chemiluminescent substrate (Pierce). For kinetic studies, the chemifluorescent ECL-plus substrate (Amersham Biosciences) was used and quantified on a Typhoon 9410 blot imager (GE Healthscience). Synthetic peptide digestions with Xlr-Flag or BMP1 conditioned media were performed in microtiter plates with increasing concentrations of the fluorogenic peptide, Mca-Y-V-A-D-A-P-K(Dnp)-OH (R&D Systems), in the presence or absence of Szl and monitored on a fluorescent plate reader (excitation = 320 nm, emission = 405 nm). Initial velocities were determined from the rate of fluorescence increase over the first 15–60 min time course.
Supplementary Material
Acknowledgments
We thank Drs. W. Wang and R. Lehrer for generous assistance with BIAcore analysis, Drs. L. Dale, C. Heldin, J.C. Hsieh, J. Nathans, and J. Larrain for reagents, and members of our laboratory for comments on the manuscript. This work was supported by the NIH (R37 HD21502-19). A.L.A. was supported by a postdoctoral fellowship of the Jonsson Cancer Center Foundation/UCLA. E.M.D.R. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental data include four figures and can be found with this article online at http://www.cell.com/cgi/content/full/124/1/147/DC1/.
References
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Banyai L, Patthy L. The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 1999;8:1636–1642. doi: 10.1110/ps.8.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bond JS, Beynon RJ. The astacin family of metalloproteinases. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L, Sun B, Collins-Racie L, LaVallie E, McCoy J, Sive H. Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev Biol. 2000;227:118–132. doi: 10.1006/dbio.2000.9873. [DOI] [PubMed] [Google Scholar]
- Byers PH. Disorders of collagen biosynthesis and structure. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Diseases. New York: McGraw-Hill; 2001. pp. 5241–5285. [Google Scholar]
- Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- De Robertis E, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M, Webb EC. Enzymes. New York: Academic Press; 1979. [Google Scholar]
- Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- Greenspan DS. Biosynthetic processing of collagen molecules. Top Curr Chem. 2005;247:149–183. [Google Scholar]
- Hammerschmidt M, Mullins MC. Dorsoventral patterning in the zebrafish: bone morphogenetic proteins and beyond. Results Probl Cell Diff. 2002;40:72–95. doi: 10.1007/978-3-540-46041-1_5. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Hojima Y, van der Rest M, Prockop DJ. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. J Biol Chem. 1985;260:15996–16003. [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci USA. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann Organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn U, Schulte-Merker S. The ventralized ogon mutant phenotype is caused by a mutation in the Zebrafish homologue of Sizzled, a secreted Frizzled-related protein. Dev Biol. 2003;260:58–67. doi: 10.1016/s0012-1606(03)00221-5. [DOI] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. Bioessays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio V, Carmany-Rampey A, Furthauer M, Gonzalez EM, Thisse C, Thisse B, Halpern ME, Solnica-Krezel L. Maternal and zygotic activity of the zebrafish ogon locus antagonizes BMP signaling. Dev Biol. 1999;214:72–86. doi: 10.1006/dbio.1999.9384. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signaling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- Pera E, De Robertis EM. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech Dev. 2000;96:183–195. doi: 10.1016/s0925-4773(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engstrom U, Heldin CH, Funa K, ten Dijke P. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 1998;434:83–87. doi: 10.1016/s0014-5793(98)00954-5. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Nusse R. The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling. EMBO J. 2005;24:3493–3503. doi: 10.1038/sj.emboj.7600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn M, Pihlajaniemi T, Hofmann K, Bucher P. The frizzled motif: in how many different protein families does it occur? Trends Biochem Sci. 1998;23:415–417. doi: 10.1016/s0968-0004(98)01290-0. [DOI] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. Reciprocal regulation of Admp and Bmp2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW. Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development. 1997;124:4739–4748. doi: 10.1242/dev.124.23.4739. [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Stöker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincins-topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Philos Trans R Soc Lond A. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DS, Mullins MC. Modulation of BMP activity in dorsal-ventral pattern formation by the chordin and ogon antagonists. Dev Biol. 2002;245:109–123. doi: 10.1006/dbio.2002.0614. [DOI] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- Xu YK, Nusse R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr Biol. 1998;8:405–406. doi: 10.1016/s0960-9822(98)70262-3. [DOI] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.