Abstract
Substance P (SP) is thought to play a cardinal role in emesis via the activation of central tachykinin NK1 receptors during the delayed phase of vomiting produced by chemotherapeutics. Although the existing supportive evidence is significant, due to lack of an appropriate animal model, the evidence is indirect. As yet, no study has confirmed that emesis produced by SP or a selective NK1 receptor agonist is sensitive to brain penetrating antagonists of either NK1, NK2, or NK3 receptors. The goals of this investigation were to demonstrate: 1) whether intraperitoneal (i.p.) administration of either SP, a brain penetrating (GR73632) or non-penetrating (e.g. SarMet – SP) NK1 receptor agonist, an NK2 receptor agonist (GR64349), or an NK3 receptor agonist (Pro7-NKB), would induce vomiting and/or scratching in the least shrew (Cryptotis parva) in a dose-dependent manner; and whether these effects are sensitive to the above selective receptor antagonists; 2) whether an exogenous emetic dose of SP (50 mg/kg, i.p.) can penetrate into the shrew brain stem and frontal cortex; 3) whether GR73632 (2.5 mg/kg, i.p.)-induced activation of NK1 receptors increases Fos-measured neuronal activity in the neurons of both brain stem emetic nuclei and the enteric nervous system of the gut; and 4) whether selective ablation of peripheral NK1 receptors can affect emesis produced by GR73632. The results clearly demonstrated that while SP produced vomiting only, GR73632 caused both emesis and scratching behavior dose-dependently in shrews, and these effects were sensitive to NK1-, but not NK2- or NK3-receptor antagonists. Neither the selective, non-penetrating NK1 receptor agonists, nor the selective NK2- or NK3-receptor agonists, caused a significant dose-dependent behavioral effect. An emetic dose of SP selectively and rapidly penetrated the brain stem but not the frontal cortex. Systemic GR73632 increased Fos expression in the enteric nerve plexi, the medial subnucleus of nucleus tractus solitarius, and the dorsal motor nucleus of the vagus, but not the area postrema. Ablation of peripheral NK1 receptors attenuated the ability of GR73632 to induce a maximal frequency of emesis and shifted its percent animals vomiting dose-response curve to the right. The NK1-ablated shrews exhibited scratching behavior after systemic GR73632-injection. These results, for the first time, affirm a cardinal role for central NK1 receptors in SP-induced vomiting, and a facilitatory role for gastrointestinal NK1 receptors. In addition, these data support the validation of the least shrew as a specific and rapid behavioral animal model to screen concomitantly both the CNS penetration and the antiemetic potential of tachykinin NK1 receptor antagonists.
Section: Regulatory Systems
Keywords: NK1 receptor, GR73632, emesis, scratching, Substance P, nucleus tractus solitarius, dorsal motor nucleus of the vagus, area postrema
1. INTRODUCTION
Although emetic circuits are not yet fully defined anatomically, control of vomiting appears to involve both central and peripheral mechanisms [58]. The medullary dorsal vagal complex (DVC) in the brain stem, including the nucleus tractus solitarius (NTS), the dorsal motor nucleus of the vagus (DMNX), and the area postrema (AP) are involved in the central mediation of emesis. Emetic afferents to the DVC arise from diverse brain nuclei, and from peripheral structures via the vagus nerve, including the gastrointestinal tract (GIT).
Neurochemically, recent evidence implicates Substance P (SP) in modulating emesis. SP, and neurokinins A and B, are members of the tachykinin neuropeptide family, which preferentially interact with three related G-protein-coupled receptors, the NK1, NK2, and NK3 receptors respectively [50]. SP has been implicated as a primary afferent neurotransmitter via NK1 receptors in various noxious stimuli including some emetogens [1]. NK1 receptors are found on neurons throughout the brain and in the gut and are densely expressed in a number of brain nuclei including the DVC [28,29,34,65]. Indeed, SP-like immunoreactivity and high concentrations of the peptide are found in several anatomical substrates of emesis: 1) the DVC, 2) the peripheral enteric nervous system, and 3) the enterochromaffin cells (EC) of the intestinal mucosa [1,52]. The latter is of particular importance since serotonin and SP release by EC cells could play important roles in the induction of the immediate and delayed phases, respectively, of emesis produced by chemotherapeutic drugs [58]. Also, administration of SP induces emesis in awake dogs or ferrets [8,21], while application to the AP elicits retching even in anesthetized ferrets [1]. While many animal studies have demonstrated a potent antiemetic effect for NK1 receptor antagonists against diverse emetogens, in clinical studies such antagonists lack full efficacy, and can only potentiate the ability of traditional antiemetics in cancer patients receiving chemotherapy [1,35,42,51,58].
The antiemetic action of NK1 receptor antagonists appears central because: 1) brain penetration is required for activity [49]; 2) central injection of CNS penetrant or non-penetrant NK1 receptor antagonists prevents emesis produced by the peripheral administration of cisplatin [21,54]; and 3) there is strong correspondence in the rank order of potency between NK1 receptor antagonists’ ID50s for their antiemetic activity in ferrets, and their ability to suppress foot tappings in gerbils induced by centrally injected NK1 receptor selective agonists [35,49,51]. These models have been used concomitantly as indices for CNS penetration, antagonist activity, and CNS-mediated antiemetic potential of NK1 receptor antagonists. Significant differences between rodent and human NK1 receptors forced the use of alternative animal models such as gerbils, whose NK1 receptors have a tissue receptor affinity profile similar to that of humans [13,49].
Despite this evidence, the question of a central and/or peripheral emetic action for tachykinins is clouded by inconsistent data from current animal models. SP is a potent emetogen in the dog, but only when administered i.v. [8,63]. However, in ferrets the opposite effect was noted [21,32]. Intravenous administration of the NK1 receptor antagonist RPR100893 was effective against cisplatin-induced emesis in ferrets, but did not reduce foot-tapping in gerbils [49]. These disparate findings suggest a peripheral component in addition to the proposed central action. Indeed, centrally- and/or peripherally-acting NK1 receptor antagonists can both prevent emesis produced by systemic administration of cisplatin [1,37,60], and reduce vagal afferent discharge produced peripherally [37,38].
A major factor that has hampered further progress in understanding of clinical aspects of vomiting and their application to chemotherapy-induced vomiting is the lack of a rapid and specific animal model for both the study of the emetogenic potential of SP and related NK1 agonists, and for teasing out the contribution of peripheral NK1 receptor activation. One such possible model is found in the emesis model species, the least shrew (Cryptotis parva) [10]. In rodent models, central administration of NK1 receptor agonists induces a specific behavior analogous to foot-tapping in gerbils called scratching [43]. We had previously observed in the least shrew that the serotonergic 5-HT2A receptor agonist DOI also induced centrally-mediated head-twitch and scratching behaviors analogous to those seen in DOI-treated rodents [11,62], which were sensitive to both 5-HT2A and NK1 receptor antagonists [11,12,62], and our preliminary studies indicated that i.p. injection of the NK1 receptor agonist GR73632 can rapidly induce both emesis and scratching behavior in the least shrew.
When coupled with initial observations of SP-induced emesis [8], and GR73632-induced emesis and scratching, and with the need for a better animal model of emesis, we hypothesized that the least shrew could be used not only as a model for emetic behaviors, but also as a model for behaviorally distinguishing between CNS-penetrating and non-penetrating drugs. Thus, the intent of this study was to validate the least shrew as the model system described above, and to address some of the discussed questions in current emesis-related literature. This was accomplished by: 1) investigating whether peripheral administration of SP, or of brain penetrating and non-penetrating NK1 receptor agonists, can induce emesis and scratching dose-dependently; 2) pharmacologically deciphering which tachykinin receptor is responsible for the induction of these behaviors via the utilization of selective receptor agonists and antagonists; 3) determining whether intraperitoneally-administered SP at emetic doses can enter the brain by analyzing the tissue levels of exogenous SP in the brain stem and frontal cortex; 4) examining Fos-measured neuronal activity in the DVC and GIT enteric neurons following systemic administration of GR73632; and 5) demonstrating the possible role of peripheral NK1 receptors in emesis following their selective peripheral ablation in the gut.
2. RESULTS
Dose-response emesis and scratching studies with tachykinin receptor agonists and antagonists
Intraperitoneal administration of SP (0, 10, 25, 50 and 100 mg/kg) increased the frequency of vomiting [(KW (4, 40) = 25.7, P < 0.0001)] (Fig 1A). Dunn’s multiple comparisons posthoc test showed that relative to the vehicle-treated control group, significant increases in the frequency of vomiting occurred in groups injected with the 50 (382%, P < 0. 01) and 100 (322%, P < 0.05) mg/kg doses of SP. The 10 and 25 mg/kg doses of SP were inactive. The onset of first emesis was rapid, within 1–2 min of SP injection, and the majority of episodes occurred within the first 5 minutes, except one animal which vomited at 25 minutes. Fisher’s exact test showed that the percentage of shrews vomiting in response to SP administration increased in a dose-dependent manner [(χ2 (4, 40) = 27.7, P < 0.0001)] (Fig. 1B). Significant increases (82 and 78%, respectively) in the number of shrews vomiting were seen at 50 (P < 0.001) and 100 mg/kg (P < 0.001) doses of SP. Although in our initial dose-response studies not all shrews vomited in response to either 50 or 100 mg/kg doses of SP, in our subsequent drug interaction studies, all vehicle-pretreated animals vomited in response to 50 mg/kg SP injection. At the doses tested, SP caused no other overt behavioral effect (e.g. scratching).
Figure 1.
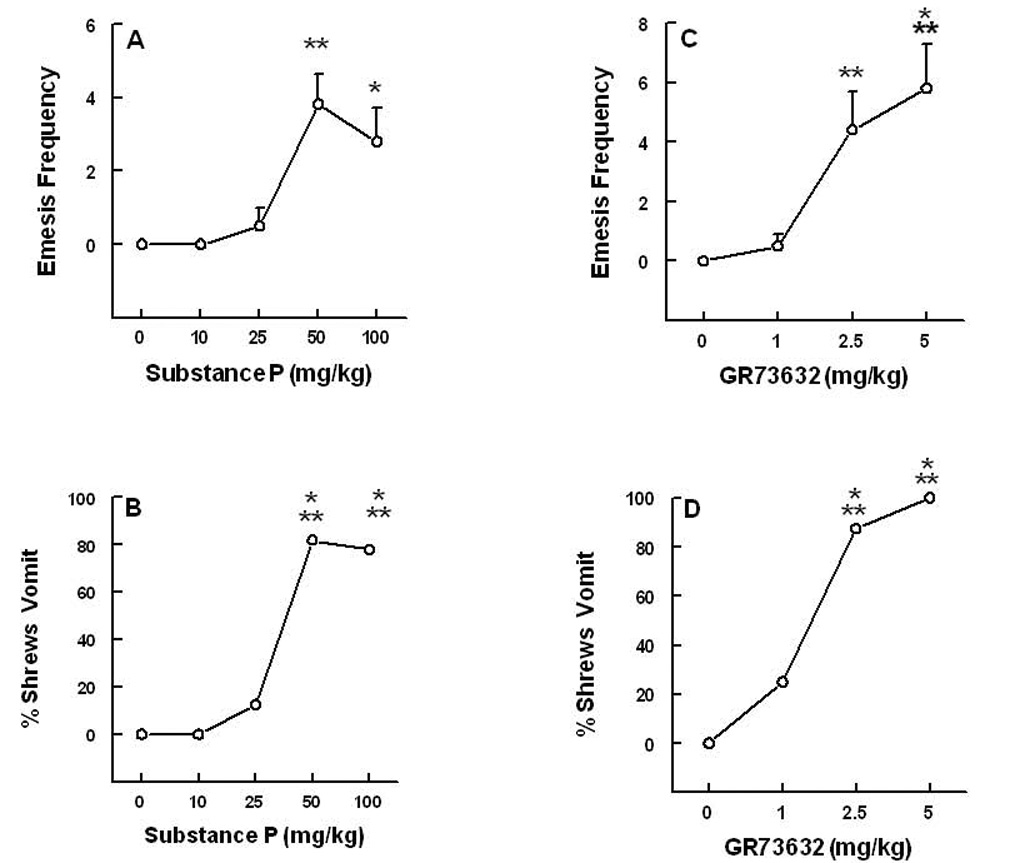
The dose-response emetic effects of varying doses of intraperitoneally-administered substance P (Graphs A and B) and the brain penetrating NK1 receptor selective agonist GR73632 (graphs C and D), during the 30 min post-injection observation period in the least shrew. Graphs A and C depict increases in the frequency of emesis (mean ± S.E.M.), whereas graphs B and D show the percentage of shrews vomiting. Significantly different from corresponding vehicle control (0 mg/kg) at P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***).
The brain penetrating and selective NK1 receptor agonist GR73632 (0, 1, 2.5 and 5 mg/kg) increased the frequency of vomiting in a dose-dependent manner [(KW (3, 32) = 24.9, P < 0.0002)] (Fig 1C). Significant increases in emesis frequency occurred at 2.5 (438%, P < 0.01) and 5 mg/kg (575%, P < 0.001) doses. The percentage of shrews vomiting also increased in a dose-dependent fashion [(χ2 (3, 32) = 26.5, P < 0.0001)] and significant increases in the number of shrews vomiting were observed at 2.5 (87.5%, P < 0.001) and 5 mg/kg (100%, P < 0.001) doses (Fig. 1D). The onset of first emesis was rapid and generally occurred within 3–4 minutes of GR73632 administration and the remaining episodes occurred in the next 15 minutes. Although SP failed to cause scratchings, intraperitoneal injection of GR73632 also caused dose-dependent increases in the frequency of scratching behavior (KW [(3, 30) = 24, P < 0.0001)] (Fig. 2A). Significant increases were seen at 2.5 (P < 0.001) and 5 mg/kg doses (P < 0.001) (Fig. 2A). The CNS non-penetrating NK1 receptor agonists produced minimal emetic and scratching behaviors which were not significantly different from their corresponding vehicle-treated controls. Thus, ASMSP caused emesis in 37% (3 of 8 shrews vomiting), 50% (4 of 8), and 50% (3 of 6) of tested shrews at its 5, 10, and 20 mg/kg doses, respectively. The ASMSP-induced vomiting frequencies (mean ± SEM) were 0.75 ± 0.4 (5 mg/kg dose), 1.23 ± 0.73 (10 mg/kg), and 1.8 ± 0.83 (20 mg/kg) vomits. It also caused 10–15 scratchings, but the effect was neither significant nor dose-dependent relative to the vehicle-treated control group (4.9 ± 2 scratchings, N=10). The third selective NK1 receptor agonist, SarMet-SP, at doses of 1, 5, and 10 mg/kg, caused emesis in 20% (1 of 5 shrews vomiting), 33% (2 of 6), and 0% (0 of 4) of shrews, respectively. SarMet-SP induced vomiting frequencies of 0.6 ± 0.6 (1 mg/kg dose), 1 ± 0.8 (5 mg/kg), and 0 ± 0 (10 mg/kg) vomits, respectively. Likewise, it induced 4–10 scratches which were not dose-dependent. The saporin analog of the latter agent was tested at a 1.2 mg/kg dose and caused emesis in 11% (1 of 9) of tested shrews.
Figure 2.
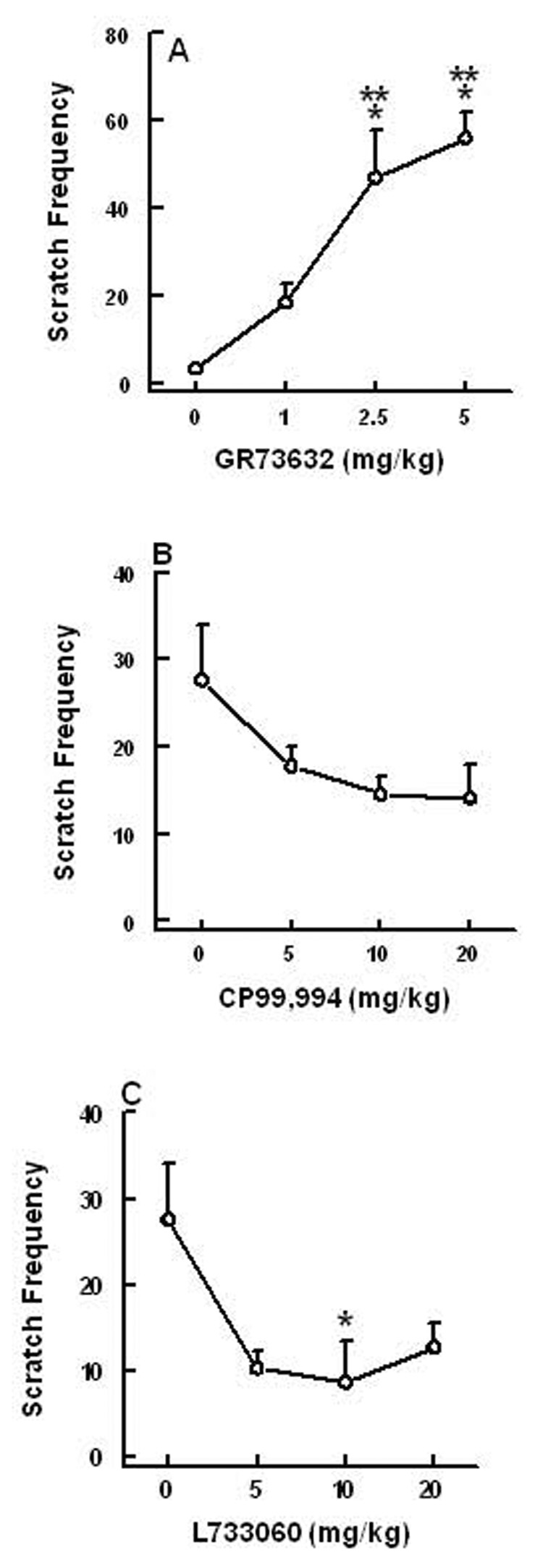
The ability of varying doses of intraperitoneally administered GR73632 (a selective NK1 receptor agonist) to induce dose-dependent increases in the frequency of scratching behavior (mean ± S.E.M.) during a 30 minute observation period in the least shrew (Graph A). Graphs B and C show the ability of varying doses of two structurally diverse but selective NK1 receptor antagonists (CP99,994 and L733060) in suppressing the scratching behavior produced by a 5 mg/kg intraperitoneal dose of GR73632. Significantly different from corresponding vehicle-pretreated control at P < 0.05 (*) and P < 0.001 (***).
In Figure 3, graphs A and B demonstrate the antivomiting activity of the NK1 receptor antagonist CP99,994 (0, 5 and 10 mg/kg, i.p.) against a 50 mg/kg (i.p.) emetic dose of SP. CP99,994 significantly attenuated (84%, P < 0 .01) the frequency of induced emesis [(KW (2, 26) = 13.6, P < 0.001)] as well as protecting (70%, P < 0.003) shrews from emesis [(χ2 (2, 26) = 14, P < 0.001)] at a dose of 10 mg/kg (Fig. 3A, B). CP99,994 (0, 5, 10 and 20 mg/kg) also dose-dependently reduced both the frequency (61, 87 and 97% respectively) [(KW (3, 42) = 21.2, P < 0.0001)] and the percentage of (2.9, 60 and 73% respectively) shrews vomiting [(χ2 (3, 42) = 26.41, P < 0.0001)] in response to a 5 mg/kg dose GR73632 (Fig. 3C, D). Indeed, significant reductions in both GR73632-induced emetic parameters were seen at its 10 (P < 0.01) and 20 mg/kg (P < 0.001) doses. Although CP99,994 (0, 5, 10 and 20 mg/kg) also caused up to 49% reduction in the frequency of scratching, the reduction failed to attain significance (Fig. 2B). Likewise, the second tested NK1 receptor antagonist, L733060 (0, 5, 10 and 20 mg), attenuated both the frequency of vomiting [(KW (3, 40) = 14.4, P< 0.002)] and the percentage of shrews vomiting [(χ2 (3, 40) = 14.1, P < 0.02)] in response to GR73632, at 5 (62%, P > 0.05; 33%, P > 0.05), 10 (74 %, P > 0.05; 53%, P < 0.01) and 20 mg/kg (95%, P < 0.01; 73%, P < 0.001), respectively, (Fig. 3E, F). Both CP99,994 and L733060 only delayed the onset of first emesis induced by GR73632 at their highest tested effective antiemetic doses. L733060 also reduced (63, 69 and 54%, respectively) the GR73632-induced scratchings [(KW (3, 40) = 3, P < 0.043)], and a statistically significant reduction was seen at its 10 mg/kg dose (P < 0.05) (Fig. 2C).
Figure 3.
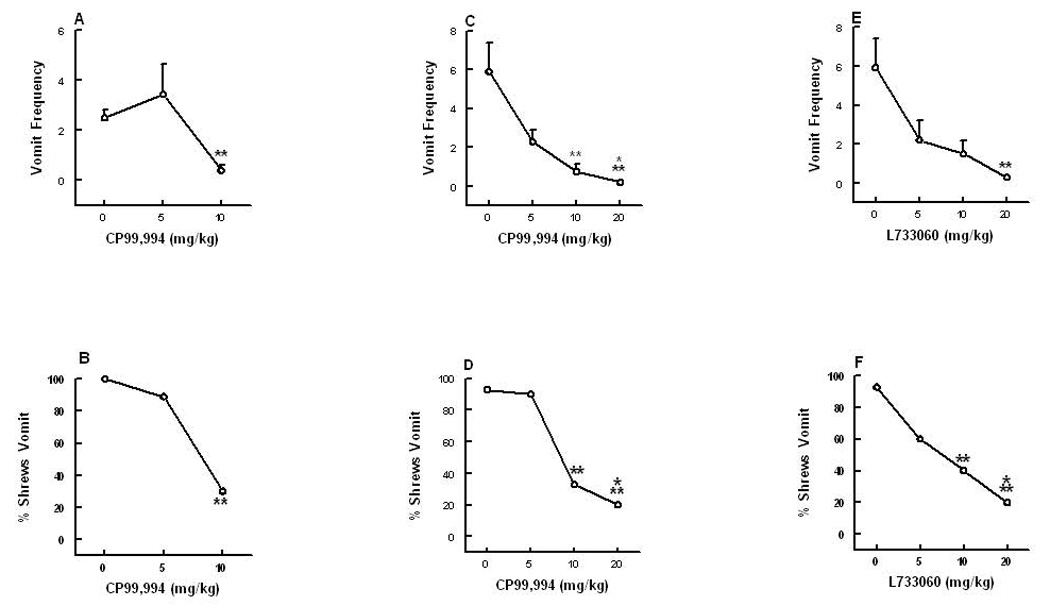
The antiemetic effects of the neurokinin NK1 receptor selective antagonist CP99,940 against substance P (graphs A and B)- and GR73632 (graphs C and D)-induced emesis in the least shrew. Graphs E and F show the ability of another NK1 receptor selective antagonist L733060 to suppress emesis produced by GR73632. Different groups of shrews received i.p. vehicle (0 mg/kg), or varying doses of CP99,994 (5, 10 or 20 mg/kg) or L733060 (5, 10 and 20 mg/kg), 30 min prior to an emetic dose of either substance P (50 mg/kg) or GR73632 (5 mg/kg). Emetic parameters were recorded for 30 min post emetic injection. Graphs A, C and E depict attenuations in the frequency (mean ± S.E.M.) of emesis, whereas graphs B, D and F show reductions in the percentage of shrews vomiting. Significantly different from vehicle control at P < 0.01 (**) and P < 0.001 (***).
Pretreatment with either the NK2 - (GR159897; 20 mg/kg i.p.) or the NK3- (SB218795; 20 mg/kg, i.p.) receptor antagonists did not significantly affect the frequency of either emesis (Fig. 4A) or scratching (Fig. 4C) behaviors induced by a 5 mg/kg dose of the NK1 receptor agonist GR73632. These antagonists also failed to affect (P > 0.05) the percentage of shrews vomiting in response to GR73632 (Fig. 4B). Moreover, these antagonists did not produce any behavioral effect by themselves at a 20 mg/kg dose. In addition, neither the vehicle (N=10), nor the selective NK2- (GR64349) or NK3- (Pro7-NKB) receptor agonists, produced emesis or significant scratchings at 5 or 10 mg/kg doses. Indeed, 1 out of 8 animals vomited in response to either dose of GR64349, while none vomited (0 out of 6) in response to doses of 5 or 10 mg/kg Pro7-NKB.
Figure 4.
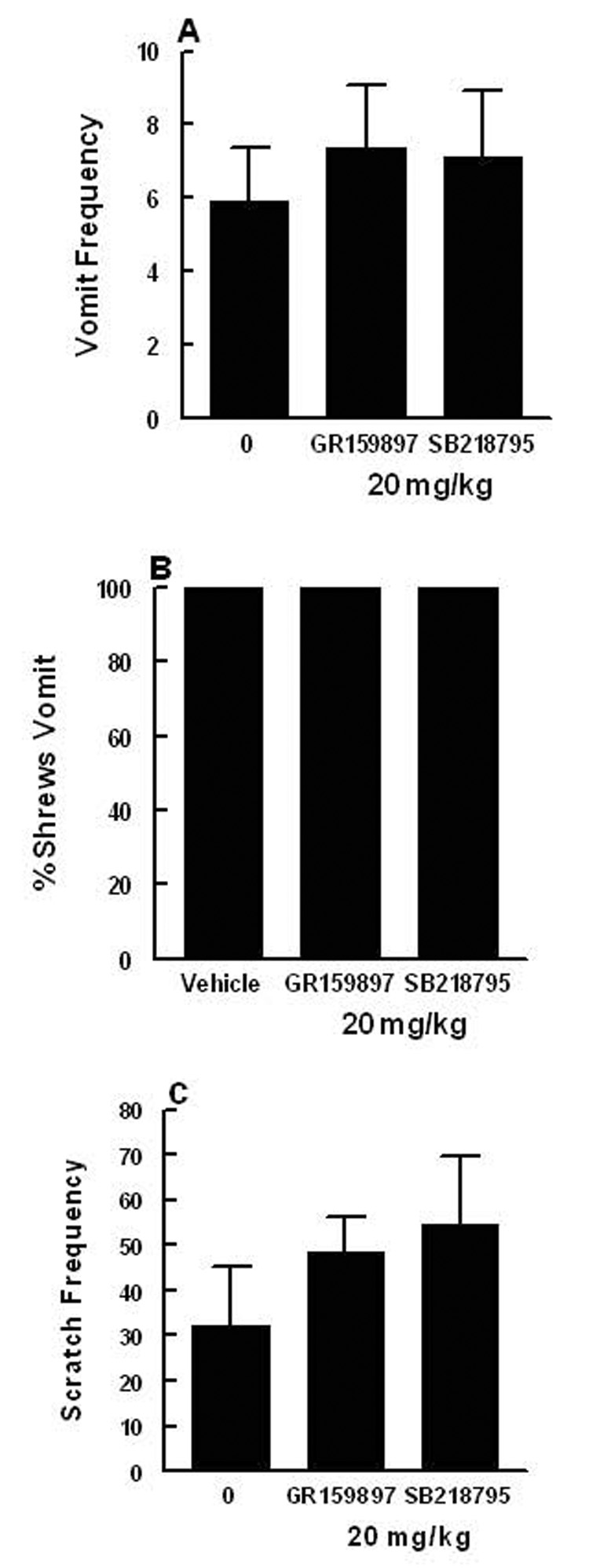
The lack of effect of either a selective NK2 receptor antagonist GR159897 (20 mg/kg, i.p.), or a selective NK3 receptor antagonist SB218795 (20 mg/kg, i.p.), on the ability of a 5 mg/kg intraperitoneal dose of the selective NK1 receptor agonist GR73632 to produce emesis and scratching behavior. Graph A represents the frequency of emesis (mean ± S.E.M.), graph B depicts the percentage of shrews vomiting, and graph C shows the frequency of scratching (mean ± S.E.M.).
Analysis of SP in shrew serum, gut and brain
Intraperitoneal administration of a 50 mg/kg dose of exogenous SP initially significantly increased the basal level of brain stem SP in a time dependent manner [(F (3,21) = 13.8 P < 0.0001)] (Fig. 5A). Indeed, significant increases occurred at 5 (P < 0.05) and 15 minutes (P < 0.01) post-injection, which returned to basal tissue level by 30 minutes. On the other hand, the basal frontal cortex SP concentration concomitantly decreased (P > 0.05, P < 0.05, and P < 0.01 at 5, 15, and 30 minutes respectively) in a time-dependent fashion [(F (3,21) = 5.3, P < 0.01)] (Fig 5A). Both duodenal (P < 0.01) and jejunal (P < 0.01) tissue levels significantly increased within 5 minutes of exogenous SP injection, which then either remained unchanged or decreased by 15 minutes post-administration, but still were significantly (P < 0.01 and P < 0.05, respectively) higher than their basal values by several fold [(F (2,17) = 8.3, P < 0.003)] and [(F (2, 17) = 8.9, P < 0.002)], respectively (Fig. 5B). Likewise, the basal SP blood serum level dramatically increased at 5 minutes post-injection and rapidly declined towards baseline values by 10 minutes [(F (3,15) = 29.7, P < 0.0001)] (Fig. 5C).
Figure 5.
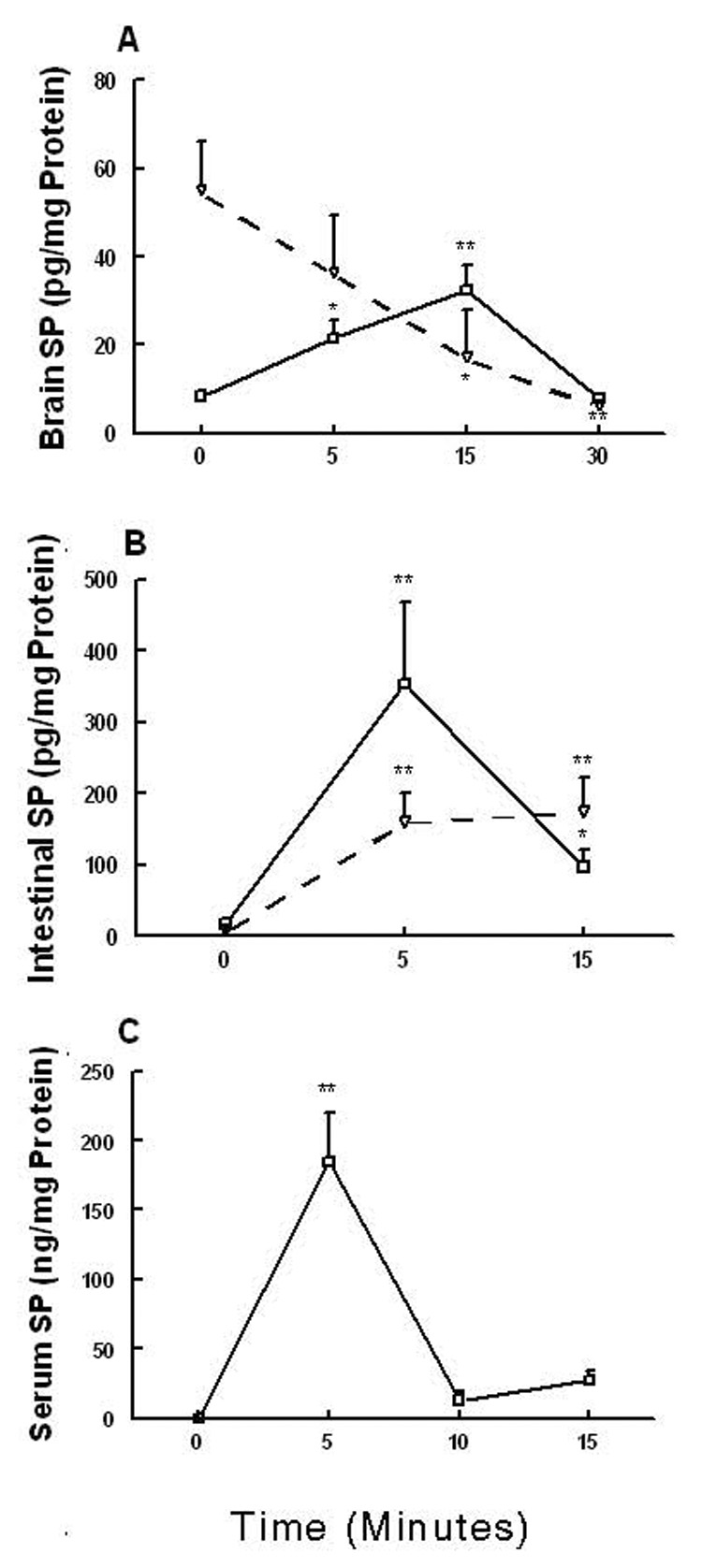
Demonstrates time-dependent distribution of exogenously administered substance P (50 mg/kg, i.p.) in: A) brain stem (▬) and frontal cortex (----), B) duodenum (▬) and jejunum(----), and C) blood serum. Significantly different from corresponding basal level at P < 0.05 (*) and P < 0.01 (**).
Fos-immunoreactivity following GR73632 injection
Intraperitoneal injections of the NK1 receptor agonist GR73632 (2.5 mg/kg) induced vomiting in 5 of 6 shrews and retching in the sixth shrew, whereas saline-injected controls (N = 6) did not vomit or retch at all. Photomicrographs of Fos immunoreactivity (Fos-IR) in GR73632 and saline-injected shrews are shown in Fig. 6. Table 1 enumerates the mean number of Fos-IR nuclei (mean ± SEM) per region of interest in GR73632-injected or saline-injected shrews, and of Fos-IR nuclei per 1 cm sliced length of enteric nervous system (i.e. small intestine, see Methods). Significant differences between GR73632 and saline injected control group were found in the NTS, the DMNX and the enteric nervous system. No significant effect was observed in the AP between the control and treated shrews.
Figure 6.
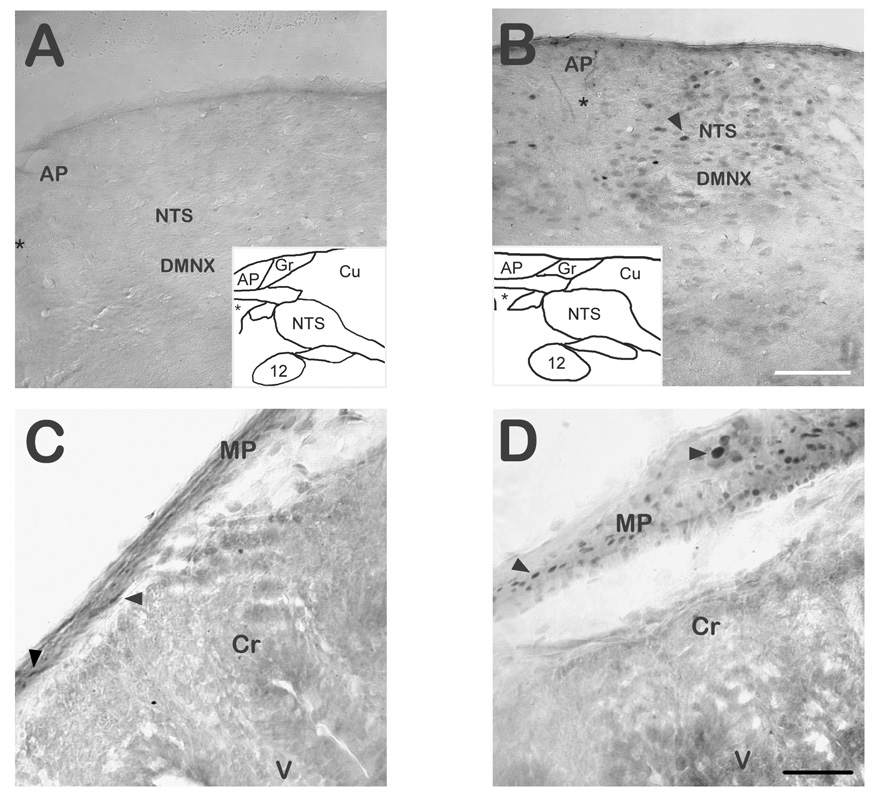
Fos Immunoreactivity (Fos-IR) in the DVC of GR73632 injected shrews and controls. A) Coronal hemisection of the dorsal vagal complex (DVC) area of a non-vomiting least shrew (saline control) stained for Fos-IR. B) Fos-IR stained coronal section of the DVC in a shrew which vomited after being given 2.5 mg/kg (i.p.) GR73632. Insets in A and B diagrammatically represent the coronal level studied, and asterisks represent the central canal in the image and corresponding inset. The NTS shows a strong induction of Fos-IR, the DMNX exhibits weaker induction, while the AP is devoid of it following either saline or GR72632 injection. Scale bar for A and B = 100 µm. C) Fos-IR in the myenteric plexus and intestinal wall of a control shrew. Cells in crypts and villi did not produce Fos-IR, but scattered Fos-IR nuclei were found in the myenteric plexus of shrews following either saline (C) or GR73632 (D) injection. D) Fos-IR is greatly enhanced in the myenteric layers (arrowheads) of the GR73632-injected shrew. Scale bar for C and D = 40 µm. Abbreviations: 12 – 12th (hypoglossal) cranial nerve nucleus; AP – area postrema; Cr - intestinal crypts; Cu – cuneate nucleus and fiber tract; DMNX – dorsal motor nucleus of the vagus nerve; Gr – gracile nucleus; MP – intestinal wall layers including myenteric plexus; NTS – nucleus of the solitary tract; V – intestinal villi.
Table 1.
Comparison of Fos-IR Nuclei per Region of Interest Following Injection of GR73632 or Saline. Data is presented (mean ± SEM) for each group and each region of interest. Significance level was determined by two-tailed Student’s T-test between groups, and was considered statistically significant when p ≤ 0.05. Significant differences between GR73632 and saline injected groups (N=6 per group) were found in the NTS, the DMNX, and the ENS.
| Region of Interest | GR73632 | Saline | Significance level |
|---|---|---|---|
| PFC | 116.8 ± 27.9 | 173 ± 26.4 | 0.25 |
| NTS | 20.5 ± 2.8 | 8.1 ± 2.9 | 0.004 |
| AP | 4.8 ± 1.7 | 2.0 ± 1.2 | 0.178 |
| DMNX | 4.9 ± 1.2 | 1.8 ± 0.7 | 0.047 |
| ENS | 11.8 ± 3.2 | 5.2 ± 2.6 | 0.05 |
Abbreviations: AP – area postrema; DMNX – dorsal motor nucleus of the vagus nerve; ENS – enteric nervous system; NTS – nucleus of the solitary tract; PFC – prefrontal cortex.
Histological analysis of SSP-Saporin lesions
Results of immunolabeling following saporin-based immunolesion are presented in Fig. 7. There was a clear loss of NK1 receptor-IR in the gut (Fig. 7C’), but not the brain (Fig. 7A’), of the SSP-saporin injected shrews versus corresponding saline-treated controls (Fig. 7C, A). SP-IR in the brain appeared similar to that of controls (Fig. 7B/B’), but intestinal SP-IR (Fig. 7D’) was reduced relative to saline-treated controls (Fig. 7D). Although somata in the nerve plexi were generally intact, fibers and terminal-like structures normally penetrating the villi and crypts of the intestinal wall appeared less dense to varying degrees. Fos-positive nuclei following GR73632 administration were found in saline-preinjected controls in the nerve plexi and the medullary DVC (Fig. 6 B/D), but only in the DVC in SSP-saporin-preinjected shrews (Fig. 7B’ inset). NK1 receptor-IR and SP-IR in shrews injected with saporin or blank-saporin were not different from that found in control shrews (data not shown). SP-IR in the brain appeared similar to that of controls (Fig. 7B/B’).
Figure 7.
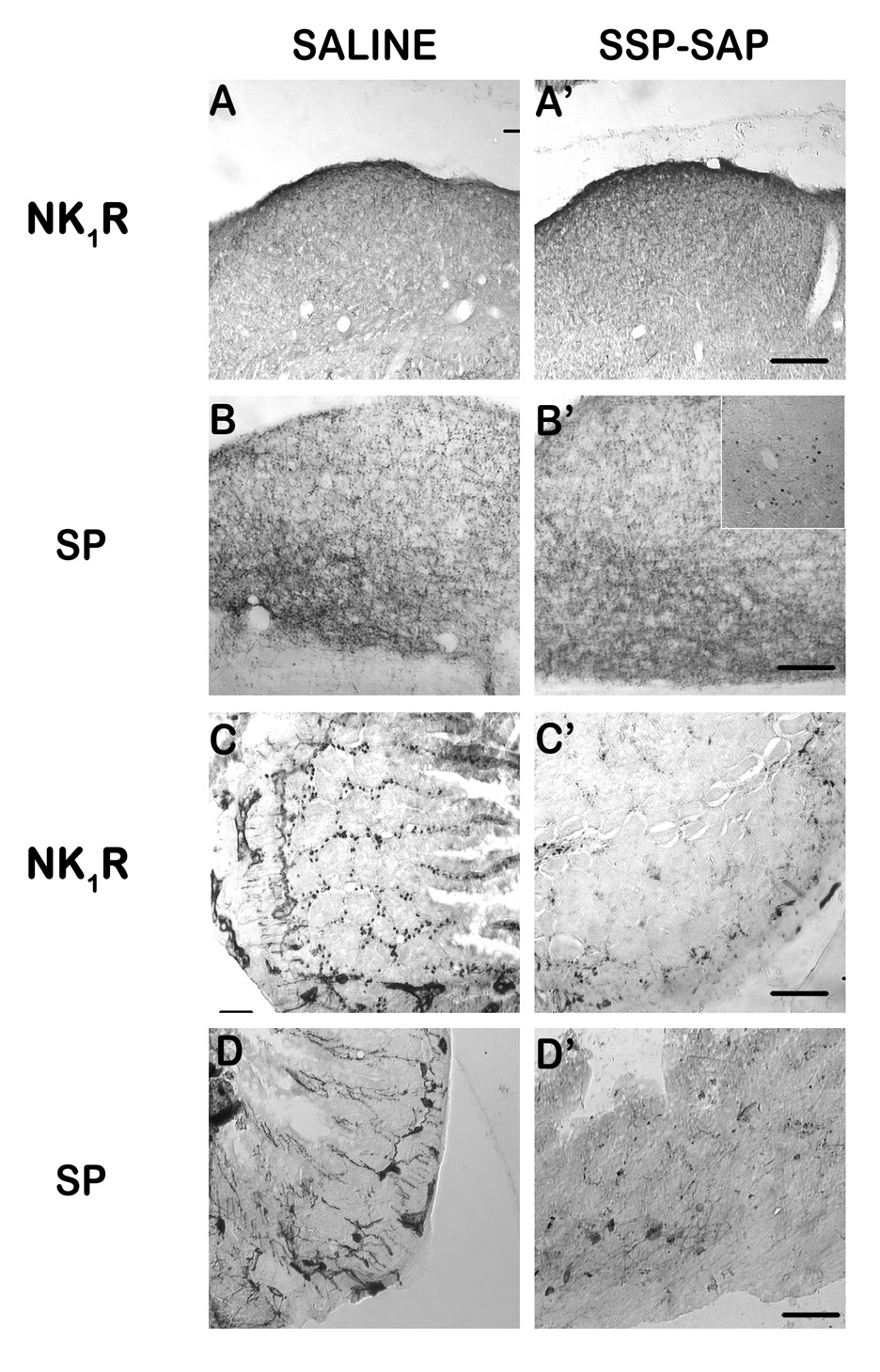
Immunohistochemical analysis of immunotoxin lesion. SSP-SAP (1.2 mg/kg, i.p.) was injected to lesion NK1 receptor-containing cells in the gut. Immunolabeling for NK1 receptors and Substance P (SP) was used to assess the lesion. A–B) Labeling in the dorsal vagal complex (DVC) of saline-(A/B) or SSP-SAP-injected (A’/B’) shrews appeared normal for both NK1 receptor (A/A’) and SP (B/B’). C–D) Relative to saline control (C), labeling in the small intestine showed a distinct and extensive loss of NK1 receptor (C’) containing cell bodies and fibers in the myenteric plexus, crypts, and villi, although the loss was not complete. SP-containing cell bodies in the intestinal nerve plexi were present in both saline (D) and SSP-SAP (D’) treated shrews, but fibers extending into the intestinal villi and crypts appeared to be reduced in number relative to controls. The inset in B’ shows the presence of Fos-IR within the DVC following i.p. injection of GR73632 and vomiting in a SSP-SAP-injected shrew, demonstrating that the NTS is still functionally responsive to emesis. Scale bars (Except inset) = 50 µm.
Emesis and scratching studies following NK1 receptor immunotoxic lesions with SSP-saporin
Challenge administration of different doses of GR73632 (0, 1, 2.5, and 5 mg/kg) in different groups of NK1 receptor-intact control shrews pretreated with a single injection of saline 4 days prior to the challenge test procedure caused dose-dependent increases in the frequency of emesis [(KW (3, 31) = 20.4, P < 0.00014)] and scratchings [(KW (3, 31) = 18.2, P < 0.0004)] as well as the percentage of shrews vomiting (0, 44, 62.5 and 100%) [(χ2 (3, 31) = 18.5, P < 0.0001)] (Fig. 8A, B, C). Post hoc analysis indicated that the emesis frequency, the percentage of shrews vomiting and the number of scratches were respectively increased at 1 (P < 0.02, P < 0.05, P < 0.001), 2.5 (P < 0.006, P < 0.013, P < 0.001), and 5 mg/kg doses (P < 0.001 for all cases). GR73632 challenge in saporin-pretreated NK1 receptor-ablated shrews also significantly increased the frequency of emesis [(KW (3, 26) = 19.4, P < 0.0001)], the percentage of shrews vomiting (0, 0, 28 and 100%, respectively) [(χ2 (3, 26) = 22.7, P < 0.0001)], and the number of scratchings [(KW (3, 26) = 13.2, P < 0.004)] (Fig. 8A, B, C). However, post hoc analysis indicated while the frequency of emesis and the percentage of shrews vomiting increased significantly only at 5 mg/kg (P < 0.001 in both cases), the number of scratches increased in a significant manner at all tested doses of GR73632 (P < 0.02, P < 0.001 and P < 0.001, respectively). In addition, significant differences were observed among the NK1 intact control and NK1- ablated shrews for some doses of GR73632 in emesis parameters but not in the scratching behavior. Indeed, the 1 mg/kg GR73632 dose caused about 2 vomits in 44% of normal shrews, but was unable to induce emesis in any of the NK1 ablated shrews (P < 0.041 and P < 0.04, respectively) (Fig. 8A, B). Although relative to normal shrews a smaller proportion of NK1-ablated shrews vomited in response to 2.5 mg/kg GR73632, the difference did not attain significance and both groups produced nearly identical mean frequencies of emesis. On the other hand, the 5 mg/kg dose caused emesis in all of the tested animals, however, the NK1 ablated shrews exhibited significantly less bouts of vomiting (P < 0.046). Overall, the percent shrew vomit dose-response curve in NK1 ablated shrews seems to be shifted to the right of its corresponding control curve in normal shrews (Fig. 8B).
Figure 8.
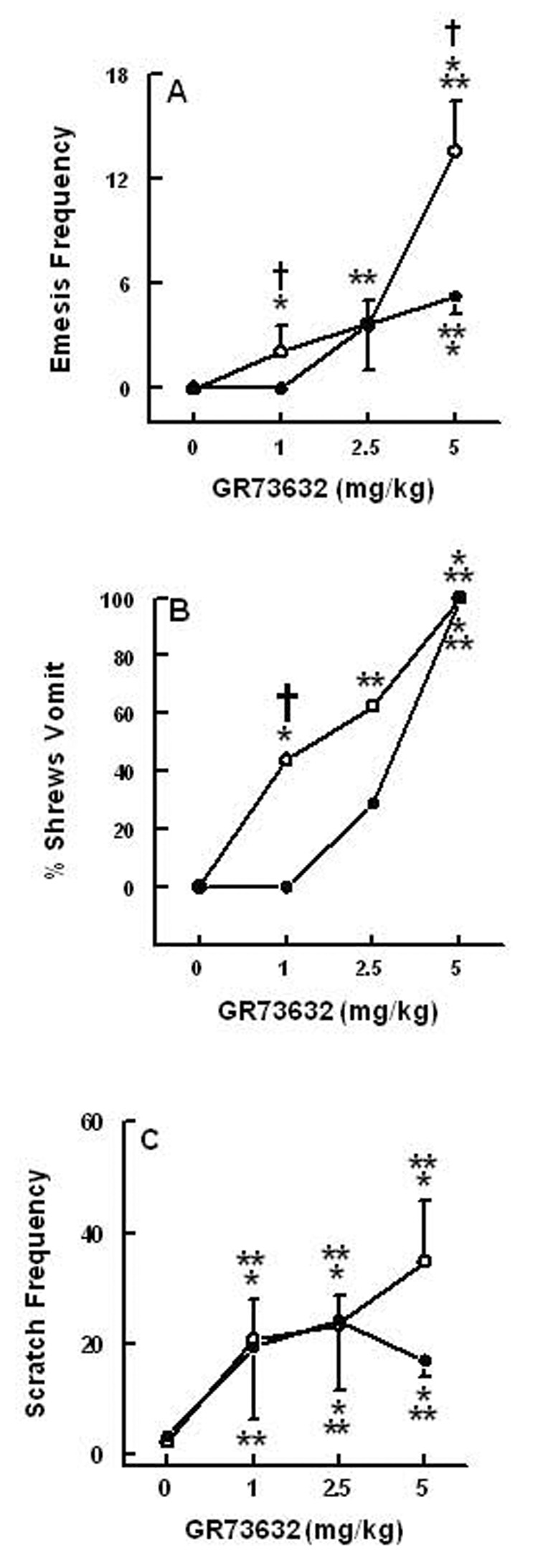
The emetic and scratching dose-response effects of intraperitoneally administered doses of the brain penetrating selective NK1 –receptor agonist GR63762 in normal (O) and in peripherally NK1-receptor ablated (•) shrews during the 30 min observation period immediately following NK1 agonist injection. On day 1 groups of shrews were treated i.p. either with saline (control) or 1.2 mg/kg SSP-saporin (peripheral NK1 receptor-ablated shrews) and on day 4 were challenged with varying doses of GR73632. Graph A shows dose-dependent increases in the frequency of emesis (mean ± S.E.M.), whereas graph B depicts the percentage of shrews vomiting. Graph C presents dose-dependent increases in the frequency of (mean ± S.E.M.) scratching behavior. Significantly different from corresponding vehicle control at P < 0.01 (**) and P < 0.001 (***); or significantly different from corresponding dose in NK1 receptor -intact shrew control group (normal, O) at P < 0.05†.
The most dramatic effect of NK1 receptor ablation on GR73632-induced emesis was qualitative. Indeed, in the NK1 receptor-intact animals, the duration of emesis with corresponding rhythmic abdominomuscular retching movements in a cephalad direction and accompanying opening of the mouth to expel gastrointestinal contents was approximately 2–4 seconds. On the other hand, in NK1 receptor-ablated shrews, the process of expulsion of food/liquid was much more prolonged lasting 15–30 seconds. Thus, the discussed retching movements with corresponding ondulatory mouth openings were continuously present until emesis occurred but these animals seemed unable to initiate a significant retroperistaltic gastrointestinal movement to expel the vomit.
3. DISCUSSION
In the absence of a suitable animal model, the emetic ability of SP and the role of brain stem NK1 receptors in vomiting has mainly been based upon indirect evidence. This study is the first report to systematically characterize the direct emetic effects of systemic SP, and to correlate brain penetrance and central vs. peripheral NK1 receptor activity with emesis in a vomiting species.
BEHAVIORAL EFFECTS OF SUBSTANCE P RELATE TO CNS PENETRATION
Intraperitoneal administration of SP caused dose-dependent increases in emesis, confirming published data that i.v.-administered SP is a robust emetogen [8]. There is evidence that peripheral peptides can influence the brain directly in that systemically-administered SP induces centrally-mediated effects in rodents [6,27,46]. Although unlikely to pass the blood-brain-barrier under physiological conditions, SP may gain entrance rapidly by a specific transport mechanism [9,18], through the AP or other circumventricular organs (CVO’s) in the CNS. The AP is located dorsal to the NTS, outside the blood-brain and cerebrospinal fluid barriers, and possesses active influx and efflux transport proteins [3] and sensitivity to bloodborne chemicals [17]. Furthermore, capillaries from the AP make vascular links with the NTS, which itself has fenestrated capillaries with high permeability [23,47]. Exogenously administered SP can be degraded rapidly in larger species [19,41] and particularly quickly in the least shrew, given that a large emetic dose (50 mg/kg, i.p.) attained maximal blood serum concentration within 5 minutes of injection, and quickly declined towards basal levels within 5 more minutes. Although maximal duodenal and jejunal tissue concentrations occurred within 5 minutes of injection, levels remained significantly above baseline for up to 15 minutes. Baseline concentrations of brain tissue SP (8.5-55 pg/mg protein) were similar to those seen in rat brain (8–80 pg/mg protein) [14]. Brain stem SP-tissue levels required a longer time to reach maximum (15 minutes) as well as to decline to basal levels (also 15 minutes), likely due to brain penetration being limited by access to CVO’s. SP levels increased time-dependently in the brain stem, but not in the frontal cortex, indicating SP entry was selectively confined to the DVC area, a result also seen following intracarotid injection in rats [33]. These findings correspond well with SP-induced emesis, in that the onset of emesis occurred within 1–2 minutes of injection, and the remaining episodes mainly occurred within 5 minutes of injection. These data support a rapid entry of SP into the brain stem without conflicting with published data [3,9,18,33] describing a specific, carrier-mediated transport mechanism for SP.
Since SP can be rapidly transported into the brain, we investigated the emetic effects of NK1 receptor selective agonist analogs of SP whose varied compositions alter their CNS-penetrating abilities. SarMet-SP is an undecapeptide, while ASMSP is a modified hexapeptide, and GR73632 is a modified pentapeptide. The first two agonists caused no more than a couple of vomiting episodes, in 30–50% of tested shrews, and with few scratchings. None of these effects were statistically significant or dose-dependent. While there may be species differences involved because of NK1 receptor differences, the inability of the latter compounds to significantly induce such behaviors appears to be due to poor penetration into the brain stem [9], a mechanism supported by the fact that such agonists must be centrally administered to induce motor behaviors [16,64]. Indeed, species differences do not appear to affect the potency/efficacy of NK1 receptor agonists [16]. Despite the likely low affinity for the SP active transport system, small amounts of the relatively non-penetrant agonists could still pass into the brain at large doses, and this leakage could produce the weakly emetic response seen in some shrews. In the current study, in addition to emesis, systemic administration of the brain-penetrating NK1 receptor agonist GR73632 [25] in the least shrew concomitantly produced scratching behavior in a dose-dependent manner. This behavior, analogous to mouse ear-scratching, is mediated centrally in both rodents and shrews via the stimulation of NK1 or serotonergic 5-HT2A receptors [11,12,16,62]. The crosstalk between the two neurotransmitter systems is demonstrable, in that blockade of NK1 receptors prevents serotonergically induced scratching and head-twitching behavior in mice [12]. Foot tapping in gerbils, a similar centrally-mediated behavior, can be induced by systemic GR73632 as well [49]. Since SP is metabolized primarily in the liver [1], the amount reaching the brain from the periphery is limited. Three lines of evidence support the cardinal role of brain penetration in correlating NK1 receptor activity and behavior: 1) systemic GR73632 caused dose-dependent emesis at less than 5% of the dose of systemic SP; 2) systemic SP failed to induce scratching; and 3) the measured basal SP concentration in shrew frontal cortex (a possible locus for scratching behavior [62]) actually decreased, indicating exogenous SP did not penetrate the telencephalon. One possible cause for a decrease in telencephalic SP is activation of inhibitory somatodendritic NK1 receptors on serotonergic dorsal raphe neurons [48]. It is possible that the high levels of exogenous SP in the brainstem/midbrain activated these receptors, and thus reduced endogenous SP levels in the frontal cortex via a negative feedback mechanism similar to that described for serotonin [22]. Indeed, NK1 receptor antagonists seem to potentiate serotonin tissue levels in the frontal cortex via such a mechanism [24].
NK1 RECEPTORS MEDIATE EMESIS AND SCRATCHING
Since SP can simultaneously activate all three neurokinin receptors at the doses used here, we investigated the emetic effects of selective agonists for all three NK receptor subtypes. Our results demonstrate that SP appears to induce vomiting via the activation of NK1 receptors. The NK1 receptor selective agonist GR73632 caused significant emesis and scratching at 1 and 2.5 mg/kg, and maximal effects at 5 mg/kg, while the NK2- and NK3-receptor agonists (GR64349 and Pro7-neurokinin B, respectively) were without effect at 10 mg/kg (see figure 1, figure 4, and figure 8). Emesis induced by SP itself was sensitive to the selective nonpeptide NK1 receptor antagonist, CP99,994. Only selective NK1 receptor antagonists (CP99,994 and L733060) fully prevented GR73632-induced emesis (10 –20 mg/kg) in a dose dependent fashion, while a 20 mg/kg dose of selective NK2- and NK3-receptor nonpeptide antagonists (GR159897 and SB18795, respectively) failed to affect the induced vomiting. The antiemetic effects of some NK1 receptor antagonists (e.g. GR205171 and CP99,994) have also been confirmed against other emetogens in a larger species of shrews, Suncus murinus [20,55]. Although only a single dose of NK2 or NK3 antagonist was used in this study, this dose was based on literature and pilot studies, and was chosen on the basis of being a relatively high dose, but not so high as to induce nonselective effects. Thus, these results for the first time affirm a direct role of NK1 receptors in SP-induced emesis.
Although both of the tested NK1 receptor antagonists were fully effective against vomiting, these agents reduced but did not completely prevent GR73632-induced scratching. CP99,994 tended to attenuate the scratching frequency but the reduction just failed to attain significance, while L733060 significantly reduced scratchings. Several factors can account for the difference: 1) scratching is a highly variable behavior and the large standard error within groups would influence the statistical outcome; and 2) the duration of CNS action of CP99,994 is very short due to rapid metabolism and brain efflux [49,59]. Indeed, CP99,994 can prevent foot tapping in gerbils when administered i.v. just before, but not when administered p.o. one hour prior to, centrally-administered GR73632 [49]. Neither of the tested NK2- or NK3- receptor agonists induced scratching behavior, nor did their selective antagonists modify GR73632-induced scratching. Thus, the induced scratching behavior is also mediated by NK1 receptor activation in the least shrew.
CENTRAL AND PERIPHERAL MECHANISMS CONTRIBUTE TO EMETIC BEHAVIOR
Although the effect of an NK1 receptor agonist on emesis-related Fos induction in the DVC of an emetic species has not yet been reported, other emetic stimuli induce strong Fos expression in neurons in the medial subnucleus of the NTS, and less robustly but significantly in the DMNX, of vomiting species including the house musk shrew [4,31,36,45,57]. Analysis of Fos-IR demonstrated that systemic administration of an emetic dose of GR73632 produced a similar expression pattern in the medial subnucleus of the NTS and in the DMNX of the least shrew. The NTS as a whole is not functionally related to emesis, and in least shrews the entire NTS is approximately 240 µm long [44]. Thus, in coronal sections, only rarely did more than one section contain the medial NTS. However, the differences in Fos-IR between vomiting and non-vomiting control groups in those sections were both visually clear and statistically significant. Not surprisingly, the absolute numbers of nuclei are not large, given the small size of the brain. However, the magnitude of the increase in DVC Fos-IR following GR73632 injection was comparable or nearly so to that following cisplatin in the ferret [57]. The minor differences are likely due to the different emetogens used. The discussed studies also indicate that while some emetic stimuli induce Fos expression in the AP, others don’t [31,36]. In the current study, GR73632 had no significant effect on Fos-IR in the AP. This apparent lack of stimulation suggests that GR736332 is acting directly in the NTS and/or DMNX, or on afferent terminals within these nuclei, to induce vomiting following its penetration into the brain stem. One drawback to Fos-based studies is the broad range of Fos-activating stimuli, which can leave unanswered the question of whether the Fos-IR is related to the induction, or to the expression (e.g. motor output), of the behavior. This question is not easily resolved without alternate methodologies (e.g. electrophysiological recording), but hypotheses can be drawn based on previous data regarding the function of the area in question. The NTS is impacted by numerous emesis-mediating afferents (e.g. vagal afferents) and serves as a major integrating site for diverse emetic stimuli [2,5,31,57], but does not directly generate motor activity. Thus, increased Fos-IR in the NTS is more likely to be related to induction of emesis rather than the motoric expression of vomiting. The DMNX, however, has both motor output and local circuit neurons, and thus Fos-IR in the DMNX could be due to either the motor output or to stimulation of local circuit neurons.
The GIT can be another potential anatomical substrate of GR73632-induced emesis. NK1 receptors are present on vagal afferents, in the enteric nervous system (ENS), and in intestinal tissue [1,26,28,30], and may directly or indirectly stimulate intestinal motility [7,15,28,29]. Indeed, Fos-IR was frequently noted in the ENS independent of emesis. However, in vomiting shrews, a modest but significant increase in Fos-IR in the ENS was found. These findings, combined with the ability of SP to generate retroperistalsis [40], and to relax the lower esophageal sphincter (an event occurring in emesis) via NK1 receptors [53], are compelling evidence for involvement of peripheral NK1 receptors in vomiting.
In addition to quantifying Fos-IR, we used i.p.-administered SSP-saporin to specifically lesion the gastrointestinal NK1 receptor system. Previous studies have shown both the effectiveness and specificity of this immunotoxin when injected directly into the CNS in other animal models [39,56,61]. Our results show that peripherally-administered SSP-saporin does not penetrate the blood-brain barrier, even via the AP, as evidenced by the completely normal immunoreactivity for both NK1 receptor and SP in the DVC. The 1.2 mg/kg dose used is a low dose, but large enough to eliminate NK1 receptor-IR within at least the segment of small intestine harvested at perfusion. Peripheral NK1 receptor ablation caused profound quantitative and qualitative changes in the ability of GR73632 to induce emesis. In addition to a reduction in the number of shrews vomiting in response to varying doses of GR73632, the NK1 receptor-ablated shrews also exhibited significantly smaller mean frequencies of vomits. Interestingly, while the largest tested dose (5 mg/kg) still induced emesis in all ablated shrews, these animals were unable to execute each vomit normally. Rather, the rhythmic retching movements with corresponding mouth openings, which normally required 2–4 seconds to expel the vomit in naive shrews, required 15–30 seconds for the completion of each ejection, possibly because these animals were unable to generate a significant retroperistaltic intestinal movement [40] to expel the vomit. Despite the demonstrable peripheral lesion, i.p. SSP-saporin neither eliminated brain SP-IR, nor completely eliminated GR73632-induced emesis. Furthermore, scratchings in ablated shrews were similar in number to those in saline-injected control shrews. One exception was the 5 mg/kg dose in ablated shrews, which tended to cause fewer scratchings than expected, albeit not significantly fewer (P > 0.05). These animals spent extensive periods of time trying to vomit, which may have interfered with the expression of scratching behavior. Thus, these results provide solid evidence for a mixed central/peripheral activity for SP on the emetic reflex. They also indicate that activation of gastrointestinal NK1 receptors is not required for the initiation of the vomiting process, but they are required to rapidly execute vomit expulsion (i.e. for normal emesis-related intestinal motility).
CONCLUSIONS
In summary, via the utilization of diverse behavioral, biochemical and immunohistochemical techniques this study has demonstrated: 1) production of emesis via the activation of NK1 receptors using SP and the brain penetrating tachykinin NK1 receptor selective agonist GR73632; 2) a cardinal role for the induction of emesis for central NK1 receptors, presumably in the NTS and DMNX emetic nuclei of the DVC, as well as a facilitatory role for the gastrointestinal NK1 receptors to rapidly expel the vomit; and 3) the validation of the least shrew as a specific and rapid NK1 receptor behavioral model, to screen concomitantly both the CNS penetration and the antiemetic potential of tachykinin NK1 receptor antagonists.
4. EXPERIMENTAL PROCEDURES
Animals and drugs
Shrews (C. parva) were bred and maintained in our animal facilities. Both male and female shrews (4–5g, 35–60 days old) were used. The feeding and maintenance of shrews are fully described elsewhere [10]. All animal protocols were approved by the Western University Institutional Animal Care and Use Committee, and followed the current guidelines recommended by NIH.
Substance P and ASMSP were purchased from Sigma/RBI (St. Louis, MO). The following drugs were purchased from Tocris Cookson Inc. (Ellisville, MO): GR73632, GR159897, GR64349, L733060, SB218795, and SarMet-SP. CP99,994 was obtained from Pfizer. Inc. (Groton, CT). The water-insoluble drugs GR159897, CP99,994, L733060, and SB218595 were initially dissolved to twice the stated concentrations in a 1:1:18 solution of ethanol:emulphor:0.9% saline, and this solution diluted with an equal volume of saline. The rest were dissolved in H2O and diluted in saline prior to administration. All drugs were administered at a volume of 0.1 ml/10g of body weight.
Dose-response emesis and scratching studies with tachykinin receptor agonists and antagonists
The present protocols were based upon our preliminary dose-response studies as well as published findings in the least shrew [10]. Shrews were acclimated to the laboratory for at least one hour prior to experimentation, then offered four mealworms (Tenebrio sp.) 30 min prior to experimentation, to help identify ejection of food. Different groups of shrews were injected with varying doses of either SP (0, 10, 25, 50 or 100 mg/kg, N = 7–11 shrews per group), GR73632 (0, 1, 2.5 or 5 mg/kg, N = 8–12 shrews per group), ASMSP (0, 5, 10 or 20 mg/kg, N = 6–10 per group), SarMet-SP (0, 1, 5 or 10 mg/kg, N = 4–10 per group), GR64349 (0, 5, or 10 mg/kg, N = 8–10 per group), or Pro7-NKB (0, 5, or 10 mg/kg, N = 6–10 per group). Immediately following injection, each shrew was placed in an observation cage and the frequencies (mean ± S.E.M.) of both vomiting (oral ejections of food or liquid, or the act of vomiting which did not result in actual ejection of food or liquid due to an empty stomach) and scratching behaviors were recorded for the next 30 min. Scratches within a bout were counted and scored cumulatively, with a new bout starting either: 1) after a 2 sec interval without scratching; or 2) after the shrew would switch to scratching with the opposing limb no matter how long an interval had passed since the previous bout (see also [11]). Based on these results, i.p. administration of 50 mg/kg SP or 5 mg/kg of GR73632 caused a maximal frequency of emesis, and in the case of GR73632 numerous scratchings, so these doses were chosen for subsequent antagonist interaction studies.
For the drug interaction studies, different doses (i.p.) of either the specific NK1 receptor antagonists CP99,994 (0, 5, 10 or 20 mg/kg, N = 10–14 shrews per group) or L733060 (0, 5, 10 or 20 mg/kg, N = 10–14 shrews per group); the specific NK2 receptor antagonist GR159897 (0 or 20 mg/kg, N = 8 per group); or the specific NK3 receptor antagonist SB218795 (0 or 20 mg/kg, N = 7–8 per group) were administered to different groups of shrews. Each shrew was offered four mealworms immediately following antagonist (or vehicle) injection and 30 min later received an emetic dose (5 mg/kg, i.p.) of GR73632. The frequencies of both emesis and scratching behaviors (mean ± S.E.M.) were recorded for 30 min immediately following GR73632 administration as described above. The antiemetic effect of CP99,994 (0, 5 or 10 mg/kg, N = 9–10 animals per group) was also investigated in a similar manner against SP (50 mg/kg, i.p.)-induced emesis.
Quantification of SP in serum, gut and brain tissues following injection (i.p.) of an emetic dose of SP
Groups of shrews were injected with 50 mg/kg SP (i.p.) and were euthanized at 5, 15 or 30 min (N = 5–6 animals per group) post injection. Samples of serum, forebrain tissue at the level of prefrontal cortex, brain stem, duodenum, and jejunum were collected for the determination of SP levels relative to a saline-treated shrew control group (N = 6). Brain SP concentration was determined for all exposure times, whereas gut and serum SP concentrations were analyzed for the 5 or 15 min exposure periods. An additional 10 min exposure group was analyzed for serum SP.
To collect blood, shrews were euthanized with an overdose of isoflurane (Vedco, St. Joseph, MO) one at a time. Each shrew was laid on its back and taped to a metal tray, and the ribcage cut off and lifted away. Blood was drawn intraventricularly until the descending aorta cleared (50–100 µl volume), and rapidly transferred to a non-heparinized tube for centrifugation to remove erythrocytes. Other tissue samples were rapidly dissected following blood collection. Brain and serum samples performed normally with the enzyme immunoassay (EIA) detection kit. However, gut samples spiked with known levels of SP showed degradation resistant to several cocktails of protease inhibitors (pP8340 from Sigma-Aldrich or #04693116001 from Roche Applied Science, Indianapolis, IN). Thus, following dissection the entire intestine was immediately immersed in a 70° C preheated solution of normal saline containing 10 mm EDTA at pH 8.0 to neutralize protease activity. After a 5 min exposure, each gut was thrice flushed and rinsed with the saline-EDTA solution, then allowed to return to room temperature. As measured beginning 2 cm away from the pyloric sphincter, two 1 cm-long gut samples (duodenum and jejunum, respectively) were excised and placed separately in test tubes containing 1 ml assay buffer. Frontal cortex and brain stem samples were dissected in test tubes containing 0.5 ml assay buffer. Each sample was homogenized using a tissue tearor (Biospec) at level 5 for 15 sec. Homogenates were centrifuged at 17,000g for 15 min at 4° C. Supernatants were collected and saved at −80° C until used for the EIA. SP concentrations were determined by “Correlate-EIA” kit (Assay Designs, Ann Arbor, Michigan) per manufacturer’s instructions, using a Bio-tek microplate reader.
Immunohistochemistry of Fos and tachykininergic systems
Shrews were given four mealworms each and then injected with either GR73632 (2.5 mg/kg i.p., N = 6) or vehicle (saline, N = 6). For emesis-related Fos visualization, a shrew was transcardially perfused 65–75 min after vomiting occurred, typically 25–35 min post-injection. Thus, shrews that vomited were perfused 90–110 min post-GR73632 injection. Animals that didn’t vomit were observed and then perfused 90–100 min post-GR73632 injection.
After the appropriate time period, shrews were anesthetized with pentobarbital (100 mg/kg) and perfused transcardially via peristaltic pump with a 25 gauge blunted needle. The shrew was perfused with ice cold heparinized saline (0.9% NaCl, 60–90 s), followed by ice cold 4% paraformaldehyde/5% picric acid in pH 7.4, 0.1M phosphate buffer (PB, 10 min). Brains were cryoprotected overnight in 30% sucrose and embedded in blocks of 12% gelatin in 30% sucrose/PB. The brain block was cut on a freezing microtome (Leica) at 30 µm into 5 series, and stored in PB with 0.03% sodium azide. Gut blocks were processed similarly (i.e. 30 µm thickness), but segmented into 0.5 cm long pieces before embedding. Due to the very thin walls of the intestine in the least shrew, the gut cannot be pinned completely flat for embedding, nor can the nerve plexi of the enteric nervous system be excised from the intestinal mucosa as is done for larger animals [30]. Thus, intestine blocks were cut at oblique angles containing several layers of the intestinal mucosa, smooth muscle, and ENS.
Immunohistochemistry was performed as described previously [44]. Tissue was blocked with normal horse serum (NHS) to reduce nonspecific staining, then reacted overnight with sheep anti-Fos polyclonal (Chemicon, Temecula, CA 1:600) with 0.3% Triton X-100 and 5% NHS in PB. With 3 PB rinses between each step, tissue was then reacted in donkey anti-sheep antibody (Jackson Immunoresearch, West Grove, PA, 1:600) in the above antibody diluent for 75 min. This was followed by 60 minutes in HRP-conjugated avidin-biotin complex (Vector Labs, Burlingame, CA, diluted 1:2), and visualization in nickel-enhanced diaminobenzidine (2% nickel, 0.05% DAB) with 0.0006% hydrogen peroxide for 6 minutes.
After reacting, the tissue was rinsed thoroughly in PB and mounted onto gelatin-subbed slides. Air-dried slides were dehydrated through ascending ethanols and cleared in xylene (Fisher), then coverslipped with DEPEX (Electron Microscopy Sciences, Hatfield, PA).
Photomicrographs of regions of interest were taken with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) mounted to a Nikon Eclipse E600 microscope. A calibrated scale bar was added and the photos exported to Adobe Photoshop 7, where a translucent, 55% threshold-filtered image was superimposed. Fos+ nuclei, defined as a cluster of connected pixels above the threshold, were then manually counted. This threshold was chosen based on pilot photomicrographs used to correlate counts of above-threshold and visually-counted nuclei. Fos+ nuclei were counted using straightforward counting by an analyst blind to the source animal. Relevant structures were identified using an atlas produced in lab [44]. To minimize counting errors from areas outside of the medial NTS, the mild cellular background “counterstaining” generated during IHC processing was used to identify the point where the medial longitudinal fasciculus essentially contacts the floor of the 4th ventricle. From this reference point, the medial NTS (as seen in atlas Nissl sections) lies caudally 0.65 - 0.8 mm. Only the section(s) from this range was counted.
SSP-Saporin NK1 receptor ablation behavioral studies
Dose-response curves were generated for shrews pretreated either with the NK1 receptor immunotoxin SSP-saporin or saline, and challenged 4 days later with varying doses of GR73632 (0, 1, 2.5 or 5 mg/kg, i.p., N = 8–10 per group). Thirty shrews were injected i.p. with 1.2 mg/kg SSP-saporin in sterile saline, and 35 shrews were injected with sterile saline alone. In addition, 2 more shrews were injected with 1.2 mg/kg unconjugated saporin (saporin; ATS, San Diego, CA) and 2 more with 1.2 mg/kg of saporin conjugated to a nonspecific peptide sequence (Blank-saporin; ATS). Shrews were returned to home cages for 3 days and supplied with food and water ad lib. Saporin-injected and Blank-saporin-injected shrews were perfused four days post-injection, and brain and gut harvested as described for immunohistochemical staining. The SSP-saporin and saline-injected shrews were divided into different groups on day 4 post-injection, and each group injected i.p. with doses of 0, 1, 2.5, or 5 mg/kg GR73632. Each shrew was monitored for 30 min post-injection and vomiting and scratching behaviors quantified. Three shrews from each group were then perfused 90–120 min post-injection as described previously for Fos immunohistochemistry, and brain and gut harvested. IHC was performed for either 1) rat anti-Substance P monoclonal antibody (Chemicon, 1:400), 2) rabbit anti-NK1 receptor polyclonal (Santa Cruz Biotech, Santa Cruz, CA, 1:500), or 3) sheep Fos, using the avidin-biotin-peroxidase method described above. IHC for SP and NK1 receptor was done on the saporin- and Blank-saporin-injected shrews to determine the extent of lesion and verify the specificity of SSP-saporin and was not quantified.
Statistical analyses
The data on the frequency of emesis and scratchings were analyzed by Kruskal-Wallis (KW) nonparametric one-way analysis of variance (ANOVA) and post hoc analysis by Dunn’s multiple comparisons test or Mann-Whitney test. A P value of < 0.05 was considered statistical significance. The incidence of emesis (number of shrews vomiting) was analyzed by Fisher’s Exact test to identify differences between groups. When appropriate, pairwise comparisons were also made by this method. Two-way ANOVA using the Kruskal-Wallis or Fisher’s Exact tests were initially utilized to respectively analyze the dose-response results of GR73632 on emesis and scratching behaviors, and percentage of shrews vomiting in saporin- and saline-pretreated control shrews. However, the data did not converge, so the described one-way ANOVA statistical methods were used to analyze the data. For some emesis data, the two-tailed Mann-Whitney test was used. The SP tissue concentration data were analyzed by a one-way ANOVA followed by post hoc Dunnett’s t-test. Fos-IR was quantified by counting immunopositive nuclei within each hemisection containing the region of interest (ROI). The number of nuclei within the ROI for each animal was obtained, and the group mean and standard error calculated. Variance was checked by one way ANOVA to ensure the groups were not statistically different, and a 2-tailed Student’s t-test was used for significant differences between group means.
ACKNOWLEDGEMENTS
This work was supported by NIH grant #R01CA115331 from the National Cancer Institute. The authors thank Mrs. Nona Williamson for typing the manuscript.
ABBREVIATIONS
- SP
Substance P
- ASMSP
acetyl- [Arg, Sar9, Met (O2)11] – SP (6–11)
- GR73632
[delta Ava [L – Pro9, N-MeLeu10] SP (7–11)]
- GR159897
[5-Flouro- 3- [2-[4-methoxy -4- [[(R) – phenylsulphinyl] methyl] – 1 – piperidinyl] ethyl] – 1H – indole]
- GR64349
[[Lys3, Gly8-R-gamma-lactam-leu9] Neurokinin-A (3–10)]
- L733060 hydrochloride
[(2S, 3S) – 3- [[3,5 - bis (Trifluoromethyl) phenyl] methoxy] – 2- phenylpiperidine hydrochloride]
- SB218795
[[[2- (Phenyl -4- quinolinyl) carbonyl] amino] – methyl ester benzeneacetic acid]
- SarMet-SP
[Sar9, Met (O2)11] – Substance P
- CP99,994
[(2S, 3S) – Cis – 3 - (2-methoxybenzylamino -2- phenylpiperidine)]
- Pro7-NKB
Pro7-Neurokinin-B
Footnotes
Parts of this paper are based on presentations at the 15th Annual Symposium of the International Cannabinoid Research Society (2005), Clearwater Beach, Florida, P56 and the 2007 Annual Neuroscience Meeting, San Diego, California P 86.12.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Andrews PLR, Rudd JA. Handbook of Experimental Pharmacology. Vol. 164. Berlin: Springer-Verlag; 2004. The role of tachykinins and the tachykinin NK1 receptor in nausea and emesis; pp. 359–440. [Google Scholar]
- 2.Bailey CP, Maubach KA, Jones RS. Neurokinin-1 receptors in the rat nucleus tractus solitarius: pre- and postsynaptic modulation of glutamate and GABA release. Neuroscience. 2004;127:467–479. doi: 10.1016/j.neuroscience.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Begley DJ. The blood-brain barrier: principles for targeting peptides and drugs to the central nervous system. J Pharm Pharmacol. 1996;48:136–146. doi: 10.1111/j.2042-7158.1996.tb07112.x. [DOI] [PubMed] [Google Scholar]
- 4.Boissonade FM, Davison JS. Effect of vagal and splanchnic nerve section on Fos expression in ferret brain stem after emetic stimuli. Am J Physiol. 1996;271:R228–R236. doi: 10.1152/ajpregu.1996.271.1.R228. [DOI] [PubMed] [Google Scholar]
- 5.Boissonade FM, Davison JS, Egizii R, Lucier GE, Sharkey KA. The dorsal vagal complex of the ferret: anatomical and immunohistochemical studies. Neurogastroenterol Motil. 1996;8:255–272. doi: 10.1111/j.1365-2982.1996.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 6.Boix F, Mattioli R, Adams F, Huston JP, Schwarting RK. Effects of substance P on extracellular dopamine in neostriatum and nucleus accumbens. Eur J Pharmacol. 1992;216:103–107. doi: 10.1016/0014-2999(92)90215-p. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil. 2004;16 Suppl 1:34–38. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter DO, Briggs DB, Strominger N. Behavioral and electrophysiological studies of peptide-induced emesis in dogs. Fed Proc. 1984;43:2952–2954. [PubMed] [Google Scholar]
- 9.Chappa AK, Audus KL, Lunte SM. Characteristics of substance P transport across the blood-brain barrier. Pharm Res. 2006;23:1201–1208. doi: 10.1007/s11095-006-0068-1. [DOI] [PubMed] [Google Scholar]
- 10.Darmani NA. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neural Transm. 1998;105:1143–1154. doi: 10.1007/s007020050118. [DOI] [PubMed] [Google Scholar]
- 11.Darmani NA, Mock OB, Towns LC, Gerdes CF. The head-twitch response in the least shrew (Cryptotis parva) is a 5-HT2- and not a 5-HT1C-mediated phenomenon. Pharmacol Biochem Behav. 1994;48:383–396. doi: 10.1016/0091-3057(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 12.Darmani NA, Pandya DK. Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J Neural Transm. 2000;107:931–945. doi: 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- 13.Duffy RA, Varty GB, Morgan CA, Lachowicz JE. Correlation of neurokinin (NK) 1 receptor occupancy in gerbil striatum with behavioral effects of NK1 antagonists. J Pharmacol Exp Ther. 2002;301:536–542. doi: 10.1124/jpet.301.2.536. [DOI] [PubMed] [Google Scholar]
- 14.Duval P, Lenoir V, Kerdelhue B. Ovarian steroid modulation of neurokinin contents in hypothalamus, pituitary, trigeminal nucleus, and cervical spinal cord of the ovariectomized female rat. J Neuroendocrinol. 1998;10:823–828. doi: 10.1046/j.1365-2826.1998.00267.x. [DOI] [PubMed] [Google Scholar]
- 15.El-Mahmoudy A, Matsuyama H, Khalifa M, Shimizu Y, Takewaki T. Tachykinins mediate non-adrenergic, non-cholinergic excitatory neurotransmission to the hamster ileum via NK1 and NK2 receptors. Life Sci. 2003;73:1939–1951. doi: 10.1016/s0024-3205(03)00545-9. [DOI] [PubMed] [Google Scholar]
- 16.Engberg S, Ahlstedt I, Leffler A, Lindstrom E, Kristensson E, Svensson A, Pahlman I, Johansson A, Drmota T, von Mentzer B. Molecular cloning, mutations and effects of NK1 receptor antagonists reveal the human-like pharmacology of gerbil NK1 receptors. Biochem Pharmacol. 2007;73:259–269. doi: 10.1016/j.bcp.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Ermisch A, Ruhle HJ, Landgraf R, Hess J. Blood-brain barrier and peptides. J Cereb Blood Flow Metab. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- 18.Freed AL, Audus KL, Lunte SM. Investigation of substance P transport across the blood-brain barrier. Peptides. 2002;23:157–165. doi: 10.1016/s0196-9781(01)00592-7. [DOI] [PubMed] [Google Scholar]
- 19.Freed AL, Cooper JD, Davies MI, Lunte SM. Investigation of the metabolism of substance P in rat striatum by microdialysis sampling and capillary electrophoresis with laser-induced fluorescence detection. J Neurosci Methods. 2001;109:23–29. doi: 10.1016/s0165-0270(01)00397-1. [DOI] [PubMed] [Google Scholar]
- 20.Gardner C, Perren M. Inhibition of anaesthetic-induced emesis by a NK1 or 5-HT3 receptor antagonist in the house musk shrew, Suncus murinus. Neuropharmacology. 1998;37:1643–1644. doi: 10.1016/s0028-3908(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 21.Gardner CJ, Bountra C, Bunce KT, Dale TJ, Jordan CC, Twissel DJ, Ward P. Antiemetic activity of neurokinin receptor antagonists is mediated centrally in the ferret. British Journal of Pharmacology. 1994;112:516. [Google Scholar]
- 22.Gobbi G, Blier P. Effect of neurokinin-1 receptor antagonists on serotoninergic, noradrenergic and hippocampal neurons: comparison with antidepressant drugs. Peptides. 2005;26:1383–1393. doi: 10.1016/j.peptides.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 24.Guiard BP, Przybylski C, Guilloux JP, Seif I, Froger N, De Felipe C, Hunt SP, Lanfumey L, Gardier AM. Blockade of substance P (neurokinin 1) receptors enhances extracellular serotonin when combined with a selective serotonin reuptake inhibitor: an in vivo microdialysis study in mice. J Neurochem. 2004;89:54–63. doi: 10.1046/j.1471-4159.2003.02304.x. [DOI] [PubMed] [Google Scholar]
- 25.Hagan RM, Ireland SJ, Jordan CC, Beresford IJ, Deal MJ, Ward P. Receptor-selective, peptidase-resistant agonists at neurokinin NK-1 and NK-2 receptors: new tools for investigating neurokinin function. Neuropeptides. 1991;19:127–135. doi: 10.1016/0143-4179(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 26.Harrington AM, Hutson JM, Southwell BR. Immunohistochemical localization of substance P NK1 receptor in guinea pig distal colon. Neurogastroenterol Motil. 2005;17:727–737. doi: 10.1111/j.1365-2982.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 27.Hasenohrl RU, Schwarting RK, Gerhardt P, Privou C, Huston JP. Comparison of neurokinin substance P with morphine in effects on food-reinforced operant behavior and feeding. Physiol Behav. 1994;55:541–546. doi: 10.1016/0031-9384(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 28.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 29.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol Ther. 1997;73:219–263. doi: 10.1016/s0163-7258(96)00196-9. [DOI] [PubMed] [Google Scholar]
- 30.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556:521–530. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Induction of Fos protein in neurons in the medulla oblongata after motion- and X-irradiation-induced emesis in musk shrews (Suncus murinus) Auton Neurosci. 2003;107:1–8. doi: 10.1016/S1566-0702(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 32.Knox AP, Strominger NL, Battles AH, Carpenter DO. Behavioral studies of emetic sensitivity in the ferret. Brain Res Bull. 1993;31:477–484. doi: 10.1016/0361-9230(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 33.Landgraf R, Klauschenz E, Bienert M, Ermisch A, Oehme P. Some observations indicating a low brain uptake of [3H]Nle11-Substance P. Pharmazie. 1983;38:108–110. [PubMed] [Google Scholar]
- 34.Mazzone SB, Geraghty DP. Characterization and regulation of tachykinin receptors in the nucleus tractus solitarius. Clin Exp Pharmacol Physiol. 2000;27:939–942. doi: 10.1046/j.1440-1681.2000.03365.x. [DOI] [PubMed] [Google Scholar]
- 35.Megens AA, Ashton D, Vermeire JC, Vermote PC, Hens KA, Hillen LC, Fransen JF, Mahieu M, Heylen L, Leysen JE, Jurzak MR, Janssens F. Pharmacological profile of (2R-trans)-4-[1-[3,5-bis(trifluoromethyl)benzoyl]-2-(phenylmethyl)-4-piper idinyl]-N-(2,6-dimethylphenyl)-1-acetamide (S)-Hydroxybutanedioate (R116301), an orally and centrally active neurokinin-1 receptor antagonist. J Pharmacol Exp Ther. 2002;302:696–709. doi: 10.1124/jpet.102.034348. [DOI] [PubMed] [Google Scholar]
- 36.Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994;14:871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minami M, Endo T, Kikuchi K, Ihira E, Hirafuji M, Hamaue N, Monma Y, Sakurada T, Tan-no K, Kisara K. Antiemetic effects of sendide, a peptide tachykinin NK1 receptor antagonist, in the ferret. Eur J Pharmacol. 1998;363:49–55. doi: 10.1016/s0014-2999(98)00784-5. [DOI] [PubMed] [Google Scholar]
- 38.Minami M, Endo T, Yokota H, Ogawa T, Nemoto M, Hamaue N, Hirafuji M, Yoshioka M, Nagahisa A, Andrews PL. Effects of CP-99, 994, a tachykinin NK(1) receptor antagonist, on abdominal afferent vagal activity in ferrets: evidence for involvement of NK(1) and 5-HT(3) receptors. Eur J Pharmacol. 2001;428:215–220. doi: 10.1016/s0014-2999(01)01297-3. [DOI] [PubMed] [Google Scholar]
- 39.Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol. 2006;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- 40.Niel JP. Rôle de la Sustance P dans le contrôle nerveux de la motricite digestive., Association des Physiologists. Nancy. 1991:A65–A76. doi: 10.3109/13813459109145918. [DOI] [PubMed] [Google Scholar]
- 41.Palmieri FE, Ward PE. Mesentery vascular metabolism of substance P. Biochim Biophys Acta. 1983;755:522–525. doi: 10.1016/0304-4165(83)90259-3. [DOI] [PubMed] [Google Scholar]
- 42.Pendergrass K, Hargreaves R, Petty KJ, Carides AD, Evans JK, Horgan KJ. Aprepitant: an oral NK1 antagonist for the prevention of nausea and vomiting induced by highly emetogenic chemotherapy. Drugs Today (Barc) 2004;40:853–863. doi: 10.1358/dot.2004.40.10.863745. [DOI] [PubMed] [Google Scholar]
- 43.Ravard S, Betschart J, Fardin V, Flamand O, Blanchard JC. Differential ability of tachykinin NK-1 and NK-2 agonists to produce scratching and grooming behaviours in mice. Brain Res. 1994;651:199–208. doi: 10.1016/0006-8993(94)90698-x. [DOI] [PubMed] [Google Scholar]
- 44.Ray AP, Darmani NA. A histologically derived stereotaxic atlas and substance P immunohistochemistry in the brain of the least shrew (Cryptotis parva) support its role as a model organism for behavioral and pharmacological research. Brain Res. 2007;1156:99–111. doi: 10.1016/j.brainres.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694) Brain Res. 1991;565:231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- 46.Richter R, Oehme P. Effects of substance P and shorter analogs on body temperature in the rat. Acta Biol Med Ger. 1982;41:725–727. [PubMed] [Google Scholar]
- 47.Roth GI, Yamamoto WS. The microcirculation of the area postrema in the rat. J Comp Neurol. 1968;133:329–340. doi: 10.1002/cne.901330304. [DOI] [PubMed] [Google Scholar]
- 48.Rupniak NM. New insights into the antidepressant actions of substance P (NK1 receptor) antagonists. Can J Physiol Pharmacol. 2002;80:489–494. doi: 10.1139/y02-048. [DOI] [PubMed] [Google Scholar]
- 49.Rupniak NM, Tattersall FD, Williams AR, Rycroft W, Carlson EJ, Cascieri MA, Sadowski S, Ber E, Hale JJ, Mills SG, MacCoss M, Seward E, Huscroft I, Owen S, Swain CJ, Hill RG, Hargreaves RJ. In vitro and in vivo predictors of the anti-emetic activity of tachykinin NK1 receptor antagonists. Eur J Pharmacol. 1997;326:201–209. doi: 10.1016/s0014-2999(97)85415-5. [DOI] [PubMed] [Google Scholar]
- 50.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 51.Singh L, Field MJ, Hughes J, Kuo BS, Suman-Chauhan N, Tuladhar BR, Wright DS, Naylor RJ. The tachykinin NK1 receptor antagonist PD 154075 blocks cisplatin-induced delayed emesis in the ferret. Eur J Pharmacol. 1997;321:209–216. doi: 10.1016/s0014-2999(96)00950-8. [DOI] [PubMed] [Google Scholar]
- 52.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 53.Smid SD, Lynn PA, Templeman R, Blackshaw LA. Activation of non-adrenergic non-cholinergic inhibitory pathways by endogenous and exogenous tachykinins in the ferret lower oesophageal sphincter. Neurogastroenterol Motil. 1998;10:149–156. doi: 10.1046/j.1365-2982.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 54.Tattersall FD, Rycroft W, Francis B, Pearce D, Merchant K, MacLeod AM, Ladduwahetty T, Keown L, Swain C, Baker R, Cascieri M, Ber E, Metzger J, MacIntyre DE, Hill RG, Hargreaves RJ. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology. 1996;35:1121–1129. doi: 10.1016/s0028-3908(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 55.Tattersall FD, Rycroft W, Marmont N, Cascieri M, Hill RG, Hargreaves RJ. Enantiospecific inhibition of emesis induced by nicotine in the house musk shrew (Suncus murinus) by the neurokinin1 (NK1) receptor antagonist CP-99,994. Neuropharmacology. 1995;34:1697–1699. doi: 10.1016/0028-3908(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 56.Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- 57.Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- 58.Veyrat-Follet C, Farinotti R, Palmer JL. Physiology of chemotherapy-induced emesis and antiemetic therapy. Predictive models for evaluation of new compounds. Drugs. 1997;53:206–234. doi: 10.2165/00003495-199753020-00003. [DOI] [PubMed] [Google Scholar]
- 59.Ward P, Armour DR, Bays DE, Giblin GMP, Peggy NA, Watson SP, Middlemiss D, Vinader V, Evans B, Naylor A, Evans DC, Bountra C, Gardiner CJ, Twissell DJ. Neurokinin NK1 antagonists: A new approach to the treatment of emesis. Discovery of a potent, orally bioavailable drug candidate; 8th RSC-SCI Medicinal Chemistry Symposium; Cambridge, UK. 1995. [Google Scholar]
- 60.Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PL. The antiemetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol. 1995;115:84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiley RG, Lappi DA. Targeting neurokinin-1 receptor-expressing neurons with [Sar9,Met(O2)11 substance P-saporin. Neurosci Lett. 1999;277:1–4. doi: 10.1016/s0304-3940(99)00846-0. [DOI] [PubMed] [Google Scholar]
- 62.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- 63.Wu M, Harding RK, Hugenholtz H, Kucharczyk J. Emetic effects of centrally administered angiotensin II, arginine vasopressin and neurotensin in the dog. Peptides. 1985;6 Suppl 1:173–175. doi: 10.1016/0196-9781(85)90028-2. [DOI] [PubMed] [Google Scholar]
- 64.Yip J, Chahl LA. Distribution of Fos-like immunoreactivity in guinea-pig brain following administration of the neurokinin-1 receptor agonist, [SAR9,MET(O2)11]substance P. Neuroscience. 1999;94:663–673. doi: 10.1016/s0306-4522(99)00283-3. [DOI] [PubMed] [Google Scholar]
- 65.Yip J, Chahl LA. Localization of NK1 and NK3 receptors in guinea-pig brain. Regul Pept. 2001;98:55–62. doi: 10.1016/s0167-0115(00)00228-7. [DOI] [PubMed] [Google Scholar]


