Abstract
Protein kinase C (PKC) is involved in a wide array of cellular processes such as cell proliferation, differentiation and apoptosis. Phosphorylation of both turn motif (TM) and hydrophobic motif (HM) are important for PKC function. Here, we show that the mammalian target of rapamycin complex 2 (mTORC2) has an important function in phosphorylation of both TM and HM in all conventional PKCs, novel PKCɛ as well as Akt. Ablation of mTORC2 components (Rictor, Sin1 or mTOR) abolished phosphorylation on the TM of both PKCα and Akt and HM of Akt and decreased HM phosphorylation of PKCα. Interestingly, the mTORC2-dependent TM phosphorylation is essential for PKCα maturation, stability and signalling. Our study demonstrates that mTORC2 is involved in post-translational processing of PKC by facilitating TM and HM phosphorylation and reveals a novel function of mTORC2 in cellular regulation.
Keywords: Akt, mTOR, PKC, TORC2
Introduction
Protein kinase C (PKC) is one of the most extensively studied kinase family and has been implicated in cell proliferation, differentiation, apoptosis, tumour promotion and neuronal activity (Griner and Kazanietz, 2007). On the basis of their structure and regulation, PKCs can be categorized into conventional, novel and atypical PKC (cPKC, nPKC and aPKC) (Mellor and Parker, 1998; Toker, 1998). Both cPKCs (α, β, γ) and nPKCs (δ, ɛ, θ, η) are activated by diacylglycerol (DAG). The cPKCs, but not the nPKCs, are also activated by calcium. In contrast, aPKCs (ζ, ι) are not activated by DAG, but they have important functions in cell polarity and asymmetric cell division (Etienne-Manneville and Hall, 2003).
In addition to regulation by intracellular second messengers, PKCs are controlled by phosphorylation in the activation loop (A-loop) within the kinase domain, the turn motif (TM) and hydrophobic motif (HM) in the C-terminal region (Parekh et al, 2000; Newton, 2003). These modifications are highly conserved in PKCs with the exception of the aPKCs, which have acidic residues in the corresponding HM sites. Phosphorylation of the activation loop is catalysed by the 3-phosphoinositide-dependent protein kinase 1 (PDK1) and is important for PKC activity (Chou et al, 1998; Dutil et al, 1998; Le Good et al, 1998). However, the kinase responsible for the TM and HM phosphorylation is less clear. There is evidence that autophosphorylation may be responsible for the TM and HM phosphorylation, whereas the question whether autophosphorylation is truly responsible for these two sites in vivo still remains (Parekh et al, 2000; Newton, 2003).
Protein kinase C belongs to the AGC family, including Akt (also known as PKB). Akt has an important function in cell growth, proliferation and inhibition of apoptosis (Lawlor and Alessi, 2001). A similar pattern of phosphorylation also occurs in Akt (Alessi and Cohen, 1998). However, phosphorylation appears to have an important function in Akt activation. Phosphorylation of the A-loop and HM is stimulated by growth factors through phosphatidylinositol 3-kinase (PI3K) and directly contributes to Akt activation (Alessi et al, 1996). In contrast, phosphorylation of the TM is not affected by growth factors, although this phosphorylation is important for Akt function (Bellacosa et al, 1998; Hauge et al, 2007). In addition, phosphorylation of the HM is also associated with Akt activation. Recent studies have shown that the mammalian target of rapamycin complex 2 (mTORC2) is responsible for Akt HM phosphorylation, whereas the TM kinase has not been identified (Sarbassov et al, 2005).
Mammalian target of rapamycin (mTOR) is a central cell growth controller (Hay and Sonenberg, 2004; Wullschleger et al, 2006). mTOR exists in two different complexes, mTORC1 and mTORC2 (Loewith et al, 2002; Sabatini, 2006). The two TOR complexes have distinct physiological functions and are regulated differently. mTORC1 activity is sensitive to inhibition by rapamycin, whereas mTORC2 activity is resistant at least to short-term treatment. One of the best-characterized physiological substrates of mTORC1 is S6K, which is also a member of the AGC kinase family. mTORC1 phosphorylates the HM in S6K, thereby promoting phosphorylation of the A-loop by PDK1 (Collins et al, 2003). mTORC2 consists of mTOR, Rictor, mLST8 and Sin1 (Sabatini, 2006). Deletion of mTORC2-specific subunits, such as Rictor or Sin1, abolishes the HM phosphorylation of Akt but not S6K, indicating the high substrate specificity of the two TOR complexes (Guertin et al, 2006; Jacinto et al, 2006; Shiota et al, 2006; Yang et al, 2006). It has also been reported that deletion of Rictor abolishes the HM phosphorylation in PKCα (Sarbassov et al, 2004; Guertin et al, 2006). However, the immunoprecipitated mTORC2 can directly phosphorylate the HM in Akt but not PKC in vitro (Sarbassov et al, 2004, 2005). Therefore, the exact function of mTORC2 in PKC regulation remains to be resolved.
In this report, we discovered a novel function of mTORC2 in the regulation of PKC and Akt. In Rictor−/− or Sin1−/− cells, PKCα protein levels and phosphorylation are dramatically decreased. We found that Rictor and Sin1 have important functions in the TM and HM phosphorylation of cPKCs and nPKCɛ and of Akt. Inhibition of mTOR by RNA interference knockdown and specific inhibitors blocks the TM and HM phosphorylation of both PKC and Akt. Interestingly, Rictor preferentially interacts with the unphosphorylated PKCα, suggesting a direct role of Rictor in PKC regulation, although we were unable to detect a direct phosphorylation of PKC by immunoprecipitated mTORC2 in vitro. We also observed that PKC kinase activity and substrate phosphorylation are impaired in Rictor−/− or Sin1−/− cells. The mTORC2-dependent TM and HM phosphorylation of PKC are critical for the kinase stability and function. Our study reveals an essential physiological function of mTORC2 in the regulation of PKC and Akt by promoting phosphorylation and maturation of the kinases.
Results
Rictor is required for phosphorylation of TM and HM in PKC and Akt
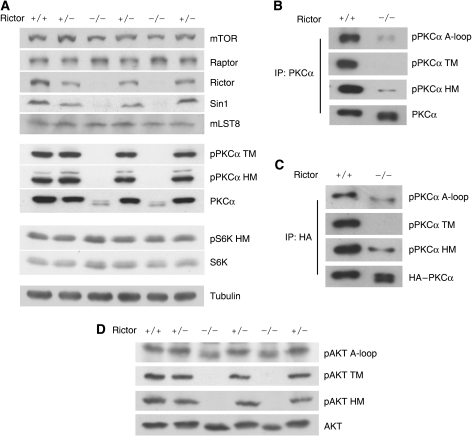
To study the function of TORC2 in AGC family kinase regulation, we examined Rictor−/− embryos, which showed no Rictor protein and a diminished Sin1 protein level (Figure 1A). We found that PKCα protein level was dramatically decreased in Rictor−/− embryos compared with that in Rictor+/+ embryos, whereas S6K protein level was unaffected (Figure 1A). Furthermore, PKCα protein migrated significantly faster in the Rictor−/− than Rictor+/+ embryos, suggesting that PKCα was dephosphorylated in the Rictor−/− embryos. As expected, phosphorylation of HM (S657) in PKCα was largely decreased in the Rictor−/− embryos (Figure 1A). In contrast, phosphorylation of HM in S6K was intact. Previous studies have indicated that phosphorylation of the PKCα TM is important for protein stability (Newton, 2003). Therefore, we examined PKCα TM (T638) phosphorylation and found that PKCα TM phosphorylation was completely abolished in the Rictor−/− embryos (Figure 1A). This observation is interesting and important because regulation of TM phosphorylation has not been clearly defined, although it is highly conserved in PKC and the AGC family.
Figure 1.
Rictor is required for TM and HM phosphorylation of PKCα and Akt. (A) Deletion of Rictor gene decreases PKCα protein levels and phosphorylation. E10.5 embryos were lysed and used for immunoblot analysis. Immunoblotting was performed for PKCα and S6K phosphorylation states and mTOR complex components in lysates prepared from individual Rictor +/+, +/− and −/− embryos. (B) Phosphorylation of PKCα TM is eliminated in Rictor−/− embryos. Endogenous PKCα proteins were immunoprecipitated and a similar protein level was loaded. Phosphorylation of A-loop, TM and HM was determined by immunoblotting. (C) A-loop, TM and HM phosphorylation of transfected PKCα. HA–PKCα plasmid was transfected into Rictor+/+ or −/− MEF and HA–PKCα protein was immunoprecipitated and probed for phosphorylation. (D) Deletion of Rictor gene eliminates Akt TM and HM phosphorylation. E10.5 embryos were analysed for Akt phosphorylation.
To ascertain the decreased phosphorylation of PKCα in the Rictor−/− embryos, PKCα proteins were immunoprecipitated from lysates of Rictor+/+ and −/− embryos, and a similar level of PKCα was loaded. We found that phosphorylation of the TM was completely abolished and HM phosphorylation was significantly reduced in the Rictor−/− embryos (Figure 1B). Interestingly, the phosphorylation of PKCα A-loop (T497), the PDK1 phosphorylation site, was also diminished in Rictor−/− embryos (Figure 1B). This observation was confirmed by transfection of PKCα into Rictor+/+ or −/− murine embryonic fibroblast (MEF) cells. We found that TM phosphorylation of the ectopically expressed PKCα was completely abolished, whereas the phosphorylation of HM and A-loop were decreased in the Rictor−/− cells (Figure 1C). Our data indicate that Rictor is critically required for phosphorylation of TM and is also involved in A-loop and HM in PKCα.
To examine whether a similar regulation was operating in Akt, we monitored Akt phosphorylation. As expected, phosphorylation of the Akt HM (S473) was abolished in Rictor−/− embryos, whereas phosphorylation of the activation loop (A-loop, T308) was not affected (Figure 1D). It is worth noting that Rictor deletion dramatically decreased PKCα A-loop, but not Akt A-loop phosphorylation, although both kinases are phosphorylated by PDK1. Therefore, the effect of Rictor on PKCα A-loop phosphorylation might be indirect. Interestingly, phosphorylation of the TM (T450) in Akt was also abolished in Rictor−/− embryos. Similar results were observed in Rictor−/− MEF cells (Supplementary Figure S1). Our study provides the first strong genetic evidence that phosphorylation of TM in both PKCα and Akt is dependent on Rictor.
Sin1 but not PDK1 is required for TM phosphorylation in both PKC and Akt
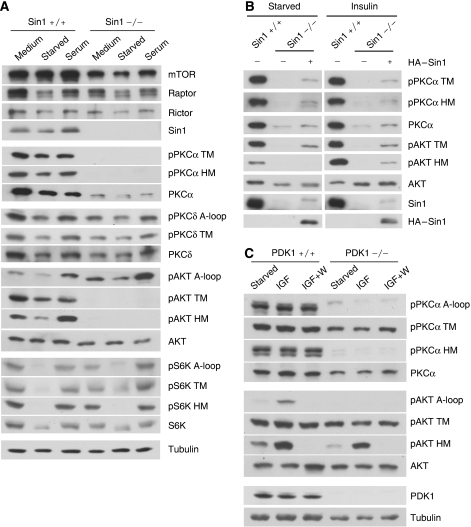
We and other groups have recently identified Sin1 as an essential subunit of mTORC2 (Frias et al, 2006; Jacinto et al, 2006; Yang et al, 2006; Vander Haar et al, 2007). To determine the function of Sin1 in PKC and Akt regulation, we analysed Sin1−/− MEFs. Deletion of Sin1 gene was confirmed by the lack of Sin1 protein expression (Figure 2A). Sin1 was also required for phosphorylation of both the TM and HM in both PKCα and Akt (Figure 2A). In contrast, the A-loop phosphorylation in Akt was independent of Sin1. In addition, PKCα protein level was also significantly decreased in Sin1−/− cells. However, lack of Sin1 had no effect on S6K or PKCδ phosphorylation. These results establish that both Rictor and Sin1 have important functions in the phosphorylation of the TM and HM in PKCα and Akt.
Figure 2.
Sin1 but not PDK1 is required for PKCα and Akt TM phosphorylation. (A) Phosphorylation of the TM and HM in PKCα and Akt, but not PKCδ and S6K, is eliminated in Sin1−/− MEF. Sin1+/+ or −/− MEF cells were cultured in normal medium (medium), serum-free medium for 12 h (starved) or restimulated with serum for 30 min (serum). Immunoblottings were performed using indicated antibodies. (B) Reintroduction of Sin1 in Sin1−/− MEF restores phosphorylation. Sin1−/− MEFs were infected with either empty vector or HA-Sin1-containing retroviruses. The cells were treated with or without 400 nM insulin for 30 min. (C) PDK1 is required for PKCα HM but not TM phosphorylation. Immunoblotting of lysates from PDK1+/+ and −/− ES cells was performed for PKCα and Akt phosphorylation. The cells were serum-starved for 3 h and then stimulated with or without 100 nM IGF for 30 min in the presence or absence of pretreatment with 100 nM wortmannin (W) for 30 min.
To confirm that the defect of the TM and HM phosphorylation in both PKCα and Akt was a consequence of Sin1 deletion, we reintroduced an HA-Sin1 plasmid into the Sin1−/− cells. HA-Sin1 expression partially restored TM and HM phosphorylation of both PKCα and Akt. The partial restorations of TM and HM phosphorylation of PKCα and Akt are likely due to the low expression levels of HA-Sin1 protein in Sin1−/− MEF (Figure 2B).
PDK1 is an important upstream kinase for the A-loop phosphorylation in both PKC and Akt (Mora et al, 2004). To examine the role of A-loop phosphorylation for TM and HM phosphorylation, we analysed PDK1−/− murine embryonic stem (ES) cells. As expected, phosphorylation of the A-loop in both PKCα and Akt was abolished; however, the TM phosphorylation was slightly affected in PDK1−/− ES cells (Figure 2C). Interestingly, the HM phosphorylation in PKCα, but not in Akt, was abolished in PDK1−/− cells, suggesting that the regulations of HM phosphorylation in PKCα and Akt are distinct. A possible interpretation is that A-loop phosphorylation by PDK1 activates PKCα, which might autophosphorylate its HM. Stimulation with insulin-like growth factor (IGF) or inhibition of PI3K by wortmannin caused a corresponding increase or decrease of Akt phosphorylation in the A-loop and HM, but not the TM (Figure 2C). However, IGF or wortmannin had a mild effect on PKCα phosphorylation.
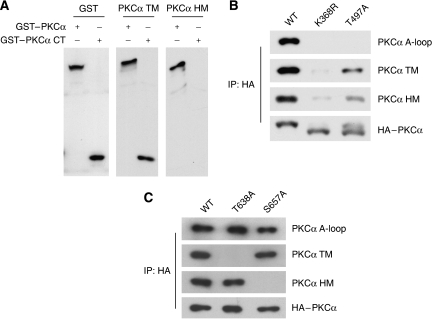
To test whether PKCα TM and HM are regulated by intramolecular autophosphorylation as reported previously (Behn-Krappa and Newton, 1999), we analysed the phosphorylation status of various PKCα mutants. The PKCα construct that contains only C-terminal fragment (amino acid 603–672) was phosphorylated on TM but not HM when expressed in 293T cells (Figure 3A). Interestingly, phosphorylation of TM, HM and A-loop were all decreased in PKCα kinase-deficient mutant (K368R) (Figure 3B), whereas phosphorylation of Akt TM was comparable in both wild-type and kinase-inactive Akt (data not shown) (Alessi et al, 1996). These data suggest that TM in both PKCα and Akt is not directly phosphorylated by intramolecular autophosphorylation. However, it is possible that kinase activity of PKCα is important for the overexpressed full-length PKCα to sustain proper localization or conformation to become a substrate for phosphorylation. Interestingly, PKCα HM phosphorylation was reduced but considerably sustained in A-loop mutant (T497A), whereas it was abolished in PDK1−/− ES cells (Figures 2C and 3B). These data suggest that a PDK1- and mTORC2-dependent heterologous kinase could be involved in phosphorylation of HM in PKCα in addition to the proposed intramolecular autophosphorylation (Behn-Krappa and Newton, 1999). Together, the above data indicate that the PKCα and Akt TM are likely to be regulated by a Rictor/Sin1-dependent heterologous kinase, but not by autophosphorylation.
Figure 3.
Relationship among different PKCα phosphorylation sites. (A) Kinase domain is not required for PKCα TM phosphorylation. HEK293T cells were transfected with indicated GST–PKCα full length or C-terminal fragment (CT, a.a. 603–672). Cell lysates were probed with antibody for GST, TM and HM as indicated. (B) Phosphorylation status of PKCα mutants. Transfected HA-tagged PKCα (in HEK293T) were immunoprecipitated with HA-antibody. Phosphorylation was determined by immunoblotting with indicated antibodies. K368R and T497A denote PKCα kinase inactive and A-loop mutant, respectively. (C) Relationship between PKCα TM and HM phosphorylation. HEK293T cells were transfected with PKCα wild type (WT), TM mutant (T638A) and HM mutant (S657A). Phosphorylation of each PKCα construct was determined.
We investigated the relationship between TM and HM phosphorylation in PKCα by examining TM and HM mutants. We found that phosphorylations of the TM and HM were independent from each other, as mutation in one motif did not affect phosphorylation of the other (Figure 3C).
Rictor and Sin1 regulate some but not all PKC family members
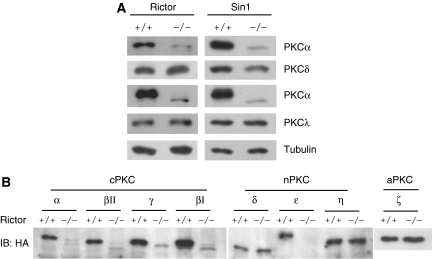
The diverse PKC isoforms are differentially regulated. We wanted to determine which PKCs are regulated by Rictor and Sin1. Both protein levels and mobility of PKCα and PKCɛ, but not PKCδ and PKCλ, were dramatically altered in Rictor−/− or Sin1−/− cells (Figure 4A). Consistent with the above-mentioned observation, TM phosphorylation of PKCδ was slightly changed in both Sin1−/− cells (Figure 2A) and Rictor−/− cells (data not shown). These data indicate that Rictor and Sin1 are required for phosphorylation and protein levels of PKCα and PKCɛ, but not PKCδ or PKCλ.
Figure 4.
Effect of Rictor and Sin1 deletion on PKC isoforms. (A) Protein levels of PKCα and ɛ but not PKCδ and λ are decreased in Rictor or Sin1−/− MEFs. Expression levels of endogenous PKCs were monitored by immunoblotting. (B) Expression levels of transfected PKC isoforms in Rictor+/+ and −/− MEFs. The Rictor+/+ and −/− MEFs were transfected with HA–PKC isoforms as indicated. Expression levels and mobility of the transfected PKCs were monitored by HA antibody.
To examine additional PKC family members, Rictor+/+ or −/− MEF cells were transfected with plasmids encoding various isoforms of PKC. We found that protein levels of all cPKCs were much lower in the Rictor−/− cells than those in the Rictor+/+ cells (Figure 4B). Furthermore, the residual cPKCs in the Rictor−/− cells showed a faster mobility, indicative of hypophosphorylation of these proteins. Among the three nPKC isoforms (δ, ɛ and η) tested, only PKCɛ protein levels and mobility were affected. Rictor deletion had little effect on the protein levels and mobility of the aPKCλ (Figure 4A) and aPKCζ (Figure 4B). Our data reveals that Rictor and Sin1 are required for phosphorylation and protein levels of all cPKCs and nPKCɛ.
mTORC2 is involved in TM and HM phosphorylation of PKC and Akt
The fact that both Rictor and Sin1, two known mTORC2 components, are required for the phosphorylation of TM and HM in both PKCα and Akt indicates a possible involvement of mTORC2 in these phosphorylations. We utilized mTOR inhibitors to explore a possible function of mTORC2 in the regulation of PKCα and Akt phosphorylation. Cells were treated with rapamycin (to inhibit mTORC1), LY294002 (to inhibit both PI3K and mTOR) and wortmannin (to inhibit PI3K) for 1 h (Brunn et al, 1996). As expected, rapamycin selectively decreased the HM phosphorylation of S6K (Supplementary Figure S2A). Both LY294002 and wortmannin inhibited phosphorylation of the A-loop and HM in Akt but not PKCα. However, TM phosphorylation in both PKCα and Akt was resistant to LY294002. LY294002 treatment for 24 h inhibited Akt TM phosphorylation but had little effect on PKCα phosphorylation (Supplementary Figure S2B). These results indicate that mTOR might not be involved in PKC phosphorylation. Alternatively, mTOR is involved, but the effect of mTOR inhibition on PKCα phosphorylation is masked by the high stability of PKC phosphorylation.
As the TM phosphorylation residues in PKCα and AKT are followed by a proline, we tested a possible role of proline-directed protein kinases. Twenty-four-hour treatment with inhibitors for ERK, JNK, p38, CDK, GSK3 and CK2 had no significant effect on Akt phosphorylation, whereas LY294002 inhibited Akt TM and HM phosphorylation (Supplementary Figure S2C). These results argue against the involvement of these proline-directed kinases in Akt TM phosphorylation.
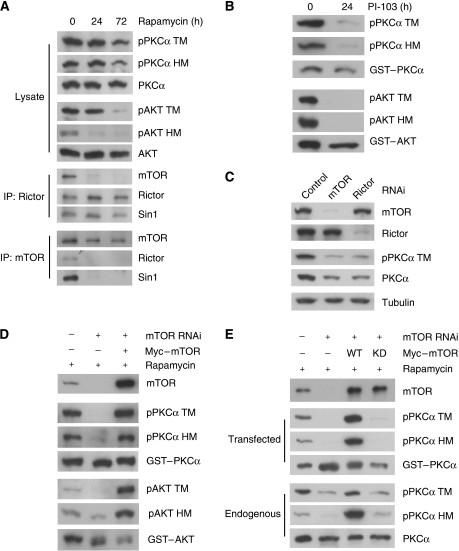
Prolonged rapamycin treatment has been reported to disrupt mTORC2 assembly and function in several cell lines (Sarbassov et al, 2006). We found that prolonged rapamycin treatment indeed disrupted mTORC2 in HepG2 cells as determined by co-immunoprecipitation of mTOR with Rictor (Figure 5A). Rapamycin treatment for 72 h caused a more complete disruption of TORC2 than the 24-h treatment. In contrast, the interaction between Rictor and Sin1 was not affected by rapamycin. Rapamycin treatment for 72 h significantly decreased both TM and HM phosphorylation in Akt and PKCα (Figure 5A). In contrast, rapamycin treatment had limited effect on TM and HM phosphorylation in PKCα and on TM in Akt phosphorylation in A549 cells whose mTORC2 function was reported to be insensitive to rapamycin (Supplementary Figure S2D) (Sarbassov et al, 2006). These observations support a possible role of mTORC2 in PKCα and Akt regulation.
Figure 5.
mTORC2 integrity and mTOR kinase activity are required for PKCα and Akt TM phosphorylation. (A) Prolonged treatment of rapamycin decreases both TM and HM phosphorylation in PKCα and Akt. HepG2 cells were treated with 100 nM rapamycin for indicated times. Total cell lysates were probed with PKC and Akt antibodies as indicated. Rictor or mTOR immunoprecipitates were analysed by immunoblotting with antibodies against TORC2 components. (B) The PI3K/mTOR inhibitor P103 inhibits phosphorylation of transfected PKCα and Akt. HEK293T cells were transfected with GST–PKCα and GST–Akt in the presence or absence of 10 μM PI-103. Immunoblottings were performed using indicated antibodies. (C) mTOR and Rictor are required for maintaining both phosphorylation and protein level of PKCα. HeLa cells stably expressing control, mTOR shRNA or Rictor shRNA were transfected with control, mTOR or Rictor siRNA oligos, respectively. (D) Knockdown of mTOR combined with prolonged rapamycin treatment abolishes TM phosphorylation. HeLa cells with mTOR knockdown using shRNA-containing lentivirus were pretreated with 100 nM rapamycin for 24 h. mTOR siRNA oligos together with GST–PKCα, GST–Akt, and siRNA-resistant Myc-mTOR were transfected as indicated under rapamycin treatment. (E) Kinase activity of mTOR is required for PKCα TM phosphorylation. Experiments were similar to Figure 5D except that the siRNA-resistant kinase dead (KD) mTOR was included. Protein levels and phosphorylation of both the transfected GST–PKCα and the endogenous PKCα were determined.
The inability of transient mTOR inhibition to abolish PKCα phosphorylation is likely due to the fact that the PKCα phosphorylation is extremely stable. To further test this possibility, we next examined the phosphorylation of newly synthesized PKCα. HEK293T cells were transfected with GST-tagged PKCα and Akt in a medium containing PI-103, which is a potent inhibitor of PI3K and mTOR (Fan et al, 2006; Knight et al, 2006). Interestingly, the TM and HM phosphorylation of the transfected GST–PKCα and Akt were dramatically reduced by PI-103 (Figure 5B). These results indicate that mTOR is required for TM and HM phosphorylation of the newly synthesized PKCα. To further investigate the function of mTOR in PKCα phosphorylation, we ablated mTORC2 components by RNA interference in HeLa cells. Knockdown of either mTOR or Rictor significantly decreased PKCα TM phosphorylation and protein level (Figure 5C).
It has been reported that combination of mTOR knockdown with prolonged rapamycin treatment efficiently inhibits mTORC2 function (Sarbassov et al, 2006). HeLa cells with shRNA-mediated mTOR knockdown were pretreated with rapamycin for 24 h and then transfected with PKCα or Akt plasmid. We found that phosphorylation of GST–PKCα TM was completely abolished and that phosphorylation of the HM was dramatically reduced (Figure 5D), results similar to those observed in the Rictor−/− or Sin1−/− cells (Figures 1 and 2A). Moreover, phosphorylation of the transfected Akt was inhibited by the combinatory treatment with mTOR knockdown and rapamycin (Figure 5D). The combination of mTOR knockdown and rapamycin treatment also significantly decreased the TM and HM phosphorylation of endogenous PKCα (Figure 5E). In contrast, phosphorylation of neither the endogenous ERK nor PKCδ was affected (Supplementary Figure S2E), indicating that the above-mentioned treatment was specific towards Akt and PKCα. Co-transfection of the siRNA-resistant wild-type mTOR, but not kinase-deficient mTOR, restored the GST–PKCα TM phosphorylation (Figure 5D and E), indicating that mTOR kinase activity is important for PKCα TM and HM phosphorylation. On the basis of these results, we conclude that mTORC2 has an essential role for the TM and HM phosphorylation of both PKCα and Akt.
PKC was not directly phosphorylated by mTORC2 in vitro but associated with Rictor
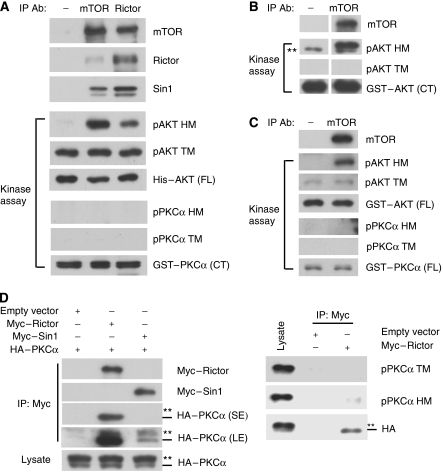
To test whether mTORC2 could phosphorylate TM in PKCα and Akt, we performed in vitro kinase assays with immunoprecipitated mTOR or Rictor. The immunoprecipitated mTOR or Rictor complex could phosphorylate the HM of His–Akt protein, which was prepared from baculovirus (Figure 6A). We consistently observed that IP complex with mTOR antibody could phosphorylate Akt HM more efficiently than that with Rictor antibody, even though the amount of precipitated Rictor was similar. This observation could be explained that not all Rictor in the cell is associated with mTOR. Alternatively, we cannot exclude the possibility that the Rictor antibody may partially interfere with the mTORC2 kinase activity.
Figure 6.
PKCα is not directly phosphorylated by mTORC2 in vitro but associated with Rictor. (A–C) Immunoprecipitated mTORC2 fails to phosphorylate PKCα in vitro. Endogenous proteins from HeLa cells were immunoprecipitated with control, mTOR or Rictor antibody. The immunoprecipitates were used to phosphorylate GST–PKCα (CT) purified from E. coli and His–Akt (FL: full-length) from baculovirus (A), GST–Akt (CT) from E. coli (B) or GST–Akt and GST–PKCα from HeLa cells (C), which had mTOR knockdown and rapamycin treatment. FL and CT denotes full-length and C-terminal fragment (a.a. 125–480 for Akt and a.a. 322–672 for PKCα), respectively. **Denotes the non-specific signal detected by phospho-Akt HM antibody. (D) Rictor preferentially interacts with hypophosphorylated PKCα. HEK293 cells were transfected with HA–PKCα and Myc–Rictor, Myc–Sin1 or empty vector as indicated. Cell lysates were immunoprecipitated with Myc antibody, and then co-immunoprecipitated HA–PKCα was determined by immunoblotting. The transfected PKCα migrates as doublet. The slow-migrating PKCα (phosphorylated form) and fast-migrating PKCα (hypophosphorylated form) are denoted by two asterisks and a dash, respectively. The faster migrating HA–PKCα was co-immunoprecipitated with Myc–Rictor (SE denotes short exposure). Longer exposure (LE) shows that a small amount of PKCα was co-immunoprecipitated with Myc–Sin1. Phosphorylation of the co-immunoprecipitated HA–PKCα was determined with indicated phospho-PKCα antibodies (right panels).
As the commercial His–Akt was phosphorylated on TM (Figure 6A), we prepared GST–Akt from E. coli as a substrate. Immunoprecipitated mTOR could phosphorylate the HM but not TM of GST–Akt (Figure 6B). In contrast, the immunoprecipitated mTOR or Rictor did not phosphorylate the GST–PKCα prepared from E. coli.
To exclude the possibility that PKCα and Akt proteins purified from bacteria may not be suitable substrates for mTORC2, we prepared dephosphorylated full-length PKCα and Akt from HeLa cells. GST–PKCα and GST–Akt were transfected into the HeLa cells with the combinatory treatment of mTOR knockdown and rapamycin. The purified GST–PKCα and GST–Akt indeed had little phosphorylation on either TM or HM (Figure 6C) and were used as substrates in the in vitro kinase reaction. Immunoprecipitated mTOR could only phosphorylate HM but not TM in GST–Akt (Figure 6C). Furthermore, neither TM nor HM in GST–PKCα was phosphorylated by the immunoprecipitated mTOR. These results suggest that mTORC2 may not be the kinase directly responsible for the TM phosphorylation in PKCα and Akt. However, our study cannot exclude the possibility that the immunoprecipitated mTORC2 misses a critical component required to phosphorylate the TM of PKCα and Akt in vitro or the substrates may miss some modifications or correct folding that are essential for the in vitro phosphorylation by mTORC2.
We tested the interaction between Rictor or Sin1 and PKCα by co-immunoprecipitation. We observed that transfected PKCα was co-immunoprecipitated with transfected Rictor (Figure 6D). It is worth noting that much less PKCα was co-immunoprecipitated with Sin1. This result suggests that the interaction between Sin1 and PKCα could be indirectly mediated by the endogenous Rictor. In contrast, transfected PKCα was not co-immunoprecipitated with the mTORC1 component Raptor (Supplementary Figure S3). We observed that the faster migrating hypophosphorylated PKCα was preferentially precipitated with Rictor (Figure 6D). Immunoblotting with phosphospecific antibodies for TM and HM confirmed that the Rictor-co-immunoprecipitated PKCα was hypophosphorylated (Figure 6D). It has been suggested that the newly synthesized PKC is unphosphorylated and phosphorylation is critical for PKC maturation (Newton, 2003). Therefore, our data indicate that Rictor may contribute to PKC phosphorylation and maturation by directly associating with the unphosphorylated immature PKC.
PKC is unstable in Rictor−/− and Sin1−/−cells
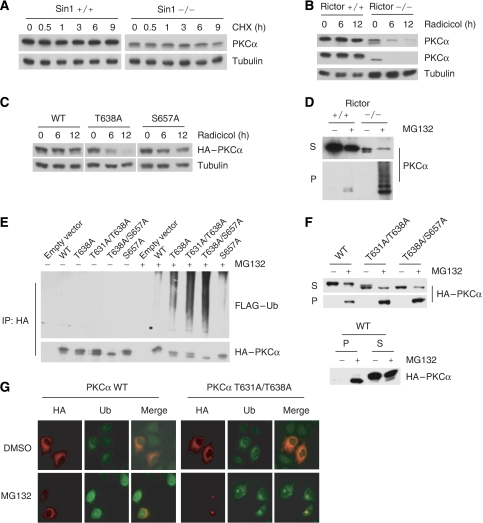
Next, we investigated the underlining mechanisms for the dramatic decrease of PKCα protein levels in the Rictor−/− and Sin1−/− cells. The fact that the protein expression level of transfected HA-PKCα was also lower in the Rictor−/− than that in the Rictor+/+ cells (Figure 4B) suggests that Rictor and Sin1 may regulate the expression of PKCα at the posttranscriptional level. Surprisingly, we found that PKCα in Sin1−/− cells was stable under cycloheximide treatment (Figure 7A).
Figure 7.
TM phosphorylation protects PKCα from proteasome-dependent degradation. (A) The residual PKCα in Sin−/− MEF cells is stable. Cells were treated with 100 μg/ml cycloheximide (CHX) for the indicated time. PKCα protein levels were determined. (B) Inhibition of Hsp90 destabilizes PKCα and PKCɛ in Rictor−/− but not in +/+ MEFs. Cells were treated with 20 μM radicicol, an Hsp90 inhibitor, for the indicated time, and PKC protein levels were monitored by immunoblotting. (C) Mutation of the TM phosphorylation site destabilizes PKCα in the presence of Hsp90 inhibition. HEK293 cells transfected with HA–PKCα wild type, TM mutant (T638A) or HM mutant (S657A) were treated with 20 μM radicicol for the indicated time. The protein levels of HA–PKCα were monitored by immunoblotting. (D) Inhibition of proteasome accumulates insoluble PKCα in the Rictor−/− but not +/+ cells. Cells were treated with 10 μM MG132 for 12 h, lysed in NP-40 buffer and then fractionated into soluble (S) and insoluble fractions (P). (E) Mutation of the PKCα TM phosphorylation site enhances ubiquitination. HEK293 cells were transfected with HA–PKCα wild type (WT), TM mutant (T638A), TM and compensation site mutant (T631A/T638A), HM mutant (S657A) or TM and HM mutant (T638A/S657A) together with FLAG–Ub. The transfected cells were treated with or without 10 μM MG132 for 12 h as indicated. Soluble fractions were immunoprecipitated with anti-HA, and ubiquitinated PKCα was determined by immunoblotting using anti-FLAG antibody. (F) The TM mutant PKCα is more prone to be in the insoluble fraction. HEK293 cells were transfected with HA–PKCα WT and TM mutants and treated with 10 μM MG132 for 12 h as described in Figure 6D. The levels of HA–PKCα in both soluble and insoluble fractions were determined by immunoblotting using anti-HA antibody. P and S denote insoluble pellet and soluble fractions, respectively. (G) PKCα TM mutant, but not the wild type, accumulates in aggresome when proteasome is inhibited. HeLa cells transfected with wild-type or mutant HA–PKCα (T631A/T638A) were treated with 5 μM MG132 for 24 h. Double immunofluorescent staining was performed using anti-HA antibody for HA–PKCα (red colour) and anti-Ub antibody for conjugated ubiquitin (green colour).
It has been reported that dephosphorylation of the TM may allow PKC to associate with heat shock protein (Hsp) (Gao and Newton, 2002), which presumably stabilizes PKC. Consistent with this notion, inhibition of Hsp90 by radicicol selectively destabilized both PKCα and PKCɛ in the Rictor−/− cells but not in the Rictor+/+ cells (Figure 7B). Similar observations were made with 17-allylamino-17-demethoxygeldanamycin (17-AAG), another Hsp90 inhibitor (Supplementary Figure S4). We observed that inhibition of Hsp90 also destabilized Akt more significantly in Rictor−/− cells than +/+ cells (data not shown). These results indicate that the residual unphosphorylated PKCα in Rictor−/− cells is stabilized by Hsp90.
To clarify whether TM or HM phosphorylation is responsible for PKCα instability induced by Hsp90 inhibitor, HEK293T cells were transfected with wild-type, TM mutant (T638A) or HM mutant (S657A) and treated with radicicol. Interestingly, only TM mutant T638A was significantly destabilized by radicicol (Figure 7C), suggesting that Hsp90 is particularly important for PKCα stability when TM is not phosphorylated.
The destabilization of PKCα in Rictor−/− cells by Hsp inhibitors, however, cannot explain why PKCα protein level is much lower in the Rictor−/− cells even in the absence of Hsp inhibitors. To determine the whereabouts of PKCα in Rictor−/− cells, MG132 was used to inhibit proteasome-dependent degradation. Surprisingly, MG132 treatment did not increase PKCα in Rictor−/− cells (data not shown). Therefore, we examined whether the unphosphorylated PKCα might be accumulated in insoluble fraction, which would not be detected by the extraction methods used in the above analyses. Interestingly, MG132 did cause a dramatic accumulation of PKCα in the insoluble fraction in Rictor−/− cells, but not in Rictor+/+ cells, even though the +/+ cells had a higher level of total PKCα (Figure 7D). Furthermore, the insoluble PKCα showed a high molecular weight ladder in the Rictor−/− cells, indicative of ubiquitination. These observations indicate that the unphosphorylated PKCα is unstable and rapidly degraded by the proteasome pathway. When proteasome activity is inhibited, the unphosphorylated PKCα is ubiquitinated and accumulated in insoluble fraction.
Lack of TM phosphorylation in PKC causes ubiquitination, degradation and aggresome formation
We determined ubiquitination of wild-type PKCα, PKCα T638A (TM mutation), PKCα T631A/T638A (mutation of the TM and the compensation site T631) (Edwards et al, 1999), PKCα S657A (HM mutation) and PKCα T638A/S657A (TM and HM double mutation) by transfection into HEK293 cells. We found that PKCα TM mutant (T638A), but not the HM mutant (S657A), was more ubiquitinated compared with wild-type protein in the presence of MG132 (Figure 7E). T631A/T638A and T638A/S657A mutants showed further increase in ubiquitination. These data strongly support the idea that the TM phosphorylation is important in preventing PKCα ubiquitination and degradation.
We also determined whether the phosphorylation-defective PKCα mutants are more prone to partition in the insoluble fraction. Fractionation followed by immunoblotting showed that a significant fraction of the transfected wild-type PKCα was present in the insoluble fraction upon MG132 treatment (Figure 7F). This observation was different from the endogenous PKCα, which had little in the insoluble fraction even in the presence of MG132 (Figure 7D). This difference can be explained by the fact that the transfected PKCα was not fully phosphorylated (Figure 6D). Consistently, only the fast-migrating, hence unphosphorylated, PKCα was preferentially partitioned in the insoluble fraction (Figure 7F). As expected, PKCα TM mutants were more prone to be found in the insoluble fraction. These data are consistent with a model wherein phosphorylation of TM stabilizes PKCα, whereas the unphosphorylated protein is rapidly ubiquitinated, thus targeted for degradation.
Proteasome-mediated degradation is the major pathway for clearance of intracellular misfolded proteins. Inhibition of proteasome often induces aggresome formation, which shows a perinuclear localization. We found that MG132 caused a marked perinuclear localization of PKCα TM mutant (T631A/T638A), but not the wild-type PKCα (Figure 7G). To confirm aggresome localization, cells were also stained with antibody against conjugated ubiquitin, which is an aggresome marker. In the presence of MG132, the PKCα TM mutant was co-localized with the aggresome marker.
Rictor−/− cells are defective in PKC signalling and kinase activity
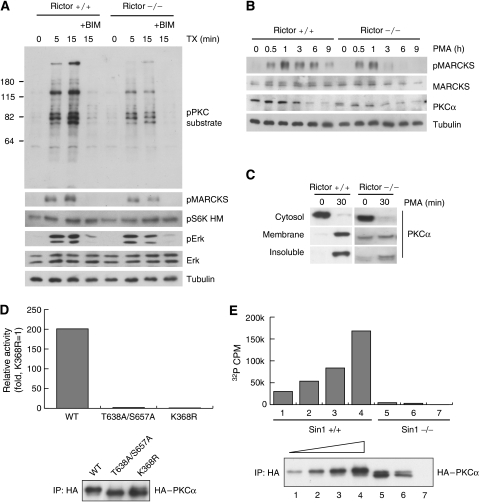
Phorbol esters, such as PMA, and their derivatives, such as thymeleatoxin (TX), are potent activators for cPKCs and nPKCs. We tested PKC activation in Rictor−/− cell by examining phosphorylation of PKC substrates in response to stimulation of TX. TX treatment caused a rapid and robust increase in phosphorylation of PKC substrates (Figure 8A). PKC substrate phosphorylation was blocked by bisindolylmaleimide I (BIM), a PKC inhibitor. Importantly, phosphorylation of PKC substrates was significantly diminished in Rictor−/− cells (Figure 8A), suggesting that PKC signalling is compromised in the Rictor−/− cells. The partial increase of PKC substrate phosphorylation was probably due to the activation of nPKCs by TX in the Rictor−/− cells because nPKCδ and nPKCη are not regulated by Rictor (Figure 4B). It is also possible that the PKC substrate antibody may recognize proteins phosphorylated by other kinases. Moreover, phosphorylation of MARCKS (myristoylated, alanine-rich PKC substrate) (Stumpo et al, 1989) was lower in the Rictor−/− cells than that in the Rictor +/+ cells. We also observed that the duration of MARCKS phosphorylation was significantly shorter in Rictor−/− MEFs than that in Rictor+/+ MEFs (Figure 8B). Similar results were observed in the Sin1−/− MEFs (Supplementary Figure S5A). On the basis of these results, our data indicate that PKC signalling is compromised in either Rictor−/− or Sin1−/− cells.
Figure 8.
PKCα functions are compromised in Rictor and Sin1−/− cells. (A) Phosphorylation of PKC substrates induced by thymeleatoxin (TX) is diminished in Rictor−/− MEFs. Rictor+/+ and −/− MEFs were treated with 100 nM thymeleatoxin (an activator for cPKC and nPKC). The presence of 1 μM bisindolylmaleimide I (BIM, pan PKC inhibitor) is indicated. Total cell lysates were prepared and immunoblotting was performed with indicated antibodies. (B) PMA-induced MARCKS phosphorylation is compromised in Rictor−/− cells. Cells were treated with 400 nM PMA. Immunoblottings were performed with indicated antibodies. (C) Effect of PMA on PKCα translocation in Rictor+/+ and −/− MEFs. Cells were treated with 400 nM PMA for 30 min. Subcellular fractionation was performed. PKCα protein was detected by immunoblotting. (D) Mutation of TM and HM in PKCα abolishes kinase activity. HeLa cells were transfected with PKCα WT, kinase inactive (K368R) and TM and HM mutants (T638A/S657A). HA–PKCα was immunoprecipitated and kinase activity was measured using a synthetic peptide as a substrate and radioactive ATP. (E) PKCα expressed in Sin1−/− MEFs is inactive. Sin1+/+ and −/− MEFs were transfected with various concentrations of HA–PKCα. HA–PKCα was immunoprecipitated and kinase activity was measured similar to Figure 7D.
Membrane translocation of PKC by PMA is important for PKC activation and phosphorylation of PKC substrates (Newton, 2003). We examined whether the hypophosphorylated PKCα could respond to PMA treatment. As expected, PMA caused a complete translocation of PKCα from cytosol to membrane- and detergent-insoluble fractions in Rictor+/+ cells (Figure 8C). In contrast, the Rictor−/− cells had a basal level of membrane-associated PKCα. Upon PMA stimulation, the cytosolic PKCα was mainly translocated to detergent-insoluble fraction with little being translocated to the membrane fraction (Figure 8C). These data show that Rictor is important for proper PKCα activation in response to intracellular second messenger. In the Rictor−/− or Sin1−/− cells, PKCα migrated as a doublet and was downshifted by PMA treatment. We found that sustained PMA treatment caused a dramatic downregulation of PKCα in Sin1+/+ but not −/− cells (Supplementary Figure S5), indicating that the PKCα did not properly respond to PMA.
We next investigated the importance of TM and HM phosphorylation in PKCα kinase activity. In vitro kinase assay showed that PKCα with mutation of both the TM and HM phosphorylation sites had little kinase activity (Figure 8D). To directly determine the function of phosphorylation in PKC activity, HA–PKCα was expressed in Sin1+/+ and −/− cells. We found that HA–PKCα precipitated from Sin1−/− cells had a much lower kinase activity than that from the Sin1+/+ cells (Figure 8E). The HA–PKCα precipitated from Sin1−/− cells also showed a faster migration than that from the Sin+/+ cells. The above-mentioned data demonstrate that the Rictor/Sin1-dependent phosphorylation of TM and HM is essential for PKCα kinase activity and signalling.
Discussion
Phosphorylation of three conserved sites (A-loop, TM and HM) is essential for the function of PKC and other AGC family kinases, including Akt and S6K (Hauge et al, 2007). However, key differences exist between PKC and Akt. Phosphorylation of both A-loop and HM in Akt is regulated by stimulation and serves as the major input for Akt activation in response to extracellular signals. For example, mitogenic growth factors stimulate phosphorylation of both A-loop and HM in Akt. In contrast, phosphorylations of all three sites in PKC are constitutive, whereas binding of second messengers, such as DAG and calcium, to regulatory domain provides the major signal input for PKC activation (Parekh et al, 2000; Newton, 2003). Then, what are the physiological functions of PKC phosphorylation, especially the TM site?
Our study supports the essential function of TM and HM phosphorylation in PKCα kinase activity, consistent with previous reports (Zhang et al, 1994; Edwards et al, 1999). In vitro kinase assays show that the unphosphorylated HA–PKCα expressed in Sin1−/− cells had little activity. Although both TM and HM are highly conserved in PKC family, it is not fully understood how phosphorylation of these two motifs is controlled. Previous studies reported that TM and HM phosphorylation of cPKCs and nPKCɛ were controlled by autophosphorylation. Growth factor-dependence HM phosphorylation was reported in nPKCɛ, whereas other reports showed that HM phosphorylation of cPKCα, nPKCδ and nPKCɛ are rapamycin sensitive and aPKCζ is the possible HM kinase for PKCδ (Behn-Krappa and Newton, 1999; Parekh et al, 1999; Ziegler et al, 1999; Cenni et al, 2002).
In this study, we demonstrate the functional importance of mTORC2 in the TM and HM phosphorylation of Akt and some PKCs (Supplementary Figure S6). Rictor, Sin1 and mTOR, hence mTORC2, are essential for TM phosphorylation of PKCα and likely for PKCβ I, β II, γ, ɛ. Inactivation of mTORC2 also decreases but does not abolish HM phosphorylation in PKCα; therefore, mTORC2 is more important for PKCα TM than HM phosphorylation. In addition, we presented data that mTORC2 is also essential for Akt TM phosphorylation. Our study demonstrates that intramolecular autophosphorylation is not required for PKCα TM phosphorylation.
It has been proposed that phosphate at the TM site in AGC kinases, including Akt and PKC, interacts with surrounding basic residues to be protected from dephosphorylation (Hauge et al, 2007). On the basis of our data, the phosphorylation on TM and HM in PKCα and Akt with the exception of Akt HM is rather stable. However, several lines of evidence presented in this study are consistent with a model wherein mTORC2 integrity and kinase activity are important for TM and HM phosphorylation of both PKCα (Supplementary Figure S6) and Akt. First, genetic ablation of Rictor or Sin1 strongly inhibits these phosphorylations. Second, disruption of mTORC2 assembly by rapamycin treatment or inhibition of mTORC2 kinase activity by inhibitors reduces the TM and HM phosphorylation in PKCα and Akt, especially the newly synthesized proteins. Third, knockdown of mTOR in combination with rapamycin treatment strongly inhibits the phosphorylation of both PKCα and Akt, especially on their TM site. We propose that mTORC2-mediated PKCα TM phosphorylation occurs during its maturation process and contributes to PKCα maturation and stability (Supplementary Figure S6).
Genetic studies in yeast have implicated a role of TOR in phosphorylation of the AGC family kinases (Kamada et al, 2005). Our data demonstrate that mTOR kinase activity is required for the phosphorylation of PKCα TM and HM and corresponding sites in Akt. However, it is worth noting that our study has yet to establish a direct phosphorylation of PKCα TM and HM and Akt TM by mTORC2. We speculate that mTORC2 may activate a kinase(s) that is responsible for PKC and Akt phosphorylation (Supplementary Figure S6). However, these data cannot exclude the possibility of autophosphorylation being responsible for PKCα HM. It is also possible that mTORC2 may directly phosphorylate the TM and HM in PKCα and Akt in vivo, but our in vitro kinase reaction could not duplicate the in vivo conditions. Regardless of the biochemical mechanism, our results have established an important novel function of mTORC2 in the phosphorylation and signalling of cPKCs, nPKCɛ and Akt.
The reduced PKCα expression in Rictor−/− cells is due to protein instability. On the basis of our data, we propose the following model. In wild-type cells, the newly synthesized PKCα is rapidly phosphorylated and then folded into a correct conformation, which is stable and ready for activation by second messengers. Phosphorylation of TM in PKCα is particularly important for this maturation process. In Rictor−/− or Sin1−/− cells, the newly synthesized PKCα is not phosphorylated. The unphosphorylated PKCα is rapidly ubiquitinated and then degraded by the proteasome (Leontieva and Black, 2004). This model is supported by our data that inhibition of proteasome by MG132 results in accumulation of ubiquitinated PKCα in the insoluble fraction in the Rictor−/−, but not in the +/+, cells (Figure 7D and E). The unphosphorylated and ubiquitinated PKCα accumulates in aggresome when proteasome-mediated degradation is blocked (Figure 7G).
A small fraction of unphosphorylated PKCα in Rictor−/− cells may escape the degradation and exist in a form that is stable but defective for signalling, such as response to PMA stimulation. The unphosphorylated PKCα is stabilized by Hsp. It is well established that activation of PKCα results in dephosphorylation (Parekh et al, 2000; Newton, 2003). Furthermore, prolonged PMA treatment causes PKCα downregulation due to ubiquitination and degradation. DAG, the physiological activator, has a much shorter half-life than PMA. Therefore, the activation of PKCα under physiological conditions is rather transient. It has been reported that the temporarily dephosphorylated PKCα after physiological activation, however, is rephosphorylated and quickly replenish cellular mature PKCα pool ready for subsequent stimulations (Newton, 2003). We hypothesize that mTORC2 not only contributes to the maturation of newly synthesized PKCα but may also have an important function in facilitating the rephosphorylation of the signal-induced and dephosphorylated PKCα.
Among the PKCs tested, the protein levels of cPKCs and nPKCɛ depend on mTORC2. In contrast, protein levels of PKCδ, λ, η and ζ are not affected by Rictor deletion, indicating that phosphorylation and maturation of these PKCs are regulated by different mechanisms. We also established that TORC2 is essential for the TM phosphorylation in Akt. One may speculate that other AGC family kinases are also regulated by either mTORC1 or mTORC2. Furthermore, we propose that phosphorylation of the conserved TM motif most probably has a similar function (facilitating protein maturation and stability) in other members of the AGC family kinases. This study demonstrated a novel function of mTORC2 in protein kinase maturation and revealed that mTORC2 has a much broader physiological function significantly beyond the current knowledge as the kinase phosphorylating Akt HM.
Materials and methods
Plasmids, antibodies and chemicals
Mammalian expression constructs of HA-tagged various PKC isoforms (α, βI, βII, γ, δ, ɛ, η, ζ) were kind gifts from Shun'ichi Kuroda (Osaka University) and Jae-Won Soh (Inha University). pPGS–HA–PKCα and pEBG–PKCα were created by subcloning rat PKCα cDNA into pPGS–CITE–neo-HA and pEBG vector. pRK7-GST–PKCα-CT was created by amplifying the C-terminal fragment (a.a. 603–672) and subcloning it into pRK7–GST vector. PKCα point mutant constructs were created using site-directed mutagenesis. shRNA lentivirus constructs and siRNA oligos were purchased from Addgene and Dharmacon, respectively. MARCKS antibody was kindly provided by Perry J Blackshear (National Institute of Environmental Health Sciences). Tubulin and FLAG (M2) antibodies were purchased from Sigma. PKCδ, ɛ, λ and Hsp90 antibodies were from BD Biosciences. HA and Myc antibodies were obtained from Covance. The conjugated ubiquitin antibody was from BIOMOL. Alexa Fluor® 594 goat anti-mouse IgG and Alexa Fluor® 488 goat anti-rabbit IgG were from Invitrogen. Other antibodies used in this study were purchased from Cell Signaling Technology. Rapamycin was obtained from Sigma. Other chemicals were from Calbiochem.
Immunoblotting, immunoprecipitation and kinase assay
PDK1+/+ and −/− ES cell lysates were kindly provided by Dario Alessi and Huang Xu (University of Dundee). For immunoblotting analysis, cells were lysed in NP-40 buffer (10 mM Tris–HCl pH 7.5, 100 mM NaCl, 1% NP-40, 50 mM NaF, 2 mM EDTA, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin). Rictor knockout mice embryos were also lysed in NP-40 buffer. For subcellular fractionation, Rictor +/+ and −/− MEFs were stimulated by PMA (400 nM) for 30 min, lysed by sonication in HEPES buffer (50 mM HEPES pH 7.4, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 1 mM DTT, 0.2 mM PMSF) and centrifuged at 100 000 g for 30 min at 4°C. The supernatant was used as cytosolic fraction, and the pellet was resuspended in HEPES buffer containing 1% Triton X-100 and centrifuged (100 000 g, 30 min, 4°C), and the supernatant (membrane) and pellet (detergent-insoluble) fractions were stored for further analyses. For preparation of soluble and insoluble fractions, the cells were lysed in NP-40 buffer and centrifuged (16 000 g, 10 min, 4°C), and the supernatant was used as soluble fraction. The pellet was sonicated with 1 × SDS sample buffer and used as insoluble fraction. For immunoprecipitation, cells were lysed in NP-40 or 0.3% CHAPS buffer as described previously (Yang et al, 2006). Antibody (1 μg) was added to each reaction and incubated for 90 min at 4°C followed by 10 ml of protein G-sepharose slurry (50%) for another hour. Immunoprecipitates were washed four times in the lysis buffer. For kinase assays, after washing with the lysis buffer, the immunoprecipitates were washed once more with kinase buffer and then incubated in 15 μl kinase assay reaction mix for 30 min at 37°C. Kinase assay reactions were designed as reported previously (Sarbassov et al, 2005). The GST–Akt (a.a. 125–480) and GST–PKCα (a.a. 322–672) purified from E. coli, full-length GST–PKCα and GST–Akt from mammalian cells, or full-length His6–Akt from baculovirus (Upstate Biotechnology), were used as substrates. To terminate the reaction, 5 μl of 4 × SDS sample buffer was added to each reaction. For PKCα kinase assay, PKC kinase assay kit (Upstate Biotechnology) was used according to the manufacturer's instruction.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Drs Dario Alessi and Huang Xu (University of Dundee), Bing Su (Yale School of Medicine), Shun'ichi Kuroda (Osaka University), Jae-Won Soh (Inha University), Alexandra Newton (University of California at San Diego) and Perry J Blackshear (NIEHS) for reagents. This work is supported by grants from NIH and DOD (KLG).
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Cohen P (1998) Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8: 55–62 [DOI] [PubMed] [Google Scholar]
- Behn-Krappa A, Newton AC (1999) The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Curr Biol 9: 728–737 [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P (1998) Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17: 313–325 [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr, Abraham RT (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15: 5256–5267 [PMC free article] [PubMed] [Google Scholar]
- Cenni V, Doppler H, Sonnenburg ED, Maraldi N, Newton AC, Toker A (2002) Regulation of novel protein kinase C epsilon by phosphorylation. Biochem J 363: 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A (1998) Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol 8: 1069–1077 [DOI] [PubMed] [Google Scholar]
- Collins BJ, Deak M, Arthur JS, Armit LJ, Alessi DR (2003) In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J 22: 4202–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil EM, Toker A, Newton AC (1998) Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr Biol 8: 1366–1375 [DOI] [PubMed] [Google Scholar]
- Edwards AS, Faux MC, Scott JD, Newton AC (1999) Carboxyl-terminal phosphorylation regulates the function and subcellular localization of protein kinase C betaII. J Biol Chem 274: 6461–6468 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2003) Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol 15: 67–72 [DOI] [PubMed] [Google Scholar]
- Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA (2006) A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell 9: 341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM (2006) mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 16: 1865–1870 [DOI] [PubMed] [Google Scholar]
- Gao T, Newton AC (2002) The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J Biol Chem 277: 31585–31592 [DOI] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 7: 281–294 [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871 [DOI] [PubMed] [Google Scholar]
- Hauge C, Antal TL, Hirschberg D, Doehn U, Thorup K, Idrissova L, Hansen K, Jensen ON, Jorgensen TJ, Biondi RM, Frodin M (2007) Mechanism for activation of the growth factor-activated AGC kinases by turn motif phosphorylation. EMBO J 26: 2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18: 1926–1945 [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137 [DOI] [PubMed] [Google Scholar]
- Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, Hall MN, Ohsumi Y (2005) Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol 25: 7239–7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM (2006) A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125: 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910 [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281: 2042–2045 [DOI] [PubMed] [Google Scholar]
- Leontieva OV, Black JD (2004) Identification of two distinct pathways of protein kinase Calpha down-regulation in intestinal epithelial cells. J Biol Chem 279: 5788–5801 [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468 [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ (1998) The extended protein kinase C superfamily. Biochem J 332 (Part 2): 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR (2004) PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170 [DOI] [PubMed] [Google Scholar]
- Newton AC (2003) Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J 370: 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh D, Ziegler W, Yonezawa K, Hara K, Parker PJ (1999) Mammalian TOR controls one of two kinase pathways acting upon nPKCdelta and nPKCepsilon. J Biol Chem 274: 34758–34764 [DOI] [PubMed] [Google Scholar]
- Parekh DB, Ziegler W, Parker PJ (2000) Multiple pathways control protein kinase C phosphorylation. EMBO J 19: 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM (2006) mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6: 729–734 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307: 1098–1101 [DOI] [PubMed] [Google Scholar]
- Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA (2006) Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell 11: 583–589 [DOI] [PubMed] [Google Scholar]
- Stumpo DJ, Graff JM, Albert KA, Greengard P, Blackshear PJ (1989) Molecular cloning, characterization, and expression of a cDNA encoding the ‘80- to 87-kDa' myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci USA 86: 4012–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A (1998) Signaling through protein kinase C. Front Biosci 3: D1134–D1147 [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323 [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484 [DOI] [PubMed] [Google Scholar]
- Yang Q, Inoki K, Ikenoue T, Guan KL (2006) Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev 20: 2820–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang L, Schwartz J, Bond RW, Bishop WR (1994) Phosphorylation of Thr642 is an early event in the processing of newly synthesized protein kinase C beta 1 and is essential for its activation. J Biol Chem 269: 19578–19584 [PubMed] [Google Scholar]
- Ziegler WH, Parekh DB, Le Good JA, Whelan RD, Kelly JJ, Frech M, Hemmings BA, Parker PJ (1999) Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr Biol 9: 522–529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information