Abstract
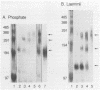
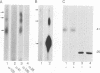
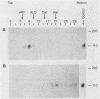
The oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein (gp120) was examined by treating infectious virions with chemical cross-linking agents and subjecting the protein to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and velocity centrifugation. Immunoblots of cross-linked samples revealed three gp120 bands and an approximately threefold shift in gp120 sedimentation. Our finding of cross-linking solely between gp120 suggests that the gp120 subunits are closely associated in the native envelope structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Earl P. L., Doms R. W., Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990 Jan;87(2):648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld D., Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 1988 May;10(2-3):113–135. doi: 10.1016/0168-1702(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Papsidero L. D., Sheu M., Ruscetti F. W. Human immunodeficiency virus type 1-neutralizing monoclonal antibodies which react with p17 core protein: characterization and epitope mapping. J Virol. 1989 Jan;63(1):267–272. doi: 10.1128/jvi.63.1.267-272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M. A., Krust B., Laurent A. G., Montagnier L., Hovanessian A. G. Characterization of human immunodeficiency virus type 2 envelope glycoproteins: dimerization of the glycoprotein precursor during processing. J Virol. 1989 Feb;63(2):647–658. doi: 10.1128/jvi.63.2.647-658.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M. A., Laurent A. G., McClure J., Krust B., Montagnier L., Hovanessian A. G. Transmembrane envelope glycoproteins of human immunodeficiency virus type 2 and simian immunodeficiency virus SIV-mac exist as homodimers. J Virol. 1990 Feb;64(2):922–926. doi: 10.1128/jvi.64.2.922-926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawaller M., Smith G. E., Skehel J. J., Wiley D. C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989 Sep;172(1):367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- Sechoy O., Philippot J. R., Bienvenue A. F protein-F protein interaction within the Sendai virus identified by native bonding or chemical cross-linking. J Biol Chem. 1987 Aug 25;262(24):11519–11523. [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Tateno M., Levy J. A. MT-4 plaque formation can distinguish cytopathic subtypes of the human immunodeficiency virus (HIV). Virology. 1988 Nov;167(1):299–301. doi: 10.1016/0042-6822(88)90084-0. [DOI] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- White J. M., Wilson I. A. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J Cell Biol. 1987 Dec;105(6 Pt 2):2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]