Abstract
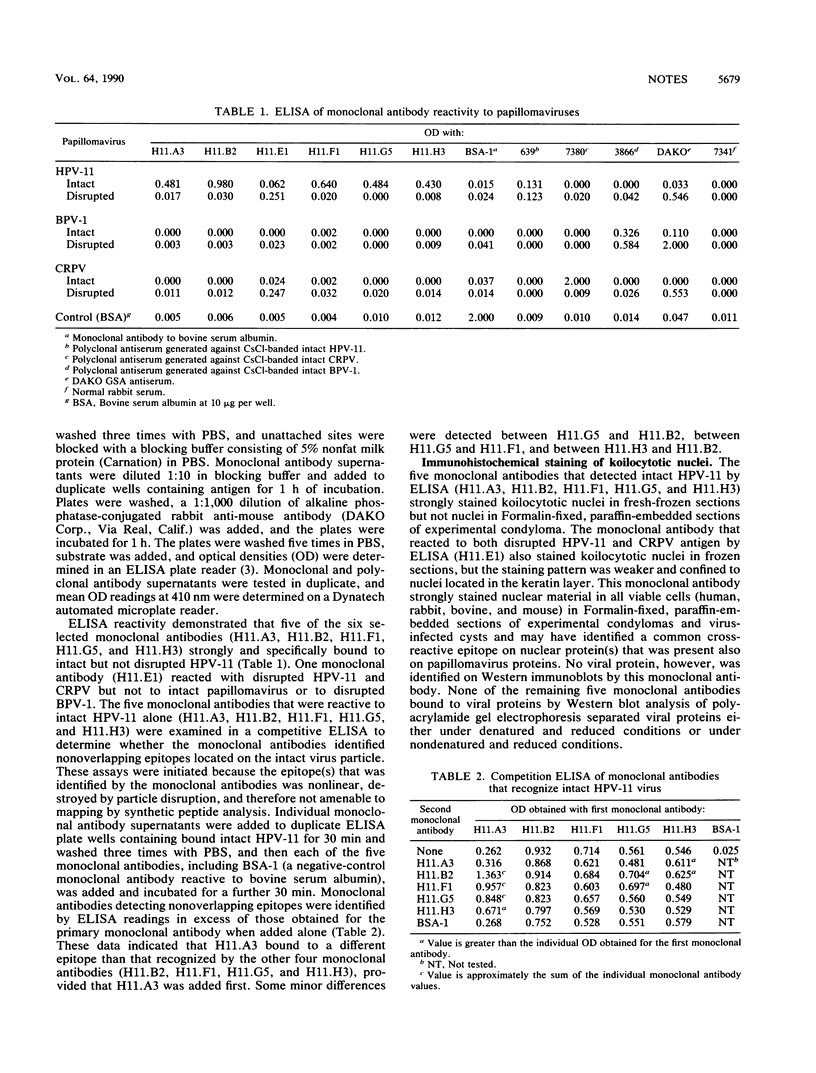
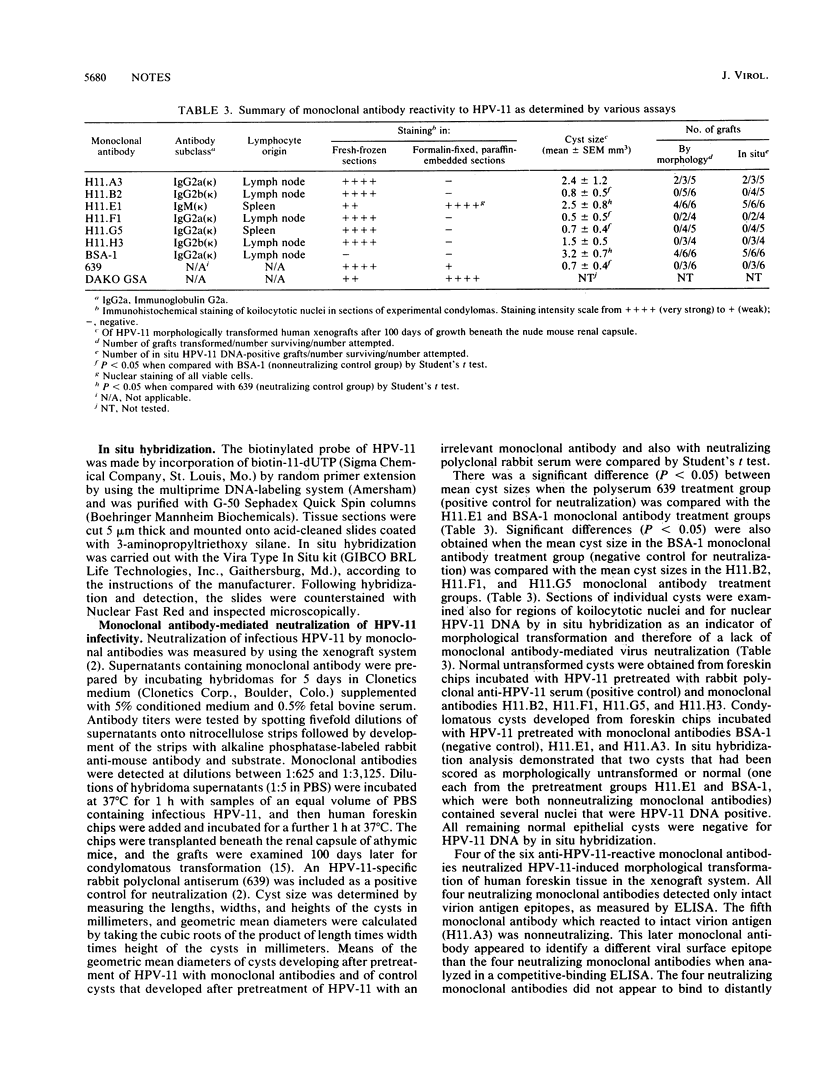
Monoclonal antibodies recognizing human papillomavirus type 11 (HPV-11) were prepared from BALB/c mice immunized with intact HPV-11 virions obtained from morphologically transformed human foreskin xenografts grown subrenally in athymic mice. Four of five monoclonal antibodies that were reactive by enzyme-linked immunosorbent assay only to intact virions neutralized HPV-11 infectivity in the athymic mouse xenograft system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett P. V., Ouldridge E. J., Rowlands D. J., Brown F., Parry N. R. Neutralizing epitopes of type O foot-and-mouth disease virus. I. Identification and characterization of three functionally independent, conformational sites. J Gen Virol. 1989 Jun;70(Pt 6):1483–1491. doi: 10.1099/0022-1317-70-6-1483. [DOI] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J Virol. 1990 Jul;64(7):3151–3156. doi: 10.1128/jvi.64.7.3151-3156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Cladel N. M., Galloway D. A. Immunological cross-reactivity to laboratory-produced HPV-11 virions of polysera raised against bacterially derived fusion proteins and synthetic peptides of HPV-6b and HPV-16 capsid proteins. Virology. 1990 Mar;175(1):1–9. doi: 10.1016/0042-6822(90)90180-y. [DOI] [PubMed] [Google Scholar]
- Cowsert L. M., Lake P., Jenson A. B. Topographical and conformational epitopes of bovine papillomavirus type 1 defined by monoclonal antibodies. J Natl Cancer Inst. 1987 Nov;79(5):1053–1057. [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Fahey K. J., Erny K., Crooks J. A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J Gen Virol. 1989 Jun;70(Pt 6):1473–1481. doi: 10.1099/0022-1317-70-6-1473. [DOI] [PubMed] [Google Scholar]
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975 May;15(5):1239–1247. doi: 10.1128/jvi.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Hsu K. H., Lonberg-Holm K., Alstein B., Crowell R. L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988 May;62(5):1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIDER J. W. STUDIES ON THE MECHANISM RESPONSIBLE FOR THE SPONTANEOUS REGRESSION OF THE SHOPE RABBIT PAPILLOMA. Cancer Res. 1963 Oct;23:1593–1599. [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Narayan O. Neutralizing antibodies to visna lentivirus: mechanism of action and possible role in virus persistence. J Virol. 1986 Jul;59(1):37–44. doi: 10.1128/jvi.59.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Leure-Dupree A. E., Zaino R. J., Weber J. A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987 Feb;61(2):590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Stoler M. H., Zaino R. J., Welsh P. Susceptibility of various human tissues to transformation in vivo with human papillomavirus type 11. Int J Cancer. 1987 Apr 15;39(4):459–465. doi: 10.1002/ijc.2910390409. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Wolfe S. A., Bartlett G. L., Zaino R. J., Sedlacek T., Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985 Oct 17;317(6038):639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Muggeridge M. I., Isola V. J., Byrn R. A., Tucker T. J., Minson A. C., Glorioso J. C., Cohen G. H., Eisenberg R. J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988 Sep;62(9):3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis P., Zibirre R., Meyer G., Kühne J., Warnecke G., Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985 Dec;66(Pt 12):2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Parry N. R., Barnett P. V., Ouldridge E. J., Rowlands D. J., Brown F. Neutralizing epitopes of type O foot-and-mouth disease virus. II. Mapping three conformational sites with synthetic peptide reagents. J Gen Virol. 1989 Jun;70(Pt 6):1493–1503. doi: 10.1099/0022-1317-70-6-1493. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]



