Abstract
Purpose
This study compared amygdala volume in children with cryptogenic epilepsy, who had complex partial seizures (CPS), with age and gender matched normal children. It also examined the relationship of amygdala volumes with seizure variables and the presence of psychopathology in the patients.
Methods
28 children with cryptogenic epilepsy, all of whom had CPS, and gender matched normal children, aged 6–16 years had magnetic resonance imaging (MRI) at 1.5 Tesla. Tissue was segmented and total brain volume and amygdala volumes obtained from manual tracings were computed.
Results
There were no significant differences in the amygdala volume of the CPS and normal groups. Within the CPS group, the children with an affective/anxiety disorder had significantly larger left amygdala volumes compared to those with no psychopathology as well as greater amygdala asymmetry. Exploring the association of seizure variables to amygdala volumes yielded no significant predictors.
Conclusions
In pediatric CPS left amygdala involvement might reflect effects of the neuropathology underlying comorbid affective or anxiety disorders on amygdala development rather than effects of on-going seizures.
Keywords: complex partial seizure disorder, childhood, magnetic resonance imaging, amygdala, seizure variables
1. Introduction
The amygdala plays an important role in the generation of epilepsy in animal models (1, 2) and in patients with temporal lobe epilepsy (TLE) (3–6). Adult magnetic resonance imaging (MRI) studies have shown amygdala volume reduction ipsilateral to the seizure focus in TLE (7–10) associated with longer duration of epilepsy but not with other seizure variables.
The amygdala has also been implicated in both depression (11, 12) and anxiety disorders (13, 14) in non-epileptic patients. Several adult studies have demonstrated amygdala volume reduction in major depressive disorder (MDD) (15–17) in keeping with neuropathologic findings of significant reduction of glial cells and glial/neuron ratio in the left amygdala of MDD patients (18). Recent childhood studies found similar amygdala volume reduction in MDD (19) and anxiety disorders (20). However, other studies have reported enlarged amygdala volumes in adults with MDD (12, 21, 22), adults with refractory partial epilepsy and comorbid affective disorders (23–25), as well as in children with MDD (26) and generalized anxiety disorder (27). These discrepant findings might be related to methodological factors, such as differences in anatomical boundaries used to measure the amygdala in these studies.
There are high rates of comorbid MDD and anxiety disorders in adults (28–33) and children with epilepsy (34–41). Amygdala volume findings, however, vary with two studies showing increased volumes in presurgical TLE adults with depression (42, 43) and two studies showing no change in volumes in individuals with TLE (44, 45). Richardson et al. (42) found a positive correlation between left and right amygdala volumes and scores on the Beck Depression Inventory (46) in individuals with TLE, including adolescents. To date, however, there have been no structural studies on amygdala volumes in children with epilepsy.
The study presented in this paper examined if amygdala volume in children with cryptogenic epilepsy who had complex partial seizures (CPS) was significantly smaller than age and gender matched normal children. Within the CPS group, we explored if those with anxiety/affective disorders would have significantly larger amygdala than those without psychopathology. In the absence of evidence regarding the relationship between seizure variables and amygdala volumes in children with CPS, we explored if in addition to duration of epilepsy, variables, such as age of onset, lateralization of EEG findings, history of prolonged and febrile seizures, as well as seizure frequency and number of antiepileptic drugs (AEDs) were associated with these volumes.
There is evidence for increased incidence of mood and anxiety disorders in families in the general population (47) and for a possible role of perinatal factors in the generation of CPS (48, 49). We, therefore, also explored if a family history of mood and anxiety disorders in first-degree relatives and a history of perinatal problems were related to amygdala volume in the CPS group.
2. Methods
2.1 Subjects
The study included 28 children with cryptogenic epilepsy, all of whom had CPS, and 30 children without epilepsy, aged 6–16 years. Table 1 describes the demographic features of the study subjects. Significantly more CPS children come from lower socioeconomic status (SES) families than the normal group based on the Hollingshead 2 factor index (50), derived from parent occupational and educational status. A score of I-III was classified as high SES while a score of IV-V was classified as low SES. The CPS group also had significantly lower mean IQ scores than the normal subjects.
Table 1.
Demographic Features of Study Groups
To be included in the study, the patients had to have a diagnosis of cryptogenic epilepsy with CPS, as defined by the International Classification of Epilepsy (51) and at least one seizure during the year prior to the child’s participation in the study. As described in this classification, children with a clinical history of CPS with or without EEG evidence for focal epileptic activity were included in the study sample. None of the children in the study had an underlying brain lesion or MRI evidence for hippocampal sclerosis.
We recruited 36% CPS subjects from tertiary centers (e.g., UCLA Pediatric Neurology services, Children’s Hospital of Los Angeles) and 64% from the community (e.g., Kaiser Sunset, Kaiser-Orange County, private pediatric neurologists, Los Angeles and San Diego branches of the Epilepsy Foundation of America). The primary pediatric neurologist at each site reviewed the clinical history, EEG records, and diagnosis of potential CPS subjects and referred them for the study. We excluded patients with a mixed seizure disorder, an MRI abnormality, an underlying neurological disorder, a metabolic disorder, a hearing disorder, and past epilepsy surgery. Of note, none of the CPS subjects in the study had hippocampal sclerosis.
A UCLA pediatric neurology investigator (W.D.S.) reviewed the history, EEG records performed at about the time of the child’s diagnosis, and diagnosis of each epileptic subject from the different recruitment sites. If he did not concur with the diagnosis or EEG findings, the child was not included in the study.
Table 2 presents information on seizure frequency during the past year, current AEDs, age of onset of seizures, duration of illness, as well as the number of febrile convulsions and number of prolonged seizures (i.e., > 5 minutes) from the parent’s and the child’s medical records. With the exception of one CPS child who was left handed, all the other patients in the study were right handed.
Table 2.
Seizure-Related Variables in CPS Group
| Seizure Variables | CPS |
|---|---|
| Frequency | |
| <=1/year | 27% |
| 2–10/year | 35% |
| >10/year | 38% |
| Age of onset | 6.3 (2.95) |
| Duration of illness | 3.6 (2.55) |
| AEDs | |
| None | 4% |
| Monotherapy | 75% |
| Polytherapy | 21% |
| Prolonged seizures | 43% |
| Febrile seizures | 19% |
Of the 28 CPS patients, 4 had non-lateralized EEG findings, 9 had a left focus, 5 a right focus, and 8 bilateral foci. EEGs were unavailable for two CPS patients. Regarding focal EEG findings, 1 child had no focal findings, 8 had interictal spikes in the temporal lobe, 11 in the frontal and temporal lobe (4 frontal and 7 frontotemporal), and 6 in other areas. 1 CPS subject had secondary generalization and 2 had background slowing. Perinatal data on the number of pregnancy and delivery complications was collected from the children’s mothers using a questionnaire modified from the Yale Neuropsycho-educational Assessment Scales (52). Of the 28 CPS subjects, 36 % had a history of delivery problems and 57 % had pregnancy problems.
We recruited the non-epileptic control subjects from four public and two private schools in the Los Angeles community after screening for neurological, psychiatric, language, and hearing disorders through a telephone conversation with a parent. We excluded from the study non-epileptic children manifesting symptoms of these disorders in the past.
2.2 Procedures
This study was conducted in accordance with the policies of the Human Subjects Protection Committees of the University of California, Los Angeles. Informed assents and consents were obtained from all subjects and their parents, respectively.
Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS)
The Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic Present and Lifetime Version (K-SADS-PL) (53) was administered to each child (those with CPS and without epilepsy) and parent by R.C. or a trained research assistant. Because the child or parent often talks about the child’s seizures during the interview, these interviewers were not blind with regards to the child’s seizure disorder (i.e., presence or absence, type). A consensus DSM-IV (American Psychiatric Association, 1994) diagnosis was reached after reviewing videotapes of the child interviews and audiotapes of the parent interviews. A child was excluded from the study if a diagnostic consensus was not reached.
Given the large number of psychiatric diagnoses relative to the number of subjects in each diagnostic group, we grouped the diagnoses as follows: “affective/anxiety” disorders included any mood or anxiety disorder including those with separation anxiety disorder, generalized anxiety disorder, specific phobia, and obsessive compulsive disorder. “Disruptive” disorders included attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder, and conduct disorder. Children with a “combined” diagnosis had both “affective/anxiety” and “disruptive” disorders.
A family history of psychopathology in relatives was obtained directly from the parents who filled out a questionnaire. Forty-three percent of the CPS subjects had a first-degree relative with a mood or anxiety disorder.
Cognition
The Wechsler Intelligence Scale for Children-III (54) administered to the children generated Full Scale, Verbal, and Performance IQ scores.
MRI Procedures
MRI Acquisition
All subjects completed MRI scanning on a 1.5 Tesla GE Signa magnetic resonance imaging scanner (GE Medical Systems, Milwaukee, WI). The imaging acquisition protocol used to obtain high resolution three-dimensional (3D) T-1 weighted spoiled grass (SPGR) sequences included a sagittal plane acquisition with slice thickness of 1.2 mm, repetition time of 14.6, echo time of 3.3, flip angle of 35, acquisition matrix of 256 × 192, FOV 24 and two excitations.
Image Preprocessing
Each scan was processed with a series of steps to assess volumes of tissue types. Initially, potential fluctuations in signal resulting from magnetic field inhomogeneities were addressed by applying a radio frequency correction (55). Next an automated brain extraction program (BET) was used to create a brain mask that separates brain tissue from non-brain tissue (skull and meninges) (56). This mask was manually modified to assure accurate separation of tissues. The automated tissue classification method of Shattuck et al. (56) was then used to segment the scans by tissue types to create gray matter, white matter and cerebrospinal fluid masks. The total intracranial volume was then automatically computed by summing the volumes of these masks including cerebellar tissue.
Amygdala Volumes
For amygdala volume analysis, the data were aligned into the Talairach co-ordinate system using the anterior commissure as the center of origin, and then reformatted into an oblique coronal plane to assure that the images were oriented in space with the long axis of the anterior hippocampus perpendicular to the coronal plane (57). These reformatting methods, described briefly here, are detailed in Bartzokis et al. (58). While viewing the image data in all three planes, the most anterior section containing the anterior commissure was marked in the coronal plane, and then located in the axial plane. Using these landmarks, the images were then rotated so that the interhemispheric fissure was perpendicular to a horizontal line at 0 degrees. Next, the sagittal image containing the most lateral slice of the anterior hippocampus was marked with a line dropped perpendicular to the anterior third of the left hippocampus. This angle was then used to obtain the oblique coronal sections used for volumetric analysis of the anterior and posterior hippocampi.
Volumes of the amygdala were then obtained on each subject by manually tracing these regions as outlined below in Figure 1. These methods have been described in detail in previously published papers (58, 59) and will be summarized briefly here. An MRI atlas was used to confirm identification of all structures (60). The most anterior slice of the amygdala was traced at the level where the thickness of the amygdala is 2.5 times the thickness of the surrounding cortex. The alveus represented the inferior boundary of the amygdala in the posterior sections, and the temporal lobe white matter represented this boundary in more anterior slices. Medially, the border included the medial temporal lobe cortex. Therefore, a small amount of medial temporal structures such as gyrus ambiens was included in the amygdala measure (57). The amygdala was traced posteriorly to the last slice where a pyramidal shape above the ventricle could easily be visualized.
Figure 1. a) and b): Manual Tracings of Amygdala.
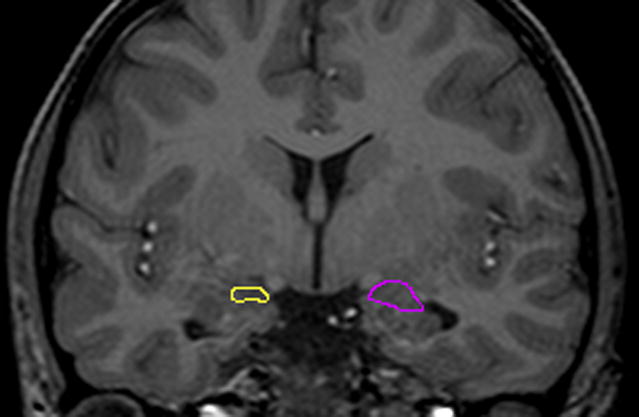
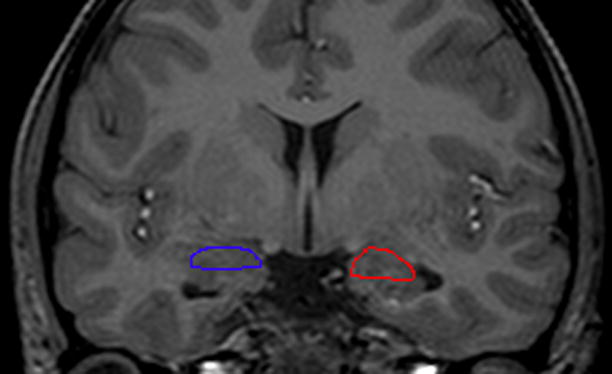
Manual tracings in the coronal plane outlining a) left (yellow) and right (purple) amygdala in a child with complex partial seizures and no psychopathology compared to b) left (blue) and right (32) amygdala in a child with complex partial seizures and comorbid affective/anxiety disorder.
Reliability of Measurements
The drawings were performed by one rater (27) and checked by a second rater (37), both without knowledge of the children’s diagnosis. A consensus drawing was then determined by agreement of the two raters about the boundaries of the regions of interest. Ten re-drawings of the medial temporal lobe regions in this study population showed intra-rater reliability > 0.9 and an inter-rater reliability of 0.95.
Data Analysis
We compared amygdala volumes (total, left and right) and amygdala asymmetries (left-right amygdala volumes) between the CPS and normal groups using ANCOVAs, controlling for total brain volume. In investigating the association of amygdala volumes with psychopathology within the CPS group, we conducted ANCOVAs with amygdala volumes as the dependent variables and presence of psychiatric diagnosis as the predictor, controlling for total brain volume. Of the 28 CPS subjects, 18 had no psychopathology. Of the other 10 CPS subjects with a psychiatric diagnosis, 6 had affective/anxiety disorder diagnoses, 2 had both anxiety disorder and disruptive disorder diagnoses and 2 had other types of diagnoses. Therefore, in the within CPS ANCOVAs looking at psychopathology, we compared only those CPS subjects with an affective/anxiety disorder (n=8) vs those without any psychopathology (n=18). Similarly, ANCOVAs were used to explore the association of amygdala volumes with family history variables, namely family history of mood and anxiety disorders and a history of perinatal problems, controlling for total brain volume.
In exploring the association of amygdala volumes with seizure variables within the CPS group, general linear models were used, with amygdala volumes as the dependent variables and age of seizure onset, duration of illness, seizure frequency, AED use (monotherapy ors polytherapy), prolonged seizures (yes or no), febrile seizures (yes or no) were used as predictors. We first included all these variables as predictors in a stepwise regression model and determined a subset of predictors which contributed significantly (p<0.1) to the variance. We then computed ANCOVAs with this subset of predictors. Total brain volume was used as a covariate in these analyses. All tests were two-tailed and an alpha level of 0.05 was adopted for all inferences.
3. Results
Between Group Differences
Table 3 presents mean amygdala volumes of the CPS and normal groups. ANCOVAs of amygdala volumes controlling for total brain volume demonstrated no significant differences between amygdala volumes in the children with CPS compared to the normal children. There were also no significant differences between the CPS and normal groups in amygdala asymmetry (left-right volumes).
Table 3.
Total Brain and Amygdala Volumes in CPS and Normal Groups
Association of Amygdala Volumes with Psychopathology and Seizure Variables
The CPS children with an affective/anxiety disorder had significantly larger left amygdala volumes compared to those with no psychopathology (1197.7 (119.3) vs 1039.1 (169.8), F1,25 = 5.11, p < .03) as well as greater amygdala asymmetry (174.3 (112.1) vs 37.4 (121.0), F1,25 = 6.63, p < .02). There were no differences in the right amygdala volumes. The other analyses exploring the association of family history and seizure variables, including temporal vs extra-temporal involvement and duration of illness, to amygdala volumes did not yield any significant predictors.
4. Discussion
Controlling for total brain volume, this study found no significant difference in amygdala volumes of the CPS compared to the normal subjects. Within the CPS group, children with an affective/anxiety disorder had significantly larger left amygdala volumes compared to those with no psychopathology. Amygdala volumes, however, were unrelated to seizure variables including age of seizure onset, duration of illness, seizure frequency, and temporal involvement vs extra-temporal disease, as well as history of perinatal problems or family history of psychopathology.
Previous adult TLE studies reported amygdala volume reduction (7–10) associated with duration of epilepsy but not with other seizure variables. It is possible that our study found no significant difference between the CPS and normal subjects in amygdala volumes because of the shorter duration of illness 3.6 (2.55) in the children compared to the adults in the other studies.
Although there have been no structural MRI studies to date on amygdala volumes in children with epilepsy, supporting our findings, recent studies reported enlarged amygdala volumes in children without epilepsy who have MDD (26) and generalized anxiety disorder (27), adults with refractory partial epilepsy with comorbid affective disorders (23–25). Similar to our study, a recent study by Richardson et al. (45) showed a significant positive relationship between amygdala volumes and depression in adolescents and adults with TLE. However, unlike our study these authors (45) found a positive relationship of both right and left amygdala volumes with depression whereas our study found this association only for left amygdala volumes and in a mixed group of affective/anxiety disorders.
Despite larger left amygdala volumes in the CPS subjects with depression and anxiety disorder diagnoses, a left focus on EEG did not appear to drive this finding. In fact, as suggested by several researchers in both imaging (11–14, 18) and neuropathology studies (18), these preliminary findings in children with CPS who have affective/anxiety disorder might reflect the effects of the neuropathology underlying depression and anxiety disorders on amygdala development.
Our findings, however, are unlike those of previous studies that, in keeping with neuropathologic findings of significant reduction of glial cells and glial/neuron ratio in the left amygdala of MDD patients (18), found amygdala volume reduction in children with MDD (19) and anxiety disorders (20), as well as in adults with MDD (15–17). The inclusion of children with both comorbid anxiety and depression in one group, the relatively small study sample size, and differences in amygdala anatomical boundaries across studies underscore the importance of replicating our findings on larger samples of CPS patients both with and without comorbid psychopathology.
Limitations of the present study include its exploratory nature with a retrospective analysis, the small sample size which precluded differentiating between the subgroup of children with depression and anxiety symptoms, a greater, albeit not significantly higher proportion of girls than boys, possible parental memory bias for seizure-related information, few CPS subjects with right epileptic activity, missing EEG data in 2 CPS subjects, and significant differences in the Full Scale IQ of the CPS and normal groups. Although the normal children in the study had high mean Full Scale IQ scores, to ensure that we do not remove illness effect, we did not control for IQ differences in the group comparisons. The study limitations underscore the need for replication of our findings.
With these limitations in mind, children with CPS and comorbid affective/anxiety disorders appear to have enlarged left amygdala volumes compared to CPS children with no psychopathology. Involvement of the left amygdala and lack of association with seizure variables imply that amygdala volumes might reflect the underlying neuropathology of the comorbid affective illness rather than the epilepsy. However, these findings need to be replicated in a prospective study of a larger sample of CPS patients both with and without depression and anxiety disorder to determine the role played by epilepsy and/or the on-going depression and anxiety disorders on amygdala development in pediatric CPS.
Acknowledgments
This study was supported by grant NS32070 (R.C.) and MH067187 (R.C.). We appreciate the technical assistance of Diana Polo-Huizar, B.A., James Lin, M.S., Erin Lanphier, Ph.D., Pamela Vona, Lesley Stahl, PhD., and Sona Hovsepian.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bertram E. Functional anatomy of spontaneous seizures in a rat model of limbic epilepsy. Epilepsia. 1997;38:95–105. doi: 10.1111/j.1528-1157.1997.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 2.Brandt C, Heile A, Potschka H, et al. Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia. 2006;47:1803–9. doi: 10.1111/j.1528-1167.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernasconi N, Bernasconi A, Caramanos Z, Bernasconi N, Bernasconi A, Caramanos Z, et al. Morphometric MRI analysis of the parahippocampal region in temporal lobe epilepsy. Ann N Y Acad Sci. 2000;911:495–500. doi: 10.1111/j.1749-6632.2000.tb06752.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65(2):223–8. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- 5.Kalviainen R, Salmenpera T, Partanen K, et al. MRI volumetry and T2 relaxometry of the amygdala in newly diagnosed and chronic temporal lobe epilepsy. Epilepsy Res. 1997;28:39–50. doi: 10.1016/s0920-1211(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 6.Salmenpera T, Kalviainen R, Partanen K, et al. MRI volumetry of hippocampus, amygdala entorhinal cortex, and perirhinal cortex after status epilepticus. Epilepsy Res. 2000;40:155–70. doi: 10.1016/s0920-1211(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 7.Bernasconi N, Bernasconi A, Caramanos Z, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126(pt 2):462–9. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 8.Cendes F, Andermann F, Gloor P, et al. MRI volumetric measurement of amygdale and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–25. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves Pereira P, Insausti R, Artacho-Perula E, Goncalves Pereira PM, Insausti R, Artacho-Perula E, et al. MR volumetric analysis of the piriform cortex and cortical amygdala in drug-regractory temporal lobe epilepsy. AJNR Am J Neuroradiol. 2005;26:319–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert MV, Brierley B, Al-Sarraj S, et al. Quantitative magnetic resonance imaging of the amygdale in temporal lobe epilepsy-clinico-pathological correlations (a pilot study) Epilepsy Research. 2003;53:39–46. doi: 10.1016/s0920-1211(02)00253-x. [DOI] [PubMed] [Google Scholar]
- 11.Frodl T, Meisenzahl E, Zetzsche T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–14. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 12.Frodl T, Meisenzahl EM, Zetzsche T, et al. Larger Amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–44. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 13.Bremner J. Brain Imaging in anxiety disorders. Expert Rev Neurother. 2004;4:278–84. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- 14.Rauch S, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 15.Caetano S, Hatch JP, Brambilla P, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141–7. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Hastings R, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–9. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 17.Sheline Y, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 18.Bowley M, Drevets WC, Ongur D, et al. Low glial numbers in the amygdala in major depressive disorders. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 19.Rosso I, Cintron CM, Steingard RJ, et al. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–6. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Milham M, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Bio Psychiatry. 2005;57:961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Altshuler L, Bartzokis G, Grieder T, et al. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–4. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 22.Bremner J, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–8. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 23.Satishchandra P, Krishnamoorthy ES, van Elst LT, et al. Mesial temporal structures and comorbid anxiety in refractory partial epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15:450–2. doi: 10.1176/jnp.15.4.450. [DOI] [PubMed] [Google Scholar]
- 24.Tebartz van Elst L, Woermann F, Lemieux L, et al. Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neurosci Lett. 2000;281:103–6. doi: 10.1016/s0304-3940(00)00815-6. [DOI] [PubMed] [Google Scholar]
- 25.Tebartz van Elst L, Woermann FG, Lemieux L, et al. Amygdala enlargement in dysthymia-a volumetric study of patients with temporal lobe epilepsy. Biol Psychiatry. 1999;46:1614–23. doi: 10.1016/s0006-3223(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 26.MacMillan S, Szeszko PR, Moore GJ, et al. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 27.DeBellis M, Casey BJ, Dahl RE, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 28.Barry J, Huynh N, Lembke A. Depression in individuals with epilepsy. Curr Treat Options Neurol. 2000;2:571–85. doi: 10.1007/s11940-000-0035-9. [DOI] [PubMed] [Google Scholar]
- 29.Gilliam F. Diagnosis and treatment of mood disorders in persons with epilepsy. Curr Opin Neurol. 2005;18:129–33. doi: 10.1097/01.wco.0000162853.29650.ec. [DOI] [PubMed] [Google Scholar]
- 30.Jacoby A, Baker GA, Steen N, et al. The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. community study. Epilepsia. 1996;37:148–61. doi: 10.1111/j.1528-1157.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 31.Mendez M, Doss RC, Taylor JL, et al. Depression in epilepsy: relationship to seizures and anticonvulsant therapy. J Nerv Ment Dis. 1993;181:444–7. [PubMed] [Google Scholar]
- 32.O’Donoghue M, Goodridge DM, Redhead K, et al. Assessing the psychosocial consequences of epilepsy: a community-based study. Br J Gen Pract. 1999;49:211–4. [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson M, Channon S, Baker J. Depressive symptomatology in a general hospital sample of outpatients with temporal lobe epilepsy: a controlled study. Epilepsia. 1994;35:771–7. doi: 10.1111/j.1528-1157.1994.tb02510.x. [DOI] [PubMed] [Google Scholar]
- 34.Caplan R, Arbelle S, Magharious W, et al. Psychopathology in pediatric complex partial and primary generalized epilepsy. Dev Med Child Neurol. 1998;40:805–811. doi: 10.1111/j.1469-8749.1998.tb12357.x. [DOI] [PubMed] [Google Scholar]
- 35.Caplan R, Siddarth P, Gurbani S, et al. Depression and Anxiety Disorders in Pediatric Epilepsy. Epilepsia. 2005;46:720–730. doi: 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- 36.Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol. 2003;45:292–5. doi: 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- 37.Dunn D, Austin JK, Huster GA. Symptoms of depression in adolescents with epilepsy. J Am Acad Child Adolesc Psychiatry. 1999;38:1132–8. doi: 10.1097/00004583-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Ettinger A, Weisbrtot DM, Nolan EE, et al. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia. 1998;39:595–9. doi: 10.1111/j.1528-1157.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 39.Oguz A, Kurul S, Dirik E. Relationship of epilepsy-related factors to anxiety and depression scores in epileptic children. J Child Neurol. 2002;17:37–40. doi: 10.1177/088307380201700109. [DOI] [PubMed] [Google Scholar]
- 40.Ott D, Caplan R, Guthrie D, et al. Measures of psychopathology in children with complex partial seizures and primary generalized epilepsy with absence. J Am Acad Child Adolesc Psychiatry. 2001;40:907–914. doi: 10.1097/00004583-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Williams J, Steel C, Sharp GB, et al. Anxiety in children with epilepsy. Epilepsy Behav. 2003;4:729–32. doi: 10.1016/j.yebeh.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 42.Richardson E, Griffith HR, Martin RC, et al. Structural and functional neuroimaging correlates of depression in temporal lobe epilepsy. Epilepsy Behav. 2007;10:242–9. doi: 10.1016/j.yebeh.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Van Elst L, Woermann FG, Lemieux L, et al. Affective aggression in patients with temporal lobe epilepsy: a quantitative MRI study of the amygdala. Brain. 2000;123(2):234–43. doi: 10.1093/brain/123.2.234. [DOI] [PubMed] [Google Scholar]
- 44.Briellmann RS, Hopwood MJ, GD J. Major depression in temporal lobe epilepsy with hippocampal sclerosis: clinical and imaging correlates. J Neurol Neurosurg Psychiatry. 2007;78:1226–1230. doi: 10.1136/jnnp.2006.104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson EJ, Griffith HR, Martin RC, et al. Structural and functional neuroimaging correlates of depression in temporal lobe epilepsy. Epilepsy & Behavior. 2007;10:242–249. doi: 10.1016/j.yebeh.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Beck A, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 47.Kanner A, Balabanov A. Depression and epilepsy: how closely related are they? Neurology. 2002;58:S27–39. doi: 10.1212/wnl.58.8_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- 48.Keller S, Wieshmann UC, Mackay CE, et al. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648–655. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theodore W, DeCarli C, Gaillard WD. Total cerebral volume is reduced in patients with localization-related epilepsy and a history of complex febrile seizures. Arch Neurol. 2003;60:250–252. doi: 10.1001/archneur.60.2.250. [DOI] [PubMed] [Google Scholar]
- 50.Hollingshead A. Medical sociology: A brief review. Milbank Mem Fund Q Health Soc. 1973;51:531–542. [PubMed] [Google Scholar]
- 51.Commission, Commission on classification and terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epilepstic seizures. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 52.Shaywitz S. The Yale Neuropsycho-educational Asssessment Scales. Schizophr Bull. 1982;8:360–424. doi: 10.1093/schbul/8.2.360. [DOI] [PubMed] [Google Scholar]
- 53.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Wechsler D. Weschsler Intelligence Scale for Children. 3. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 55.Sled J, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Trans Med Imaging. 1998;17:653–62. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- 56.Shattuck D, Sandor-Leahy SR, Schaper KA, et al. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–76. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 57.Bartzokis G, Mintz J, Marx P, et al. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magnetic Resonance Imaging. 1993:11. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- 58.Bartzokis G, Altshuler LL, Greider T, et al. Reliability of medial temporal lobe volume measurements using reformatted 3D images. Psychiatry Res. 1998;82:11–24. doi: 10.1016/s0925-4927(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 59.Levitt J, Blanton RE, Caplan R, et al. Medial temporal lobe in childhood-onset Schizophrenia. Psychiatry Research: Neuroimaging. 2001;108:17–27. doi: 10.1016/s0925-4927(01)00108-1. [DOI] [PubMed] [Google Scholar]
- 60.Duvernoy H. The Human Brain. New York: 1991. [Google Scholar]
- 61.Geidd J, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]


