Summary
Thyroid hormone (TH) has a remarkable range of actions in the development and function of the nervous system. A multigenic picture is emerging of the mechanisms that specify these diverse functions in target tissues. Distinct responses are mediated by α and β isoforms of TH receptor which act as ligand-regulated transcription factors. Receptor activity can be regulated at several levels including that of uptake of TH ligand and the activation or inactivation of ligand by deiodinase enzymes in target tissues. Processes under the control of TH range from learning and anxiety-like behaviour to sensory function. At the cellular level, TH controls events as diverse as axonal outgrowth, hippocampal synaptic activity and the patterning of opsin photopigments necessary for colour vision. Overall, TH coordinates this variety of events in both central and sensory systems to promote the function of the nervous system as a complete entity.
Keywords: Thyroid hormone, deiodinase, selenoprotein, nuclear receptor, transcription, neuronal differentiation, behavior, retina, cochlea, hearing, colour vision, photoreceptor, oligodendrocyte, mental retardation, depression, glucose utilization
Introduction
It has long been evident from human cretinism that TH is necessary for brain development (Osler, 1897, Gesell et al., 1936). The neurological retardation arising in cretinism results from TH insufficiency during critical periods of neuronal differentiation. TH insufficiency can result from impairment of the thyroid gland (congenital hypothyroidism) or from a lack of the dietary iodine necessary for biosynthesis of TH. Newborn screening and hormonal replacement have greatly reduced the occurrence of mental retardation in congenital hypothyroidism (Rovet and Daneman, 2003). Iodine deficiency remains a widespread cause of mental retardation in a number of developing countries (DeLong et al., 1985, Delange, 2001).
The symptoms of cretinism are diverse and suggest a role for TH in learning, language ability, memory, motor control and sensory function. Recent findings indicate that the spectrum of functions of TH in the mammalian nervous system is wider than may have been anticipated from general observations of cretinism and include, for example, a key role in colour vision (Ng et al., 2001a). This remarkable breadth of functions raises an obvious question: how can this hormone elicit so many different responses? Moreover, the responses in a given tissue change as development progresses such that a lack of TH produces different defects in the adult than in the fetus or infant. The question therefore becomes more complex: what determines the temporal as well as the cellular specificity of TH actions within the nervous system?
Genetic studies of model species and of human disease have pointed to some answers by identifying a number of genes that mediate or modify TH action in neural target tissues. Nervous system phenotypes have been identified in mice with mutations in the TH receptor α and β genes and in genes encoding deiodinase enzymes that metabolize TH (Forrest et al., 1996, Bernal, 2007, Galton et al., 2007). Human neurological defects have been associated with mutations in the TH receptor β gene (Refetoff et al., 1993, Chatterjee and Beck-Peccoz, 2001) and in the MCT8 gene that encodes a TH transporter (Dumitrescu et al., 2004, Friesema et al., 2004). Genetic defects that interfere with TH action at any level in the target tissue may potentially cause neurological phenotypes, as shall be discussed (Figure 1, Table 1).
Figure 1.
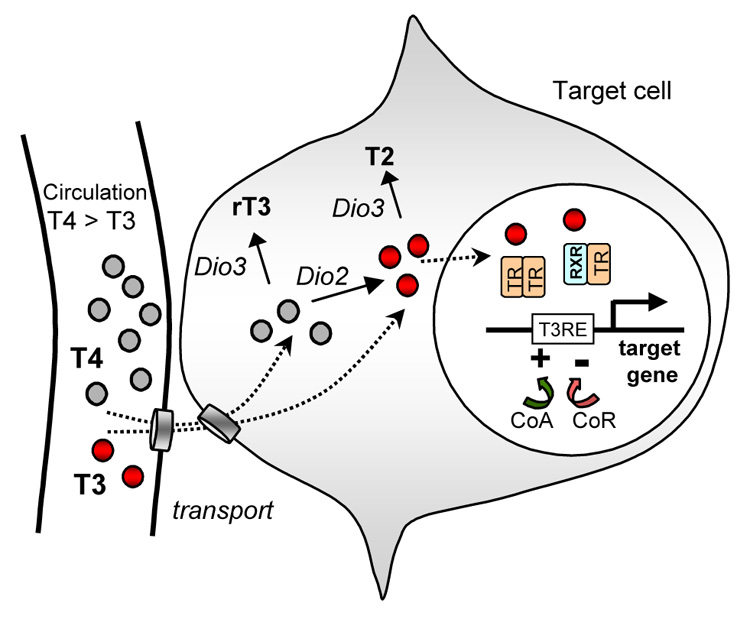
An outline of the cellular response to TH. T3 ligand (small red circles) binds a nuclear receptor (TR) that acts as a transcription factor to regulate target gene expression. The TR binds a T3 response element (T3RE) in the gene and its activity is regulated by corepressors (CoR) or coactivators (CoA). TR can bind efficiently to a given T3RE as a homodimer or heterodimer with retinoid X receptor (RXR), or less efficiently as a monomer. T3 is at lower levels than T4 (small grey circles) in the circulation but T3 levels may be amplified by conversion of T4 into T3 by type 2 deiodinase (encoded by Dio2). Ligand may be inactivated by type 3 deiodinase (Dio3) which converts T4 and T3 to rT3 and T2, respectively. T2 and rT3 have minimal biological activity. It should be noted that deiodinases may act in the target cell or in nearby cells through a paracrine-like mode of control. Deiodinases are membrane-associated within the cell. Transporters can control hormone uptake into tissues and communication between cells by hormone transfer.
Table 1. Nervous system phenotypes associated with mutations in genes that are involved in thyroid hormone action.
Nervous system phenotypes associated with mutations in mammalian genes that mediate thyroid hormone functions. Only general phenotypes are noted. See text for details.
| Gene product | Mouse gene Location | Mouse phenotypes | Human gene Location | Human disease |
|---|---|---|---|---|
| thyroid hormone receptor β |
Thrb, Nr1a2 14 |
Sensory deficits Behavioural defects |
THRB 3p24.2 |
Syndrome of resistance to thyroid hormone |
| thyroid hormone receptor α |
Thra, Nr1a1 11 |
Behavioural defects Synaptic abnormalities |
THRA 17q11.2 |
unknown |
| type 2 deiodinase |
Dio2 12 |
Deafness Behavioural defects (mild) Reduced T3 content in brain |
DIO2 14q24.2-24.3 |
unknown |
| type 3 deiodinase |
Dio3 12 |
Color visual defect (unpublished) |
DIO3 14q32 |
unknown |
| monocarboxylate TH transporter |
Mct8, Slc16a2 X |
Low uptake of T3 in brain |
MCT8 Xq13.2 |
X-linked mental retardation Allan-Herndon-Dudley syndrome |
| mu-crystallin, NADP-dependent TH binding protein |
Crym 7 |
Low retention of T3 in brain |
CRYM 16p13.11-12.3 |
Non-syndromic deafness |
For references, see text.
For details of chromosome locations, see Mouse Genome Informatics, http://www.informatics.jax.org/ and Online Mendelian Inheritance in Man, http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM
Although it is common knowledge that TH is generally required by the nervous system, our understanding of how this hormone elicits its functions is far from complete. This article reviews a number of functions of TH in the nervous system and the emerging view of the multigenic mechanisms that determine these actions. Evidence suggests that the selective cooperation of a limited number of genes encoding receptors, deiodinases and other components determines the nature, time and place of TH actions in the nervous system.
Early experimental studies
Pioneering investigations into the underlying actions of TH in the brain were performed by Eayrs (Eayrs and Taylor, 1951, Eayrs, 1966) and Legrand (Legrand, 1984) beginning half a century ago. These studies in model species, mainly the rat, revealed several features that are still pertinent to research today. (i) It was evident that TH has multiple different functions in the brain. Thus, hypothyroidism delayed cell migration, outgrowth of neuronal processes, synaptogenesis and myelin formation and also increased glial cell proliferation (gliosis) and neuronal cell death. In contrast, hyperthyroidism tended to promote premature differentiation, also with deleterious consequences on function. (ii) Many observations pointed to a general role for TH as a maturation factor in brain development. The general structure of the brain was formed but defects typically arose at later stages of terminal differentiation. (iii) Many actions of TH occurred within limited time windows of development. This is illustrated by an early study demonstrating a diminished electroencephalogram response in rats made hypothyroid as newborns by thyroidectomy (Bradley et al., 1960). However, if thyroidectomy was deferred until weaning, the electroencephalogram was preserved, indicating that in this system, TH acts within an early developmental period. Temporal sensitivity is a recurrent theme of TH action.
TH action at the cellular level
The nuclear TH receptor (TR) is a ligand-regulated transcription factor and has a central role in transducing the hormonal signal into a cellular response (Sap et al., 1986, Weinberger et al., 1986, Tata, 2006). The transcriptional activity of the TR is not the focus of this article but a few features relevant to the nervous system are mentioned here.
The TR can act as a ligand-regulated repressor or activator on different genes such that target genes fall into both positive (induced) and negative (repressed) categories. Much remains to be learned about the target genes that underlie the response of specific neuronal cell types in vivo. Screens have indicated candidate brain genes that are either up- or down-regulated by TH (Munoz et al., 1991, Lezoualc'h et al., 1992, Iglesias et al., 1996, Thompson, 1996, Anderson et al., 1997, Denver et al., 1997, Alvarez-Dolado et al., 1999, Rodriguez-Pena, 1999, Dowling et al., 2000, Poguet et al., 2003, Quignodon et al., 2007) as has been reviewed elsewhere (Dussault and Ruel, 1987, Thompson and Potter, 2000, Zoeller and Rovet, 2004, Bernal, 2007). In many cases it is still unknown if a gene responds directly or indirectly to TH. Indirect responses may be mediated for example, through intermediary factors or cell-cell signaling (Cuadrado et al., 1999, Lorenzo et al., 2002). It is likely that both direct and indirect target genes contribute to the overall tissue response to TH.
The TR has the ability to bind to chromatin in the absence or presence of ligand, which has implications for the T3-independent regulation of target genes both in normal and abnormal situations (Damm et al., 1989, Sap et al., 1989). Ligand-independent regulation may have a normal role in a nervous tissue niche where hormonal exposure is transiently limited at immature stages, as suggested in different species (Forrest et al., 1990). This has been suggested for example in amphibian eye development (Havis et al., 2006). However, there can be deleterious consequences in the brain in hypothyroid disease where the TR would be persistently locked into an unoccupied state causing long-term changes in gene expression (Hashimoto et al., 2001, Morte et al., 2002).
Transcriptional cofactors are involved in modifying TR activity when it is bound to the chromatin of a target gene. Several cofactors for the TR have been identified (Koenig, 1998) and some such as hairless (Potter et al., 2002), TBL1 (Guenther et al., 2000), Alien (Tenbaum et al., 2003) TRAP220 (Ito and Roeder, 2001) and NCoR (Jepsen et al., 2000) may modify TR activity in the nervous system. Also, mutations in the XPD subunit of the cofactor TFIIH were found to destabilize TR binding to brain target genes and to alter myelination in mice (Compe et al., 2007). XPD mutations occur in human trichothiodystrophy, which is associated with neurological defects resembling those of hypothyroidism including mental retardation, spasticity and hypomyelination.
Non-genomic responses for TH or metabolites of TH have also been proposed based largely on in vitro tissue culture studies (Siegrist-Kaiser et al., 1990). It is unknown if there is a major physiological role for such responses in the nervous system in vivo.
TH availability in the nervous system
TR activity can be controlled at several steps preceding its interaction with a target gene. The amount of TH ligand available to neural tissues is governed initially by the activity of the thyroid gland and by the iodine present in the diet. The thyroid gland releases two major forms of TH, thyroxine (3,5,3’,5’-tetraiodothyronine, T4) and 3,5,3’-triiodothyronine (T3)(Figure 1). T3 is the main active form of hormone that binds the TR although T4 is more abundant than T3 in serum. T3 is also produced locally in some tissues by deiodination of T4, which is considered to be an important mechanism in nervous tissues. T4 can also bind the TR although with much lower affinity than T3 and it is not excluded that in some situations, T4 at sufficiently high levels may directly stimulate responses, as has been discussed recently (Galton et al., 2007).
Developmentally programmed changes in TH levels in the circulation occur from early stages after conception through embryogenesis and postnatal development. Serum T4 and T3 levels are initially low in the fetus but rise in development and increase markedly around birth in humans, providing a general stimulus for many tissues (Burrow et al., 1994). TH transferred from the mother to the fetus is important, especially during early brain development before the onset of fetal thyroid function at mid-gestation in humans (Auso et al., 2004, Mitchell and Klein, 2004, Morreale de Escobar et al., 2004, Zoeller and Rovet, 2004).
The iodine supply determines the ratio of T3 and T4 synthesised by the thyroid gland (Deme et al., 1976). T4 and T3 are produced in a two-step process catalyzed by thyroid peroxidase. Tyrosine residues of thyroglobulin are iodinated yielding mono-(MIT) and diiodotyrosine (DIT) residues, which are coupled to form either T4 or T3 residues. The relative amount of T4 and T3 depends on the DIT/MIT ratio that increases with the level of iodination. In a euthyroid state, T3 is present at only 10–25% of the level of T4 within thyroglobulin but when iodine intake is low the thyroid gland produces more T3 than T4 (Deme et al., 1976). In serum, the concentration of T3 is ~50 times lower than that of T4 which depends on the rate of secretion of T3 from the thyroid gland, its shorter half-life than that of T4 and its peripheral generation or clearance rates (Nicoloff et al., 1972). In iodine-deficiency, T4 levels in serum decline but T3 levels tend to be maintained (Delange and Ermans, 1996). This response may protect some tissues but presumably not those in the nervous system that rely upon local amplification of T3 levels by deiodination from T4. The example of the auditory system is discussed below.
The sensitivity of distinct neuronal populations to hypothyroidism at different phases of development is suggested by the varying outcomes of iodine de ficiency at early or later stages of human development (Dumont et al., 1994, Morreale de Escobar et al., 2004). Deficiency at early stages in utero is associated with severe neurological cretinism with deaf-mutism whereas deficiency at later stages produces a myxedematous cretinism with growth and mental retardation and without severe auditory deficits. Iodine treatment protects the brain and neurological function from iodine deficiency if given early in fetal development but not if given in the third trimester or postnatally (Cao et al., 1994).
Thus, adequate systemic provision of TH is a prerequisite for the development and function of the nervous system. However, target tissues to a large extent govern their specific responses through deiodinases and receptors, as discussed below.
Deiodinases and the nervous system
In the vicinity of the target cell, the level of T3 can be amplified or depleted by deiodination (Köhrle, 1999, Bianco et al., 2002, Brown, 2005, St Germain et al., 2005). Deiodinases are selenocysteine-containing enzymes and are membrane-associated within the cell. Type 2 and type 3 deiodinases are conserved across vertebrate species and both are expressed in the nervous system (Bates et al., 1999, Marsh-Armstrong et al., 1999, Peeters et al., 2001, Yoshimura et al., 2003, Darras et al., 2006). T3 is generated from T4 by type 2 deiodinase (encoded by the Dio2 gene) and is inactivated by type 3 deiodinase (encoded by Dio3) which converts both T4 and T3 into minimally active metabolites. Type 1 deiodinase encoded by Dio1 has both activating and inactivating activities but is more important in homeostasis in liver, kidney and other tissues than it is in nervous tissues. Little or no type 1 deiodination is detected in mammalian brain (Campos-Barros et al., 1996, Kester et al., 2004) and no brain phenotype has been described in Dio1-deficient mice (Schneider et al., 2006). Dio1 is expressed in Xenopus tadpole brain suggesting that there is variation in deiodinase function in non-mammalian species (Morvan Dubois et al., 2006).
Nervous system tissues contain relatively high ratios of T3 / T4 compared to the circulation or non-nervous tissues. A variety of concentrating mechanisms may be at play and the local generation of T3 by type 2 deiodinase is viewed as a major factor (van Doorn et al., 1985, Campos-Barros et al., 2000, Pinna et al., 2002, Kester et al., 2004). Two commonly occurring polymorphisms have been described in the human DIO2 gene (Mentuccia et al., 2002, Peeters et al., 2005) but it is unknown if these are involved in specific changes in brain function. Polymorphisms in the DIO2 locus have been associated with mental retardation in a Chinese population in an iodine-deficient area and it was suggested that this allelic variation may influence T3 availability in an iodine-deficient environment (Guo et al., 2004). Deafness is the most pronounced neurological phenotype known to date in Dio2−/− mice, indicating a major role for type 2 deiodinase in the inner ear (Schneider et al., 2001, Ng et al., 2004) (Table 1). Dio2−/− mice are also averse to descending a vertical pole and lack urgency in escaping from water, perhaps indicating a lowered state of anxiety (Galton et al., 2007). Otherwise, Dio2−/− mice show surprisingly little neuronal dysfunction compared to that caused by hypothyroidism, suggesting that there may be important but as yet undefined means of compensation for the absence of type 2 deiodinase mice. Current evidence therefore suggests that the primary origin of T3 ligand varies in different tissues: (i) In some brain regions, T3 obtained from the circulation may be adequate; (ii) In other brain regions and in the cochlea, additional T3 generated locally by type 2 deiodinase is required.
Less is known so far of the role of type 3 deiodinase, the TH-inactivating enzyme, in the mammalian nervous system. The Dio3 gene is typically expressed earlier in brain development than is Dio2 and it is generally thought to have a protective role at immature stages. Experimental demonstration of such Dio3 functions is required but initial studies of Dio3−/− mice suggest that there are defects in colour vision (Hernandez et al., 2006)(D.F., L. N., A. Hernandez, V. Galton D. St.Germain, unpublished data). A role for type 3 deiodinase is supported by studies in non-mammalian species. During amphibian metamorphosis, type 3 deiodinase regulates retinal cell proliferation (Marsh-Armstrong et al., 1999) and in avian species, it cooperates with type 2 deiodinase in the hypothalamic control of seasonal reproductive behaviour (Yoshimura et al., 2003, Yasuo et al., 2005). Deiodinases confer adaptability on neuronal responses in vertebrate species and further research on these enzymes in the nervous system is likely to be rewarding.
Hormonal uptake and local communication between cells
The cellular uptake of TH provides another level of control of TH action. Uptake may be mediated by various types of transporters including those of the L type amino acid, organic anion and monocarboxylate families (Abe et al., 2002, Friesema et al., 2005, Taylor and Ritchie, 2007). Mutations recently identified in the MCT8 monocarboxylate transporter in human X-linked mental retardation and Allen-Herndon-Dudley syndrome suggest the importance of TH transport in neurological function (Dumitrescu et al., 2004, Friesema et al., 2004, Brockmann et al., 2005, Schwartz et al., 2005, Maranduba et al., 2006). Affected males exhibit psychomotor and speech defects. Mct8-deficient mice have reduced uptake of T3 in brain although neurological phenotypes have not been reported (Dumitrescu et al., 2006, Heuer, 2007, Trajkovic et al., 2007).
Although the deiodinase and transporter genes have been found independently to have a role in nervous tissues, an obvious implication is that their functions converge. Transporters may control not only the uptake of TH from serum or cerebrospinal fluid but also the transfer of T3 ligand between cells following the local generation of T3 by deiodination. This is suggested by the finding that in some brain tissues, glia are the main Dio2-expressing cells which presumably generate T3 whereas the main target cells are adjacent neurons (Guadaño-Ferraz et al., 1997). An even more marked physical separation of T3-generating and T3-responding cells exists in the cochlea. Dio2-expressing tissues surrounding the cochlear duct are exposed to T4 inflow in the vasculature suggesting a model whereby these supporting tissues take up T4, convert the T4 to T3, then release T3 internally to the sensory target tissues (Campos-Barros et al., 2000). Transporters would be a necessary component of these paracrine-like mechanisms. Such local hormonal communication may be the rule rather than the exception for TH action in many neuronal environments.
It is possible that other steps remain to be identified in the chain of events that lead to a cellular response to TH. The CRYM gene encodes mu-crystallin and has been proposed to act as an intracellular, cytosolic “holder” of T3 (Suzuki et al., 2007). Human CRYM mutations have been associated with non-syndromic deafness and in one case, a mutation was shown to impair the T3 binding ability of the protein (Abe et al., 2003, Oshima et al., 2006). However, unlike the human cases, Crym−/− mice have normal auditory function, a discrepancy that is not yet understood (Suzuki et al., 2007). It is unknown if these mice have brain phenotypes.
Thyroid hormone receptors in the nervous system
Functions in the nervous system have been identified for both the Thrb and Thra receptor genes (Table 2). Vertebrate species express three conserved TR isoforms and their functions have been demonstrated by targeted deletions (knockout) and change-of-function (knock-in) mutations in mice (discussed below). Thrb encodes two major isoforms, TRβ1 and TRβ2, whereas Thra encodes a single T3 receptor, TRα1. Other truncated or variant TR products have been found but only in isolated species (Harvey et al., 2007) and these non-canonical products lack known functions in the nervous system.
Table 2. Nervous system phenotypes associated with targeted mutations in thyroid hormone receptor genes in mice.
Nervous system phenotypes associated with targeted mutations in the Thrb and Thra receptor genes in mice. Compiled from studies of both null (knockout) and change-of-function (knock-in) mutations. See text for details.
| Thra, thyroid hormone receptor α | References |
|---|---|
| Reduced glucose utilization in brain | Itoh et al, 2001 |
| Synaptic impairment (whisker-barrel pathway) | Esaki et al, 2003 |
| Learning deficiency | Guadano-Ferraz et al, 2003, Wilcoxon et al, 2007 |
| Cerebellar defects | Morte et al, 2002, Venero et al, 2005 |
| Purkinje cell defects | Heuer et al, 2003 |
| Anxiety-like behaviour | Venero et al, 2005 |
| GABAergic neuron defects | Morte et al, 2003, Venero et al, 2005 |
| Oligodendrocyte differentiation defects | Billon et al, 2002, Baas et al, 2002 |
| Astrocyte differentiation defects | Morte et al, 2004 |
| Mating behaviour abnormality | Dellovade et al, 2000 |
| Neurogenesis defect | Lemkine et al, 2003 |
| Thrb, thyroid hormone receptor β | |
| Deafness | Forrest et al, 1996 |
| Color blindness | Ng et al, 2001 |
| Circadian entrainment defects | Dkhissi et al, 2007 |
| Audiogenic seizure susceptibility | Ng et al, 2001 |
| Mating behaviour abnormality | Dellovade et al, 2000 |
| Cerebellar defects | Hashimoto et al, 2001 |
| Hyperactivity, Impaired “vigilance” | Siesser et al, 2005 |
Selected references only are given. See text for additional references.
Although TRα1, TRβ1 and TRβ2 have similar transactivation properties in vitro, each isoform serves distinct functions in vivo, which is partly determined by its expression pattern (Forrest et al., 1990, Strait et al., 1990, Mellström et al., 1991, Bradley et al., 1992). TRα1 is expressed widely in the brain from early embryonic stages and it is also the major TR isoform in embryonic stem (ES) cells. TRα1 mediates neuronal-like differentiation in ES cells and in the PC12 cell line (Muñoz et al., 1993, Liu et al., 2002, Liu and Brent, 2005). Together with evidence for a role in neurogenesis (Lemkine et al., 2005), these observations suggest functions for TRα1 in neural progenitor cells, both at immature and more mature stages. The widespread expression of TRα1 also suggests other maintenance functions in the brain.
TRβ1 is generally induced later than TRα1 in the brain, suggestive of both overlapping and independent functions. TRβ2 has the most specialized expression pattern of any TR isoform, being restricted to cone photoreceptors, the cochlea, pituitary and paraventricular nucleus of the hypothalamus (Hodin et al., 1989, Sjöberg et al., 1992, Bradley et al., 1994, Abel et al., 2001). In the adult, TRα1 and TRβ1 show overlapping expression patterns in many brain regions. However, the varying proportions of TRα1 and TRβ1 in a cell type may determine whether the response is primarily governed by TRα1 or TRβ isoforms. Examples of predominant expression of a specific isoform include TRα1 in hippocampal neurons (Guadaño-Ferraz et al., 2003) and TRβ2 in retinal cones (Ng et al., 2001a). In certain situations, however, even when TRα1 and TRβ isoforms are co-expressed in a cell, there may be differences in their target gene recognition or cofactor preference that determine independent functions, as suggested in Purkinje cells (Heuer and Mason, 2003). The progressive rise in expression of TRα1 and TRβ1 is in accord with earlier reports of increasing T3 binding capacity in brain during development (Schwartz and Oppenheimer, 1978, Bernal and Pekonen, 1984). In the more mature brain, the total TR mass, regardless of its composition by TRα1 or TRβ isoforms, may be a determining factor in the responsiveness of some cell types.
Thrb functions
The Thrb gene serves a prominent role in sensory systems. Thrb mutations cause deafness in mice (Forrest et al., 1996, Griffith et al., 2002, Shibusawa et al., 2003) and are associated with hearing loss in humans (Brucker-Davis et al., 1996). Thrb-deficiency or hypothyroidism in rodents retards the maturation of many cochlear cell types including the sensory hair cells and deforms the tectorial membrane which mediates transduction of sound (Deol, 1976, Uziel, 1986). Thrb-deficiency retards the maturation of a potassium current in inner hair cells (Rüsch et al., 2001) and hypothyroidism retards the synaptic characteristics of both inner (Brandt et al., 2007, Sendin et al., 2007) and outer hair cells (Uziel et al., 1983). The auditory nerve and central pathways are also sensitive to TH (Dow-Edwards et al., 1986, Knipper et al., 1998).
It is unclear how TH coordinates the late differentiation of so many cell types in the auditory system. However, evidence indicates that although TRβ has the major role, TRα1 also contributes, suggesting that part of the answer lies in cooperation between the two TR genes and the relative levels of TRβ and TRα1 in individual cell types (Bradley et al., 1994, Winter et al., 2006). Indeed, TRβ/TRα1 combined mutants have exacerbated defects consistent with cooperative functions (Rüsch et al., 2001). This role of TH as a global timing signal for the onset of hearing has been proposed to be determined by type 2 deiodinase within the cochlea rather than by TRβ or by circulating T4 and T3 levels. Type 2 deiodinase is induced in the cochlea prior to the onset of hearing and Dio2−/− mice display a cochlear phenotype like that of Thrb−/− mice (Campos-Barros et al., 2000, Ng et al., 2004)(Figure 2). The developmental signals that induce Dio2 expression in the cochlea are currently unknown. The auditory system thus provides a paradigm of functional cooperation between receptors and deiodinases.
Figure 2.
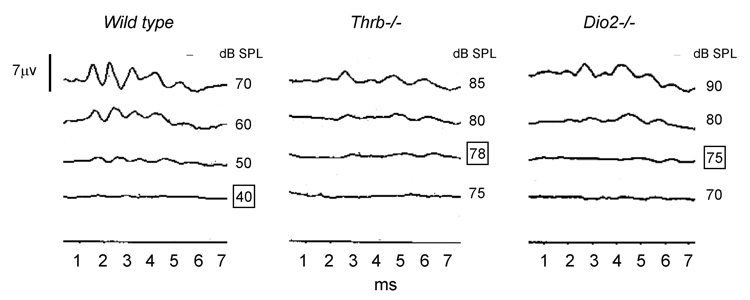
Auditory deficits caused by deletion of Thrb or Dio2 genes in mice. Comparable elevations in auditory thresholds occur in both strains of mice. Thresholds for a click stimulus assessed by the auditory-evoked brainstem response are ~40 dB sound pressure level (dB SPL) in a wild type mouse. Thresholds in Thrb−/− and Dio2−/− examples shown are 78 and 75 dB SPL, respectively (boxed).
A key role for Thrb is in the colour visual system. The TRβ2 isoform is specifically expressed in immature cone photoreceptors (Sjöberg et al., 1992, Jones et al., 2007), in which it determines the differential expression of M (medium-wave) and S (short-wave) opsin photopigments that mediate colour vision (Ng et al., 2001a, Roberts et al., 2006, Applebury et al., 2007). It has also been reported that Thrb influences circadian responses, at least in part because TRβ2 induces M opsin in cones. M cones are thought to contribute to light-dependent entrainment (Dkhissi-Benyahya et al., 2007).
Deletion of Thrb results in audiogenic seizure susceptibility, suggesting another role for TRβ in the central mechanisms that constrain the propagation of the response to sustained auditory stimulation (Ng et al., 2001b). Thrb is also associated with changes in female mating behaviour, possibly through interactions with estrogen signaling in the hypothalamus (Dellovade et al., 2000). Behavioral phenotypes have been identified using knock-in mutations in Thrb that express dominant negative TRβ proteins similar to those found in human resistance to thyroid hormone. The mutations in mice promote hyperactivity, impair learning in a vigilance test (Siesser et al., 2005) and in a water maze and cause motor deficiency and defects in cerebellar Purkinje cells (Hashimoto et al., 2001). Transgenic over-expression of dominant negative TRβ proteins in mice also produces hyperactivity (Wong et al., 1997, McDonald et al., 1998) that may be relevant to symptoms that occur in human resistance to thyroid hormone (Leonard et al., 1995).
Human resistance to thyroid hormone typically shows autosomal dominant inheritance and involves THRB mutations that generate dominant negative TRβ proteins. Neurological symptoms are common but vary in incidence and severity and include attention deficits, hyperactivity, hearing loss and mental retardation (Hauser et al., 1993, Refetoff et al., 1993, Brucker-Davis et al., 1996). As in mice, TRβ2 is detected in human fetal cones (Lee et al., 2006) but the possibility of colour visual defects in human thyroid disorders has not been studied systematically (Newell and Diddie, 1977, Mirabella et al., 2005).
Thra functions
To date, inherited mutations in the human THRA gene have not been reported. In mice, Thra mutations produce a different spectrum of phenotypes than do Thrb mutations and these include defects in behaviour and synaptic function. A Thra knock-in mutation that over-expresses a dominant negative TRα1 causes anxiety-like symptoms and learning defects together with abnormalities in hippocampal inhibitory neurons of the GABA (γ-amino butyric acid) class (Venero et al., 2005). Thra deletion also causes abnormalities in open field and fear conditioning tests (Guadaño-Ferraz et al., 2003, Wilcoxon et al., 2007). The GABAergic, parvalbumin-positive neurons in the CA1 region of the hippocampus that may underlie these defects express greater levels of TRα1 than TRβ, which could explain their being primarily regulated by TRα1 (Guadaño-Ferraz et al., 2003).
Mutations in both Thra and Thrb genes give cerebellar phenotypes resembling in part those caused by hypothyroidism with impaired arborisation of Purkinje cells and delayed migration of granule neurons (Hashimoto et al., 2001, Venero et al., 2005). Studies in explant cultures made from knockout mice suggest that the T3-dependent formation of dendrites by Purkinje cells is mediated by TRα1 intrinsic to the Purkinje cell rather than in the granule neurons (Heuer and Mason, 2003). The cerebellum represents an example of cooperation whereby TRα1 and TRβ may act partly in different cell populations to accomplish the overall differentiation programme (see section on cooperation). At the cellular level, the Thra gene also regulates differentiation of oligodendrocytes in the optic nerve (Baas et al., 2002, Billon et al., 2002) and astroglia in the cerebellum with the latter function being suggested to involve interactions between TRα1 and TRβ1 (Morte et al., 2004).
The Thra gene also regulates glucose utilization, an indicator of synaptic activity, in the brain. In mice, a dominant negative mutation in TRα1 (TRαPV/+) but not in TRβ impaired glucose utilization (Itoh et al., 2001) (Figure 3). This mutation also impaired glucose utilization following stimulation of the whisker-to-barrel pathway, a major tactile sensory function in rodents (Esaki et al., 2003). In accord with these findings, hypothyroidism delays the maturation and activity of the barrel field in rats (Berbel et al., 2001). Although the Thrb gene rather than Thra has the main known role in sensory systems, this evidence suggests that the Thra gene contributes to the central pathways that relay sensory information to other brain regions.
Figure 3.
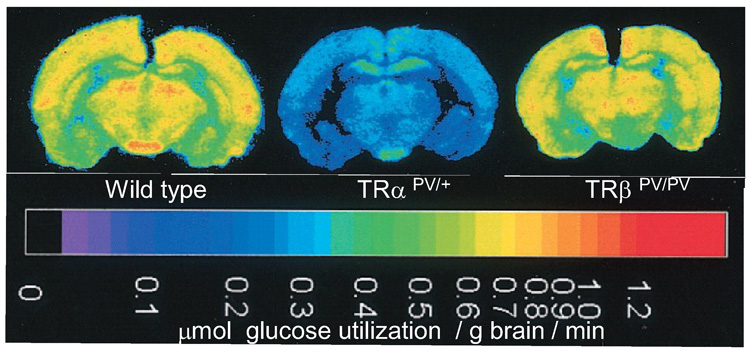
Glucose utilization in the brain of mice carrying TRα or TRβ mutations. Quantitative colour-coded autoradiographs of brain sections from wild type or TRα and TRβ mutant mice. Local rates of glucose utilization established by the deoxyglucose method are encoded in the calibrated color scale at the bottom of the figure. Redrawn (with permission) from Itoh et al Proc. Natl Acad Aci USA, 98: 9913 (2001).
It has been reported that TRα1-deficient mice are surprisingly resistant to the cerebellar defects that result from hypothyroidism (Morte et al., 2002). This result supports the proposal that TRα1 in the absence of ligand is responsible for some of the deleterious consequences of hypothyroidism. This may be explained if TRα1 is chronically locked in an unoccupied state that results in the abnormal regulation of gene networks (Bernal, 2007). Thus, some of the neurological damage in hypothyroidism may be the result of aberrant receptor activity rather than no receptor activity.
Thra and Thrb cooperative functions
Cooperation between the Thra and Thrb genes in the nervous system is likely to be more widespread than is evident from the limited studies described to date. Cooperation may occur at several levels. First, in many scenarios, an individual cell type may express some amount of both TRα1 and TRβ, such that both TR isoforms can contribute to the joint regulation the same target genes. Alternatively, in a more complex scenario, TRα1 and TRβ may act in distinct cell types in a tissue or organ to control an overall neurophysiological function. The cerebellum and auditory system provide examples, as has been discussed. Thus, the cochlea displays overlapping patterns of expression of the Thra and Thrb genes and both genes play a functional role in hearing (Bradley et al., 1994, Rüsch et al., 2001). All of the senses may be subject to some level of control by TH (Freeman and Sohmer, 1995) and it is likely that cooperative functions may be revealed in other sensory systems. Although the TR genes interact positively in many situations, this need not always be the case. An example of opposing functions concerns mating behaviour in mice in which the female lordosis response is reduced by TRα1-deficiency but is increased by TRβ-deficiency (Dellovade et al., 2000).
There is scope for further study of mouse strains lacking all known TRs in which no compensation is possible between TRβ and TRα1. Exacerbated or new phenotypes in the nervous system may be revealed, reflecting the total action of all TR isoforms (Calzà et al., 2000, Baas et al., 2002). Indeed, combined mutations in TRβ and TRα1 are known to exacerbate the phenotype in several non-nervous tissues (Göthe et al., 1999).
TH and the formation of neuronal connections
Eayrs and Legrand showed that a major role of TH in the brain is to promote the growth of axons and dendrites and the formation of synaptic densities. These findings have renewed relevance today in the light of studies of the underlying genes.
The cerebellum, the subject of many of these studies, represents a classical model of TH action in the brain (Figure 4). The cerebellar granule cells proliferate after birth in the external granular layer during the first two postnatal weeks. The granule cells then migrate and their axons, the parallel fibers, make synaptic contacts with the tertiary dendrites of the Purkinje cells. In hypothroid rats, the migration of granule cells is slowed, the granule cell bodies pile up in the molecular layer and the parallel fibers are shortened. Simultaneously, the growth of the dendritic tree of Purkinje cells is impaired (Nicholson and Altman, 1972, Legrand, 1984), the number of synapses is reduced and abnormal contacts result from the delayed interaction between neurons (Vincent et al., 1982). Hypothyroidism also impairs axonal and dendritic outgrowth in other areas including the neocortex (Eayrs, 1966), hippocampus (Rami et al., 1989) and the cochlea (Uziel et al., 1983) and in neuronal cell cultures (Couchie et al., 1986, Heuer and Mason, 2003). TH can regulate axonal outgrowth indirectly by stimulating expression of neurotrophic factors such as NGF or BDNF and of neurotrophin receptors (Clos and Legrand, 1990, Lindholm et al., 1993, Muñoz et al., 1993, Neveu and Arenas, 1996).
Figure 4.
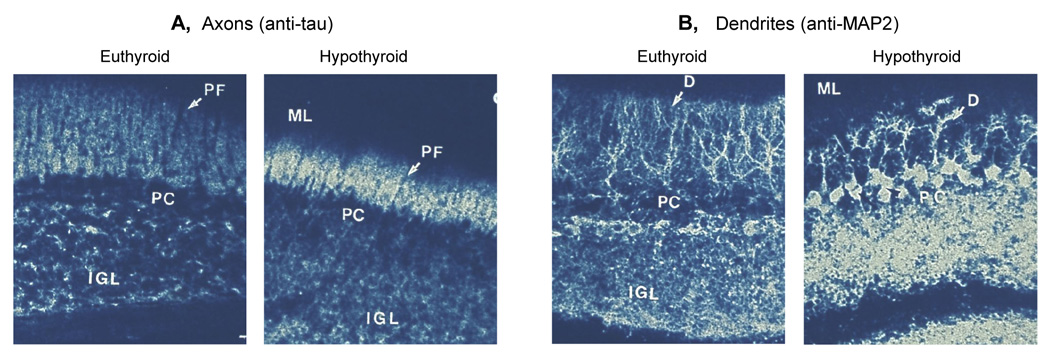
The cerebellum of 14 day old euthyroid and hypothyroid rats stained with antibodies against axon-specific Tau (A) and dendrite-specific MAP2 (B). IGL, internal granular layer, PF, parallel fibres (the axons of granule neurons), PC, Purkinje cell, D, dendrites (of Purkinje cells), ML, molecular layer. Note the shorter parallel fibres and Purkinje cell dendrites in the hypothyroid brain. (Redrawn from Nunez et al, 1989)
Axonal and dendritic outgrowth and the neuronal cytoskeleton
TH regulates axonal and dendritic outgrowth largely through the growth cone, the motile tip of growing axons. The behaviour of the growth cone is driven by dynamic reorganization of actin filaments and microtubules by signaling pathways linked to guidance cue receptors (Dickson, 2002). Microtubule assembly is promoted by microtubule-associated proteins (MAPs), including Tau, which promote polymerization of the α, β tubulin dimer and by interactions with other cytoskeletal and extracellular proteins. MAPs and Tau stabilize microtubules and Tau has a role in neuronal polarity. MAP2 is dendrite specific whereas Tau is axon specific and splice variants of both are produced in development with different polymerizing activity (Mareck et al., 1980).
Hypothyroidism decreases the number of microtubules, their shape and stability in the dendritic tree of Purkinje cells in the cerebellum (Faivre et al., 1983) and in some neurons in the cochlea (Gabrion et al., 1984). TH controls the expression of tubulins and MAPs. The tubulin multigene family expresses six α and five β isotypes leading to microtubules with unique properties. During rat brain development, TH regulates total tubulin amounts (Gonzales and Geel, 1978, Takahashi, 1984), down-regulates α1 and α2 tubulins and up-regulates β4 tubulin (Aniello et al., 1991b, Lorenzo et al., 2002).
The rate of in vitro microtubule assembly is reduced in brain extracts from hypothyroid rodents and is restored to normal by addition of either Tau or MAP2 (Francon et al., 1977). Reduced microtubule assembly and stability depends on the presence at immature stages of MAPs with a lower polymerization activity than adult species (Mareck et al., 1980). In the cortex and cerebellum of hypothyroid adult rats, the proportion of immature Tau proteins remains high (Aniello et al., 1991a). Figure 4 depicts a comparison of normal and hypothyroid rat cerebellum, showing defects in Purkinje cell dendrites and in the parallel fibers, shown by staining for Tau or MAP2 (Nunez et al., 1989). Expression of MAP2 and MAP1 is regulated by TH during development and in adulthood (Benjamin et al., 1988, Silva and Rudas, 1990). The changes induced by hypothyroidism in slow axonal transport protein (Stein et al., 1994) may relate to microtubule stability. TH also regulates expression of monomeric actin (Biswas et al., 1997) and neurofilaments that can alter axonal stability (Marc and Rabie, 1985).
Interestingly, the impairments caused by hypothyroidism partly resemble those described in neurodegenerative diseases. Mutations in adult Tau variants or changes in the ratio of Tau variants have been detected in the brain in frontotemporal dementia and Parkinsonism linked to chromosome 17, Alzheimer’s disease and progressive supranuclear palsy (Spillantini and Goedert, 1998, Andreadis, 2005, Ezquerra et al., 2007).
Synaptic activity and glucose utilization
Maturation of the mammalian brain is accompanied by profound increases in local rates of glucose utilization (Kennedy et al., 1982). In rats made hypothyroid at birth and studied later in adulthood, rates of cerebral glucose utilization are markedly depressed with the greatest decreases occuring in the cerebral cortex and auditory pathways (Dow-Edwards et al., 1986).
Glucose is the sole nutrient used by the brain to produce the energy for synaptic activity. There is a close correlation between local neural activity and glucose metabolism in the nerve endings (Sokoloff, 1981) such that the reduced glucose utilization in the hypothyroid brain might explain some of the decrease in synaptic activity. Studies in mutant mice (Itoh et al., 2001) showed that the regulation of brain glucose utilization depends primarily upon TRα1 rather than TRβ (Figure 3). Mice with a dominant negative mutation in TRα1 but not TRβ had severely impaired utilization of radiolabled glucose in many brain regions. It has been reported that hypothyroidism in rats impairs expression of the glucose transporter GLUT1 in the cerebral cortex, suggesting one type of target gene that may contribute to the reduction in glucose utilization caused by hypothyroidism or mutation in the Thra gene (Santalucia et al., 2006).
It is interesting to note that TH synchronizes the production of glucose in peripheral tissues with the requirement for glucose in the brain. Thus, enzymes involved in gluconeogenesis, glycogen synthesis and glycogenolysis are under TH control in the liver (Feng et al., 2000) and muscle (Clement et al., 2002) such that TH can contribute to increases in the circulating glucose that is required for brain synaptic activity.
Recordings of neuronal populations in hypothyroid rodents have correlated synaptic function in the hippocampus with impaired behaviour in spatial learning tasks (Gilbert and Paczkowski, 2003, Sui and Gilbert, 2003, Sui et al., 2005). These studies revealed abnormalities in hippocampal long term potentiation, a process of synaptic strengthening at the cellular level that is thought to underlie learning and memory (Gilbert and Sui, 2006). Developmental hypothyroidism in rats has been shown to impair GABAergic neurons and to diminish inhibitory synaptic function (Gilbert et al., 2007). These neurophysiological studies add a fascinating and necessary dimension to understanding how TH regulates specific cellular functions that underlie behaviour. Future studies may reveal the molecular mechanisms by which TH regulates particular forms of synaptic activity.
TH and adult brain function
TH deficiency has different consequences in adult and geriatric life than in early development. TH abnormalities in adulthood produce milder symptoms but these can have a pronounced influence on mood and anxiety. Adult abnormalities, unlike those in development, are generally reversible. The association of TH dysfunction and mood disturbance is well recognized although its prevalence is not established (Simon et al., 2002, Bunevicius et al., 2005, Jorde et al., 2006). Nonetheless, since the mood disturbances associated with hypo- and hyperthyroidism in adulthood are reversible, the evaluation of thyroid function represents a cornerstone of the assessment of patients affected by psychiatric disorders (Haggerty et al., 1993). Similarly, since hypothyroidism in the elderly can be associated with cognitive impairment, screening of thyroid function is advocated in the initial evaluation of dementia (Ladenson et al., 2000). Despite the lack of undisputed evidence of effectiveness (Siegmund et al., 2004), TH is considered a valuable adjunct therapy in the treatment of poorly responsive cases of depression (Joffe and Sokolov, 1994, Bauer et al., 2002).
In adults, it may be expected that TH influences neuronal function and maintenance rather than the more profound events that guide brain development. The nature of these adult functions is poorly defined. The question is complicated because of the difficulty in distinguishing between a developmental or adult origin of a phenotype with most inherited mutations, whether in a mouse model or human patient. However, an innovative approach used a mouse Thra mutation that produces a dominant negative TRα1 protein (Venero et al., 2005). This TRα1 protein failed to bind T3 at normal levels but the defect could be overcome with high levels of T3, allowing the mutation to be “switched-off” at a chosen age. Heterozygous mutants exhibited anxiety-like behaviour and locomotor defects. Remarkably, treatment with T3 in adults was able to reverse the anxiety-like behaviour. The mutants exhibited defects in hippocampal GABAergic neurons, suggesting that this type of inhibitory neuron is a target for TH in the adult brain.
Studies in hypothyroid adult rats have also described impairment of long term potentiation in the hippocampal-medial prefrontal cortex pathway that is thought to be involved in learning and memory (Sui et al., 2006). Other studeis of adult rats report that T4 enhances performance in a water maze and increase acetylcholinesterase levels in the prefrontal cortex and hippocampus, suggesting an interaction of TH and cholinergic pathways (Smith et al., 2002).
The sensitivity of the adult brain to TH is suggested by other studies. Hypothyroidism in adult rats reduces neurogenesis in the hippocampus (Desouza et al., 2005) and produces abnormal behaviour in a swim test, suggestive of depressive symptoms (Montero-Pedrazuela et al., 2006). Also, Thra-deficient mice show impaired cell proliferation in the the stem cells in the sub-ventricular zone of the brain, suggesting that TRα1 contributes to neurogenesis in adults (Lemkine et al., 2005). In addition, the expression of neuropeptides and re-myelination may be influenced by TH in adults (Calza et al., 2005), hinting at the range of functions that remain to be uncovered. A question deserving further study concerns the target genes of TH in the adult brain, of which little is known (Iniguez et al., 1992). Knowledge of downstream genes may help to illuminate the extent of overlap in the mechanisms by which TH acts in the developing and adult brain.
Concluding comments
Genetic, cellular and physiological studies have made fascinating progress in elucidating specific functions of TH in the nervous system. It is also interesting to reflect on these functions as a whole rather than as separate parts given that, in life, TH acts in the context of the whole organism.
It is noteworthy that although mice lacking all nuclear TH receptors are runted, they retain vital bodily functions and near normal longevity (Göthe et al., 1999). These observations point to TH as a signal that adjusts many organs from an immature to a mature functional state thereby enhancing the fitness of the individual for autonomous, adult life. In the brain, a similar generalization may apply. Thus, human individuals suffering from cretinism retain basal, primitive brain activities but display deficiencies in the more sophisticated features of mental function, i.e. memory, learning, representation, awareness and, more generally, intelligence. Evidence from animal models suggests that a number of these defects may be explained for example, by deficiencies of different types of synapses (Figure 5). In rodent models, hypothyroidism or receptor defects often prevent neural functions from progressing beyond an immature state. Thus, the brain attains sub-optimal levels of glucose utilization (Dow-Edwards et al., 1986) and the colour visual system acquires only shortwave (“blue”)-sensitive cones, which are thought to represent a rudimentary or default pathway of cone differentiation (Ng et al., 2001a).
Figure 5.
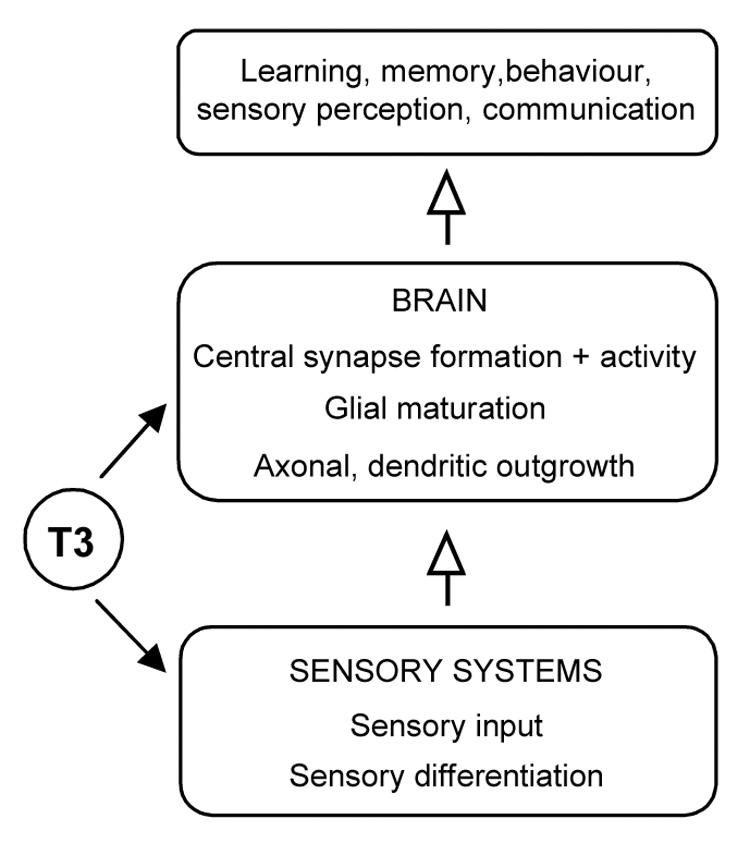
A global view of TH functions in the nervous system. TH coordinates the maturation of sensory systems and the central neuronal network in the brain to promote the function of the nervous system as a complete entity.
The senses have received less attention than the brain but also depend upon TH. TH mediates the timely onset of sensory function during critical periods of development. Thus, the acquisition of information and environmental awareness are impaired by hypothyroidism with consequences for learning, memory and communication. Moreover, sensory inflow during critical periods is thought to modify synaptic connections in central regions and ultimately to shape the maturation of the brain as a functional entity (Hensch, 2004). TH therefore serves an overall role in coordinating the development of both sensory and central systems to ensure that the nervous system as a whole acquires correct function.
The identification of key genes that mediate TH action has set the stage to understand how the integrated function of this relatively limited number of genes determines diverse neurophysiological and neurodevelopmental events. The receptor and deiodinase genes provide different but equally critical levels of control while TH transporters and transcriptional cofactors may further modify the response in specific neuronal populations. One may anticipate that new approaches will continue to reveal new functions for this hormone in the nervous system.
Acknowledgement
This work was supported by the intramural research program at NIDDK/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacques Nunez, Email: jnunez@mail.nih.gov.
Francesco S. Celi, Email: fc93a@nih.gov.
Lily Ng, Email: ngl@niddk.nih.gov.
Douglas Forrest, Email: forrestd@niddk.nih.gov.
REFERENCES
- Abe S, Katagiri T, Saito-Hisaminato A, Usami S, Inoue Y, Tsunoda T, Nakamura Y. Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet. 2003;72:73–82. doi: 10.1086/345398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Suzuki T, Unno M, Tokui T, Ito S. Thyroid hormone transporters: recent advances. Trends in Endocrinol. and Metab. 2002;13:215–220. doi: 10.1016/s1043-2760(02)00599-4. [DOI] [PubMed] [Google Scholar]
- Abel ED, Ahima RS, Boers ME, Elmquist JK, Wondisford FE. Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest. 2001;107:1017–1023. doi: 10.1172/JCI10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Ruiz M, Del Rio JA, Alcantara S, Burgaya F, Sheldon M, Nakajima K, Bernal J, Howell BW, Curran T, Soriano E, Muñoz A. Thyroid hormone regulates reelin and dab1 expression during brain development. J Neurosci. 1999;19:6979–6993. doi: 10.1523/JNEUROSCI.19-16-06979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GW, Hagen SG, Larson RJ, Strait KA, Schwartz HL, Mariash CN, Oppenheimer JH. Purkinje cell protein-2 cis-elements mediate repression of T3-dependent transcriptional activation. Mol Cell Endocrinol. 1997;131:79–87. doi: 10.1016/s0303-7207(97)00095-6. [DOI] [PubMed] [Google Scholar]
- Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739:91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Aniello F, Couchie D, Bridoux AM, Gripois D, Nunez J. Splicing of juvenile and adult tau mRNA variants is regulated by thyroid hormone. Proc Natl Acad Sci U S A. 1991a;88:4035–4039. doi: 10.1073/pnas.88.9.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniello F, Couchie D, Gripois D, Nunez J. Regulation of five tubulin isotypes by thyroid hormone during brain development. J Neurochem. 1991b;57:1781–1786. doi: 10.1111/j.1471-4159.1991.tb06381.x. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Farhangfar F, Glosmann M, Hashimoto K, Kage K, Robbins JT, Shibusawa N, Wondisford FE, Zhang H. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Dev Dyn. 2007;236:1203–1212. doi: 10.1002/dvdy.21155. [DOI] [PubMed] [Google Scholar]
- Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–4047. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- Baas D, Legrand C, Samarut J, Flamant F. Persistence of oligodendrocyte precursor cells and altered myelination in optic nerve associated to retina degeneration in mice devoid of all thyroid hormone receptors. Proc Natl Acad Sci U S A. 2002;99:2907–2911. doi: 10.1073/pnas.052482299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140:844–851. doi: 10.1210/endo.140.2.6537. [DOI] [PubMed] [Google Scholar]
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7:140–156. doi: 10.1038/sj.mp.4000963. [DOI] [PubMed] [Google Scholar]
- Benjamin S, Cambray-Deakin MA, Burgoyne RD. Effect of hypothyroidism on the expression of three microtubule-associated proteins (1A, 1B and 2) in developing rat cerebellum. Neuroscience. 1988;27:931–939. doi: 10.1016/0306-4522(88)90196-0. [DOI] [PubMed] [Google Scholar]
- Berbel P, Auso E, Garcia-Velasco JV, Molina ML, Camacho M. Role of thyroid hormones in the maturation and organisation of rat barrel cortex. Neuroscience. 2001;107:383–394. doi: 10.1016/s0306-4522(01)00368-2. [DOI] [PubMed] [Google Scholar]
- Bernal J, Pekonen F. Ontogenesis of the nuclear 3,5,3'-triiodothyronine receptor in the human fetal brain. Endocrinology. 1984;114:677–679. doi: 10.1210/endo-114-2-677. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Billon N, Jolicoeur C, Tokumoto Y, Vennström B, Raff M. Normal timing of oligodendrocyte development depends on thyroid hormone receptor α 1. EMBO J. 2002;21:6452–6460. doi: 10.1093/emboj/cdf662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SC, Pal U, Sarkar PK. Regulation of cytoskeletal proteins by thyroid hormone during neuronal maturation and differentiation. Brain Res. 1997;757:245–253. doi: 10.1016/s0006-8993(97)00225-4. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS., 3rd Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β 2-subtype, in the developing mammalian nervous system. J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS., 3rd α and β thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc Natl Acad Sci U S A. 1994;91:439–443. doi: 10.1073/pnas.91.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PB, Eayrs JT, Schmalbach K. The electroencephalogram of normal and hypothyroid rats. Electroencephalogr Clin Neurophysiol. 1960;12:467–477. doi: 10.1016/0013-4694(60)90022-5. [DOI] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Munkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J Neurosci. 2007;27:3174–3186. doi: 10.1523/JNEUROSCI.3965-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann K, Dumitrescu AM, Best TT, Hanefeld F, Refetoff S. X-linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. J Neurol. 2005;252:663–666. doi: 10.1007/s00415-005-0713-3. [DOI] [PubMed] [Google Scholar]
- Brown DD. The role of deiodinases in amphibian metamorphosis. Thyroid. 2005;15:815–821. doi: 10.1089/thy.2005.15.815. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni M-A, Koby M, Weintraub BD. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone (RTH) J. Clin. Endocrinol. Metab. 1996;81:2768–2772. doi: 10.1210/jcem.81.8.8768826. [DOI] [PubMed] [Google Scholar]
- Bunevicius R, Velickiene D, Prange AJ., Jr Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves' disease. Gen Hosp Psychiatry. 2005;27:133–139. doi: 10.1016/j.genhosppsych.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- Calza L, Fernandez M, Giuliani A, D'intino G, Pirondi S, Sivilia S, Paradisi M, Desordi N, Giardino L. Thyroid hormone and remyelination in adult central nervous system: a lesson from an inflammatory-demyelinating disease. Brain Res Brain Res Rev. 2005;48:339–346. doi: 10.1016/j.brainresrev.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Calzà L, Forrest D, Vennström B, Hökfelt T. Expression of peptides and other neurochemical markers in hypothalamus and olfactory bulb of mice devoid of all known thyroid hormone receptors. Neuroscience. 2000;101:1001–1012. doi: 10.1016/s0306-4522(00)00420-6. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Hoell T, Musa A, Sampaolo S, Stoltenburg G, Pinna G, Eravci M, Meinhold H, Baumgartner A. Phenolic and tyrosyl ring iodothyronine deiodination and thyroid hormone concentrations in the human central nervous system. J Clin Endocrinol Metab. 1996;81:2179–2185. doi: 10.1210/jcem.81.6.8964848. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci U S A. 2000;97:1287–1292. doi: 10.1073/pnas.97.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XY, Jiang XM, Dou ZH, Rakeman MA, Zhang ML, O'donnell K, Ma T, Amette K, Delong N, Delong GR. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331:1739–1744. doi: 10.1056/NEJM199412293312603. [DOI] [PubMed] [Google Scholar]
- Chatterjee V, Beck-Peccoz P. Resistance to thyroid hormone. In: Degroot L, Jameson J, editors. Endocrinology. 4th ed. Philadelphia, PA: WB Saunders Company; 2001. [Google Scholar]
- Clement K, Viguerie N, Diehn M, Alizadeh A, Barbe P, Thalamas C, Storey JD, Brown PO, Barsh GS, Langin D. In vivo regulation of human skeletal muscle gene expression by thyroid hormone. Genome Res. 2002;12:281–291. doi: 10.1101/gr.207702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos J, Legrand C. An interaction between thyroid hormone and nerve growth factor promotes the development of hippocampus, olfactory bulbs and cerebellum: a comparative biochemical study of normal and hypothyroid rats. Growth Factors. 1990;3:205–220. doi: 10.3109/08977199009043905. [DOI] [PubMed] [Google Scholar]
- Compe E, Malerba M, Soler L, Marescaux J, Borrelli E, Egly JM. Neurological defects in trichothiodystrophy reveal a coactivator function of TFIIH. Nat Neurosci. 2007;10:1414–1422. doi: 10.1038/nn1990. [DOI] [PubMed] [Google Scholar]
- Couchie D, Faivre-Bauman A, Puymirat J, Guilleminot J, Tixier-Vidal A, Nunez J. Expression of microtubule-associated proteins during the early stages of neurite extension by brain neurons cultured in a defined medium. J Neurochem. 1986;47:1255–1261. doi: 10.1111/j.1471-4159.1986.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Bernal J, Munoz A. Identification of the mammalian homolog of the splicing regulator Suppressor-of-white-apricot as a thyroid hormone regulated gene. Brain Res Mol Brain Res. 1999;71:332–340. doi: 10.1016/s0169-328x(99)00212-0. [DOI] [PubMed] [Google Scholar]
- Damm K, Thompson CC, Evans RM. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989;339:593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Darras VM, Verhoelst CH, Reyns GE, Kuhn ER, Van Der Geyten S. Thyroid hormone deiodination in birds. Thyroid. 2006;16:25–35. doi: 10.1089/thy.2006.16.25. [DOI] [PubMed] [Google Scholar]
- Delange F. Iodine deficiency as a cause of brain damage. Postgrad Med J. 2001;77:217–220. doi: 10.1136/pmj.77.906.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delange FM, Ermans A-M. Iodine deficiency. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's The Thyroid. 7th ed. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- Dellovade TL, Chan J, Vennstrom B, Forrest D, Pfaff DW. The two thyroid hormone receptor genes have opposite effects on estrogen-stimulated sex behaviors. Nat Neurosci. 2000;3:472–475. doi: 10.1038/74846. [DOI] [PubMed] [Google Scholar]
- Delong GR, Stanbury JB, Fierro-Benitez R. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism) Dev Med Child Neurol. 1985;27:317–324. doi: 10.1111/j.1469-8749.1985.tb04542.x. [DOI] [PubMed] [Google Scholar]
- Deme D, Gavaret JM, Pommier J, Nunez J. Maximal number of hormonogenic iodotyrosine residues in thyroglobulin iodinated by thyroid peroxidase. Eur J Biochem. 1976;70:7–13. doi: 10.1111/j.1432-1033.1976.tb10949.x. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Pavgi S, Shi Y-B. Thyroid hormone-dependent gene expression program for Xenopus neural development. J. Biol. Chem. 1997;272:8179–8188. doi: 10.1074/jbc.272.13.8179. [DOI] [PubMed] [Google Scholar]
- Deol MS. The role of thyroxine in the differentiation of the organ of Corti. Acta Otolaryngol. 1976;81:429–435. doi: 10.3109/00016487609107497. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D, Crane AM, Rosloff B, Kennedy C, Sokoloff L. Local cerebral glucose utilization in the adult cretinous rat. Brain Res. 1986;373:139–145. doi: 10.1016/0006-8993(86)90323-9. [DOI] [PubMed] [Google Scholar]
- Dowling AL, Martz GU, Leonard JL, Zoeller RT. Acute changes in maternal thyroid hormone induce rapid and transient changes in gene expression in fetal rat brain. J Neurosci. 2000;20:2255–2265. doi: 10.1523/JNEUROSCI.20-06-02255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- Dumont JE, Corvilain B, Contempre B. The biochemistry of endemic cretinism: roles of iodine and selenium deficiency and goitrogens. Mol Cell Endocrinol. 1994;100:163–166. doi: 10.1016/0303-7207(94)90297-6. [DOI] [PubMed] [Google Scholar]
- Dussault JH, Ruel J. Thyroid hormones and brain development. Ann. Rev. Physiol. 1987;49:321–324. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- Eayrs JT, Taylor SH. The effect of thyroid deficiency induced by methyl thiouracil on the maturation of the central nervous system. J. Anat. 1951;85:350–358. [PMC free article] [PubMed] [Google Scholar]
- Eayrs JT. Thyroid and central nervous development. Sci Basis Med Annu Rev. 1966:317–339. [PubMed] [Google Scholar]
- Esaki T, Suzuki H, Cook M, Shimoji K, Cheng SY, Sokoloff L, Nunez J. Functional activation of cerebral metabolism in mice with mutated thyroid hormone nuclear receptors. Endocrinology. 2003;144:4117–4122. doi: 10.1210/en.2003-0414. [DOI] [PubMed] [Google Scholar]
- Ezquerra M, Gaig C, Ascaso C, Munoz E, Tolosa E. Tau and saitohin gene expression pattern in progressive supranuclear palsy. Brain Res. 2007;1145:168–176. doi: 10.1016/j.brainres.2007.01.098. [DOI] [PubMed] [Google Scholar]
- Faivre C, Legrand C, Rabie A. Effects of thyroid deficiency and corrective effects of thyroxine on microtubules and mitochondria in cerebellar Purkinje cell dendrites of developing rats. Dev Brain Res. 1983;8:21–30. [Google Scholar]
- Feng X, Jiang Y, Meltzer P, Yen PM. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol. 2000;14:947–955. doi: 10.1210/mend.14.7.0470. [DOI] [PubMed] [Google Scholar]
- Forrest D, Sjöberg M, Vennström B. Contrasting developmental and tissue-specific expression of α and β thyroid hormone receptor genes. EMBO J. 1990;9:1519–1528. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor β is essential for development of auditory function. Nature Genet. 1996;13:354–357. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- Francon J, Fellous A, Lennon AM, Nunez J. Is thyroxine a regulatory signal for neurotubule assembly during brain development? Nature. 1977;266:188–190. doi: 10.1038/266188a0. [DOI] [PubMed] [Google Scholar]
- Freeman S, Sohmer H. Effect of thyroxine on the development of somatosensory and visual evoked potentials in the rat. J Neurol Sci. 1995;128:143–150. doi: 10.1016/0022-510x(94)00229-h. [DOI] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, Von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- Friesema EC, Jansen J, Milici C, Visser TJ. Thyroid hormone transporters. Vitam Horm. 2005;70:137–167. doi: 10.1016/S0083-6729(05)70005-4. [DOI] [PubMed] [Google Scholar]
- Gabrion J, Legrand C, Mercier B, Harricane MC, Uziel A. Microtubules in the cochlea of the hypothyroid developing rat. Hear Res. 1984;13:203–214. doi: 10.1016/0378-5955(84)90074-1. [DOI] [PubMed] [Google Scholar]
- Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL. Thyroid Hormone Homeostasis and Action in the Type 2 Deiodinase-Deficient Rodent Brain During Development. Endocrinology. 2007;148:3080–3088. doi: 10.1210/en.2006-1727. [DOI] [PubMed] [Google Scholar]
- Gesell A, Amatruda CS, Culotta CS. Effect of thyroid therapy on the mental and physical growth of cretinous infants. Am. J. Diseases of Children. 1936;52:1117–1138. [Google Scholar]
- Gilbert ME, Paczkowski C. Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Brain Res Dev Brain Res. 2003;145:19–29. doi: 10.1016/s0165-3806(03)00191-3. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L. Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res. 2006;1069:10–22. doi: 10.1016/j.brainres.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, Schon JP, Phani S, Goodman JH. Thyroid Hormone Insufficiency during Brain Development Reduces Parvalbumin Immunoreactivity and Inhibitory Function in the Hippocampus. Endocrinology. 2007;148:92–102. doi: 10.1210/en.2006-0164. [DOI] [PubMed] [Google Scholar]
- Gonzales LW, Geel SE. Quantitation and characterization of brain tubulin (colchicine-binding activity) in developing hypothyroid rats. J Neurochem. 1978;30:237–245. doi: 10.1111/j.1471-4159.1978.tb07057.x. [DOI] [PubMed] [Google Scholar]
- Göthe S, Wang Z, Ng L, Nilsson J, Campos-Barros A, Ohlsson C, Vennström B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AJ, Szymko YM, Kaneshige M, Quinonez RE, Kaneshige K, Heintz KA, Mastroianni MA, Kelley MW, Cheng SY. Knock-in mouse model for resistance to thyroid hormone (RTH): an RTH mutation in the thyroid hormone receptor beta gene disrupts cochlear morphogenesis. J Assoc Res Otolaryngol. 2002;3:279–288. doi: 10.1007/s101620010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Obregón M, St. Germain D, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc. Natl. Acad. Sci. USA. 1997;94:10391–10396. doi: 10.1073/pnas.94.19.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Benavides-Piccione R, Venero C, Lancha C, Vennström B, Sandi C, Defelipe J, Bernal J. Lack of thyroid hormone receptor alpha1 is associated with selective alterations in behavior and hippocampal circuits. Mol Psychiatry. 2003;8:30–38. doi: 10.1038/sj.mp.4001196. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40- repeat protein linked to deafness. Genes Dev. 2000;14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Guo TW, Zhang FC, Yang MS, Gao XC, Bian L, Duan SW, Zheng ZJ, Gao JJ, Wang H, Li RL, Feng GY, St Clair D, He L. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J Med Genet. 2004;41:585–590. doi: 10.1136/jmg.2004.019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty JJ, Jr, Stern RA, Mason GA, Beckwith J, Morey CE, Prange AJ., Jr Subclinical hypothyroidism: a modifiable risk factor for depression? Am J Psychiatry. 1993;150:508–510. doi: 10.1176/ajp.150.3.508. [DOI] [PubMed] [Google Scholar]
- Harvey CB, Bassett JH, Maruvada P, Yen PM, Williams GR. The rat thyroid hormone receptor (TR) Deltabeta3 displays cell-, TR isoform-, and thyroid hormone response element-specific actions. Endocrinology. 2007;148:1764–1773. doi: 10.1210/en.2006-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Curty FH, Borges PP, Lee CE, Abel ED, Elmquist JK, Cohen RN, Wondisford FE. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci U S A. 2001;98:3998–4003. doi: 10.1073/pnas.051454698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P, Zametkin AJ, Martinez P, Vitiello B, Matochik JA, Mixson AJ, Weintraub BD. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328:997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- Havis E, Le Mevel S, Morvan Dubois G, Shi DL, Scanlan TS, Demeneix BA, Sachs LM. Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. Embo J. 2006;25:4943–4951. doi: 10.1038/sj.emboj.7601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Mason CA. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor alpha1. J Neurosci. 2003;23:10604–10612. doi: 10.1523/JNEUROSCI.23-33-10604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H. The importance of thyroid hormone transporters for brain development and function. Best Pract Res Clin Endocrinol Metab. 2007;21:265–276. doi: 10.1016/j.beem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hodin RA, Lazar MA, Wintman BI, Darling DS, Koenig RJ, Larsen PR, Moore DD, Chin WW. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989;244:76–78. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Iglesias T, Caubin J, Stunnenberg HG, Zaballos A, Bernal J, Munoz A. Thyroid hormone-dependent transcriptional repression of neural cell adhesion molecule during brain maturation. EMBO. J. 1996;15:4307–4316. [PMC free article] [PubMed] [Google Scholar]
- Iniguez MA, Rodriguez-Pena A, Ibarrola N, Morreale De Escobar G, Bernal J. Adult rat brain is sensitive to thyroid hormone. Regulation of RC3/neurogranin mRNA. J Clin Invest. 1992;90:554–558. doi: 10.1172/JCI115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Roeder RG. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab. 2001;12:127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Kaneshige M, Suzuki H, Cook M, Sokoloff L, Cheng SY, Nunez J. Brain glucose utilization in mice with a targeted mutation in the thyroid hormone α or β receptor gene. Proc Natl Acad Sci U S A. 2001;98:9913–9918. doi: 10.1073/pnas.171319498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, Mcevilly RJ, Kurokawa R, Kumar V, Liu F, Seto E, Hedrick SM, Mandel G, Glass CK, Rose DW, Rosenfeld MG. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–763. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST. Thyroid hormones, the brain, and affective disorders. Crit Rev Neurobiol. 1994;8:45–63. [PubMed] [Google Scholar]
- Jones I, Ng L, Liu H, Forrest D. An intron control region differentially regulates expression of thyroid hormone receptor β2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol. 2007;21:1108–1119. doi: 10.1210/me.2007-0037. [DOI] [PubMed] [Google Scholar]
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145–153. doi: 10.1210/jc.2005-1775. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Sakurada O, Shinohara M, Miyaoka M. Local cerebral glucose utilization in the newborn macaque monkey. Ann Neurol. 1982;12:333–340. doi: 10.1002/ana.410120404. [DOI] [PubMed] [Google Scholar]
- Kester MH, Martinez De Mena R, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, Hume R, Morreale De Escobar G. Iodothyronine levels in 23 the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab. 2004;89:3117–3128. doi: 10.1210/jc.2003-031832. [DOI] [PubMed] [Google Scholar]
- Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- Koenig RJ. Thyroid hormone receptor coactivators and corepressors. Thyroid. 1998;8:703–713. doi: 10.1089/thy.1998.8.703. [DOI] [PubMed] [Google Scholar]
- Köhrle J. Local activation and inactivation of thyroid hormones: the deiodinase family. Mol Cell Endocrinol. 1999;151:103–119. doi: 10.1016/s0303-7207(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, Smith SA, Daniels GH, Cohen HD. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160:1573–1575. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]
- Lee TC, Almeida D, Claros N, Abramson DH, Cobrinik D. Cell cycle-specific and cell type-specific expression of rb in the developing human retina. Invest Ophthalmol Vis Sci. 2006;47:5590–5598. doi: 10.1167/iovs.06-0063. [DOI] [PubMed] [Google Scholar]
- Legrand J. Effects of thyroid hormones on central nervous system development. In: Yanai J, editor. Neurobehavioral Teratology. Amsterdam: Elsevier Science Publishers; 1984. [Google Scholar]
- Lemkine GF, Raj A, Alfama G, Turque N, Hassani Z, Alegria-Prevot O, Samarut J, Levi G, Demeneix BA. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. Faseb J. 2005;19:863–865. doi: 10.1096/fj.04-2916fje. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Martinez P, Weintraub BD, Hauser P. Magnetic resonance imaging of cerebral anomalies in subjects with resistance to thyroid hormone. Am J Med Genet. 1995;60:238–243. doi: 10.1002/ajmg.1320600314. [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F, Hassan AHS, Giraud P, Loeffler J-P, Lee SL, Demeneix BA. Assignment of the β-thyroid hormone receptor to 3,5,3'-triiodothyronine-dependent inhibition of transcription from the thyrotropin-releasing hormone promoter in chick hypothalamic neurons. Mol. Endocrinol. 1992;6:1797–1804. doi: 10.1210/mend.6.11.1480171. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Castren E, Tsoulfas P, Kolbeck R, Berzaghi Mda P, Leingartner A, Heisenberg CP, Tessarollo L, Parada LF, Thoenen H. Neurotrophin-3 induced by tri-iodothyronine in cerebellar granule cells promotes Purkinje cell differentiation. J Cell Biol. 1993;122:443–450. doi: 10.1083/jcb.122.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Tachiki KH, Brent GA. A targeted thyroid hormone receptor α gene dominant-negative mutation (P398H) selectively impairs gene expression in differentiated embryonic stem cells. Endocrinology. 2002;143:2664–2672. doi: 10.1210/endo.143.7.8906. [DOI] [PubMed] [Google Scholar]
- Liu YY, Brent GA. Thyroid hormone-dependent gene expression in differentiated embryonic stem cells and embryonal carcinoma cells: identification of novel thyroid hormone target genes by deoxyribonucleic acid microarray analysis. Endocrinology. 2005;146:776–783. doi: 10.1210/en.2004-1177. [DOI] [PubMed] [Google Scholar]
- Lorenzo PI, Menard C, Miller FD, Bernal J. Thyroid hormone-dependent regulation of Talpha1 alpha-tubulin during brain development. Mol Cell Neurosci. 2002;19:333–343. doi: 10.1006/mcne.2001.1087. [DOI] [PubMed] [Google Scholar]
- Maranduba CM, Friesema EC, Kok F, Kester MH, Jansen J, Sertie AL, Passos-Bueno MR, Visser TJ. Decreased cellular uptake and metabolism in Allan-Herndon-Dudley syndrome (AHDS) due to a novel mutation in the MCT8 thyroid hormone transporter. J Med Genet. 2006;43:457–460. doi: 10.1136/jmg.2005.035840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc C, Rabie A. Microtubules and neurofilaments in the sciatic nerve fibres of the developing rat: Effects of thyroid deficiency. Int J Dev Neurosci. 1985;3:353–358. doi: 10.1016/0736-5748(85)90069-3. [DOI] [PubMed] [Google Scholar]
- Mareck A, Fellous A, Francon J, Nunez J. Changes in composition and activity of microtubule-associated proteins during brain development. Nature. 1980;284:353–355. doi: 10.1038/284353a0. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron. 1999;24:871–878. doi: 10.1016/s0896-6273(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Mcdonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone beta1 receptor gene. Learn Mem. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- Mellström B, Naranjo JR, Santos A, Gonzalez AM, Bernal J. Independent expression of the α and β c-erbA genes in developing rat brain. Mol. Endocrinol. 1991;5:1339–1350. doi: 10.1210/mend-5-9-1339. [DOI] [PubMed] [Google Scholar]
- Mentuccia D, Proietti-Pannunzi L, Tanner K, Bacci V, Pollin TI, Poehlman ET, Shuldiner AR, Celi FS. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002;51:880–883. doi: 10.2337/diabetes.51.3.880. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, Rovet J. Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies. Pediatr Res. 2005;57:902–907. doi: 10.1203/01.PDR.0000157681.38042.B4. [DOI] [PubMed] [Google Scholar]
- Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45–U48. doi: 10.1530/eje.0.151u045. [DOI] [PubMed] [Google Scholar]
- Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernandez-Lamo I, Garcia-Verdugo JM, Bernal J, Guadano-Ferraz A. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006;11:361–371. doi: 10.1038/sj.mp.4001802. [DOI] [PubMed] [Google Scholar]
- Morreale De Escobar G, Obregon MJ, Del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Morte B, Manzano J, Scanlan T, Vennström B, Bernal J. Deletion of the thyroid hormone receptor alpha 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci U S A. 2002;99:3985–3989. doi: 10.1073/pnas.062413299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morte B, Manzano J, Scanlan TS, Vennstrom B, Bernal J. Aberrant maturation of astrocytes in thyroid hormone receptor {alpha}1 knock out mice reveals an interplay between thyroid hormone receptor isoforms. Endocrinology. 2004;145:1386–1391. doi: 10.1210/en.2003-1123. [DOI] [PubMed] [Google Scholar]
- Morvan Dubois G, Sebillot A, Kuiper GG, Verhoelst CH, Darras VM, Visser TJ, Demeneix BA. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology. 2006;147:4941–4949. doi: 10.1210/en.2006-0609. [DOI] [PubMed] [Google Scholar]
- Munoz A, Rodriguez-Pena A, Perez-Castillo A, Ferreiro B, Sutcliffe JG, Bernal J. Effects of neonatal hypothyroidism on rat brain gene expression. Mol Endocrinol. 1991;5:273–280. doi: 10.1210/mend-5-2-273. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Wrighton C, Seliger B, Bernal J, Beug H. Thyroid hormone receptor/c-erbA: control of commitment and differentiation in the neuronal chromaffin progenitor line PC12. J. Cell. Biol. 1993;121:423–438. doi: 10.1083/jcb.121.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu I, Arenas E. Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J Cell Biol. 1996;133:631–646. doi: 10.1083/jcb.133.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell FW, Diddie KR. Typical monochromacy, congenital deafness, and resistance to intracellular action of thyroid hormone. Klin Monatsbl Augenheilkd. 1977;171:731–734. [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Saltó C, Vennström B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001a;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Pedraza PE, Faris JS, Vennstrom B, Curran T, Morreale De Escobar G, Forrest D. Audiogenic seizure susceptibility in thyroid hormone receptor beta-deficient mice. Neuroreport. 2001b;12:2359–2362. doi: 10.1097/00001756-200108080-00015. [DOI] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DL, Galton VA, Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JL, Altman J. The effects of early hypo- and hyperthyroidism on the development of the rat cerebellar cortex. II. Synaptogenesis in the molecular layer. Brain Res. 1972;44:25–36. doi: 10.1016/0006-8993(72)90363-0. [DOI] [PubMed] [Google Scholar]
- Nicoloff JT, Low JC, Dussault JH, Fisher DA. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest. 1972;51:473–483. doi: 10.1172/JCI106835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez J, Couchie D, Brion JP. Microtubule assembly: regulation by thyroid hormone. In: Delong RJ Jr, Condliffe Pg, editors. Iodine and the brain. New York: Plenum Press; 1989. [Google Scholar]
- Oshima A, Suzuki S, Takumi Y, Hashizume K, Abe S, Usami S. CRYM mutations cause deafness through thyroid hormone binding properties in the fibrocytes of the cochlea. J Med Genet. 2006;43:e25. doi: 10.1136/jmg.2005.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler W. Sporadic cretinism in America. Transactions Congress of American Physicians and Surgeons. 1897:169–206. [Google Scholar]
- Peeters R, Fekete C, Goncalves C, Legradi G, Tu HM, Harney JW, Bianco AC, Lechan RM, Larsen PR. Regional physiological adaptation of the central nervous system deiodinases to iodine deficiency. Am J Physiol Endocrinol Metab. 2001;281:E54–E61. doi: 10.1152/ajpendo.2001.281.1.E54. [DOI] [PubMed] [Google Scholar]
- Peeters RP, Van Den Beld AW, Attalki H, Toor H, De Rijke YB, Kuiper GG, Lamberts SW, Janssen JA, Uitterlinden AG, Visser TJ. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab. 2005;289:E75–E81. doi: 10.1152/ajpendo.00571.2004. [DOI] [PubMed] [Google Scholar]
- Pinna G, Brodel O, Visser T, Jeitner A, Grau H, Eravci M, Meinhold H, Baumgartner A. Concentrations of seven iodothyronine metabolites in brain regions and the liver of the adult rat. Endocrinology. 2002;143:1789–1800. doi: 10.1210/endo.143.5.8770. [DOI] [PubMed] [Google Scholar]
- Poguet AL, Legrand C, Feng X, Yen PM, Meltzer P, Samarut J, Flamant F. Microarray analysis of knockout mice identifies cyclin D2 as a possible mediator for the action of thyroid hormone during the postnatal development of the cerebellum. Dev Biol. 2003;254:188–199. doi: 10.1016/s0012-1606(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Potter GB, Zarach JM, Sisk JM, Thompson CC. The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol. 2002;16:2547–2560. doi: 10.1210/me.2002-0115. [DOI] [PubMed] [Google Scholar]
- Quignodon L, Grijota-Martinez C, Compe E, Guyot R, Allioli N, Laperriere D, Walker R, Meltzer P, Mader S, Samarut J, Flamant F. A combined approach identifies a limited number of new thyroid hormone target genes in post-natal mouse cerebellum. J Mol Endocrinol. 2007;39:17–28. doi: 10.1677/JME-06-0054. [DOI] [PubMed] [Google Scholar]
- Rami A, Rabie A, Clos J. The time course of hippocampal cholinergic innervation in the developing hypothyroid rat. A combined histochemical and biochemical study of acetylcholinesterase activity. Int J Dev Neurosci. 1989;7:301–308. doi: 10.1016/0736-5748(89)90035-x. [DOI] [PubMed] [Google Scholar]
- Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocrine Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale De Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A. Oligodendrocyte development and thyroid hormone. J Neurobiol. 1999;40:497–512. doi: 10.1002/(sici)1097-4695(19990915)40:4<497::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rovet J, Daneman D. Congenital hypothyroidism: a review of current diagnostic and treatment practices in relation to neuropsychologic outcome. Paediatr Drugs. 2003;5:141–149. doi: 10.2165/00128072-200305030-00001. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21:9792–9800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santalucia T, Palacin M, Zorzano A. T3 strongly regulates GLUT1 and GLUT3 mRNA in cerebral cortex of hypothyroid rat neonates. Mol Cell Endocrinol. 2006;251:9–16. doi: 10.1016/j.mce.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B. The c-erbA protein is a high affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J, Muñoz A, Schmitt J, Stunnenberg H, Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erbA oncogene product. Nature. 1989;340:242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005;77:41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz HL, Oppenheimer JH. Ontogenesis of 3,5,3'-triiodothyronine receptors in neonatal rat brain: dissociation between receptor concentration and stimulation of oxygen consumption by 3,5,3'-triiodothyronine. Endocrinology. 1978;103:943–948. doi: 10.1210/endo-103-3-943. [DOI] [PubMed] [Google Scholar]
- Sendin G, Bulankina AV, Riedel D, Moser T. Maturation of ribbon synapses in hair cells is driven by thyroid hormone. J Neurosci. 2007;27:3163–3173. doi: 10.1523/JNEUROSCI.3974-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibusawa N, Hashimoto K, Nikrodhanond AA, Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen RN, Wondisford FE. Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J Clin Invest. 2003;112:588–597. doi: 10.1172/JCI18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, Dabers T, Kirsch G, Sanger E, Engel G, Hamm AO, Nauck M, Meng W. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14 : 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf) 2004;60:750–757. doi: 10.1111/j.1365-2265.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- Siegrist-Kaiser CA, Juge-Aubry C, Tranter MP, Ekenbarger DM, Leonard JL. Thyroxine-dependent modulation of actin polymerization in cultured astrocytes. A novel, extranuclear action of thyroid hormone. J Biol Chem. 1990;265:5296–5302. [PubMed] [Google Scholar]
- Siesser WB, Cheng SY, Mcdonald MP. Hyperactivity, impaired learning on a vigilance task, and a differential response to methylphenidate in the TRbetaPV knock-in mouse. Psychopharmacology (Berl) 2005;181:653–663. doi: 10.1007/s00213-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Silva JE, Rudas P. Effects of congenital hypothyroidism on microtubule-associated protein-2 expression in the cerebellum of the rat. Endocrinology. 1990;126:1276–1282. doi: 10.1210/endo-126-2-1276. [DOI] [PubMed] [Google Scholar]
- Simon NM, Blacker D, Korbly NB, Sharma SG, Worthington JJ, Otto MW, Pollack MH. Hypothyroidism and hyperthyroidism in anxiety disorders revisited: new data and literature review. J Affect Disord. 2002;69:209–217. doi: 10.1016/s0165-0327(01)00378-0. [DOI] [PubMed] [Google Scholar]
- Sjöberg M, Vennström B, Forrest D. Thyroid hormone receptors in chick retinal development: differential expression of mRNAs for α and N-terminal variant β receptors. Development. 1992;114:39–47. doi: 10.1242/dev.114.1.39. [DOI] [PubMed] [Google Scholar]
- Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26:45–60. doi: 10.1016/s0149-7634(01)00037-9. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. The deoxyglucose method for the measurement of local glucose utilization and the mapping of local functional activity in the central nervous system. Int Rev Neurobiol. 1981;22:287–333. doi: 10.1016/s0074-7742(08)60296-2. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- St Germain DL, Hernandez A, Schneider MJ, Galton VA. Insights into the role of deiodinases from studies of genetically modified animals. Thyroid. 2005;15:905–916. doi: 10.1089/thy.2005.15.905. [DOI] [PubMed] [Google Scholar]
- Stein S, Oates E, Hall C, Grumbles R, Fernandez L, Taylor N, Puett D, Jin S. Identification of a point mutation in the thyrotropin receptor of the hyt/hyt hypothyroid mouse. Mol. Endocinol. 1994;8:129–138. doi: 10.1210/mend.8.2.8170469. [DOI] [PubMed] [Google Scholar]
- Strait KA, Schwartz HL, Perez-Castillo A, Oppenheimer JH. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J. Biol Chem. 1990;265:10514–10521. [PubMed] [Google Scholar]
- Sui L, Gilbert M. Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology. 2003;144:4195–4203. doi: 10.1210/en.2003-0395. [DOI] [PubMed] [Google Scholar]
- Sui L, Anderson WL, Gilbert ME. Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol Sci. 2005;85:647–656. doi: 10.1093/toxsci/kfi095. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang F, Li BM. Adult-onset hypothyroidism impairs paired-pulse facilitation and long-term potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. Brain Res. 2006;1096:53–60. doi: 10.1016/j.brainres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Suzuki N, Mori J, Oshima A, Usami S, Hashizume K. micro-Crystallin as an intracellular 3,5,3'-triiodothyronine holder in vivo. Mol Endocrinol. 2007;21:885–894. doi: 10.1210/me.2006-0403. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Transplacental effects of 3,5-dimethyl-3'-isopropyl-L-thyronine on tubulin content in fetal brains in rats. Jpn J Physiol. 1984;34:365–368. doi: 10.2170/jjphysiol.34.365. [DOI] [PubMed] [Google Scholar]
- Tata JR. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol. 2006;246:10–20. doi: 10.1016/j.mce.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Taylor PM, Ritchie JW. Tissue uptake of thyroid hormone by amino acid transporters. Best Pract Res Clin Endocrinol Metab. 2007;21:237–251. doi: 10.1016/j.beem.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Tenbaum SP, Juenemann S, Schlitt T, Bernal J, Renkawitz R, Munoz A, Baniahmad A. Alien/CSN2 gene expression is regulated by thyroid hormone in rat brain. Dev Biol. 2003;254:149–160. doi: 10.1016/s0012-1606(02)00023-4. [DOI] [PubMed] [Google Scholar]
- Thompson CC. Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci. 1996;16:7832–7840. doi: 10.1523/JNEUROSCI.16-24-07832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CC, Potter GB. Thyroid hormone action in neural development. Cereb Cortex. 2000;10:939–945. doi: 10.1093/cercor/10.10.939. [DOI] [PubMed] [Google Scholar]
- Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel A, Pujol R, Legrand C, Legrand J. Cochlear synaptogenesis in the hypothyroid rat. Brain Res. 1983;283:295–301. doi: 10.1016/0165-3806(83)90186-4. [DOI] [PubMed] [Google Scholar]
- Uziel A. Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol (Stockh) 1986;429:23–27. doi: 10.3109/00016488609122726. [DOI] [PubMed] [Google Scholar]
- Van Doorn J, Roelfsema F, Van Der Heide D. Concentrations of thyroxine and 3,5,3'-triiodothyronine at 34 different sites in euthyroid rats as determined by an isotopic equilibrium technique. Endocrinology. 1985;117:1201–1208. doi: 10.1210/endo-117-3-1201. [DOI] [PubMed] [Google Scholar]
- Venero C, Guadano-Ferraz A, Herrero AI, Nordstrom K, Manzano J, De Escobar GM, Bernal J, Vennstrom B. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes Dev. 2005;19:2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Legrand C, Rabie A, Legrand J. Effects of thyroid hormone on synaptogenesis in the molecular layer of the developing rat cerebellum. J Physiol (Paris) 1982;78:729–738. [PubMed] [Google Scholar]
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav Brain Res. 2007;177:109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Braig C, Zimmermann U, Geisler HS, Franzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, Christ S, Walsh EJ, Mcgee J, Kopschall I, Rohbock K, Knipper M. Thyroid hormone receptors TRalpha1 and TRbeta differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci. 2006;119:2975–2984. doi: 10.1242/jcs.03013. [DOI] [PubMed] [Google Scholar]
- Wong R, Vasilyev V, Ting Y, Kutler D, Willingham M, Weintraub B, Cheng S. Transgenic mice bearing a human mutant thyroid hormone α1 receptor manifest thyroid function anomalies, weight reduction and hyperactivity. Mol. Med. 1997;3:303–314. [PMC free article] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Nakao N, Takagi T, Follett BK, Ebihara S, Yoshimura T. The reciprocal switching of two thyroid hormone-activating and - inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. doi: 10.1210/en.2005-0057. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]


