Abstract
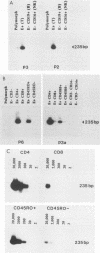
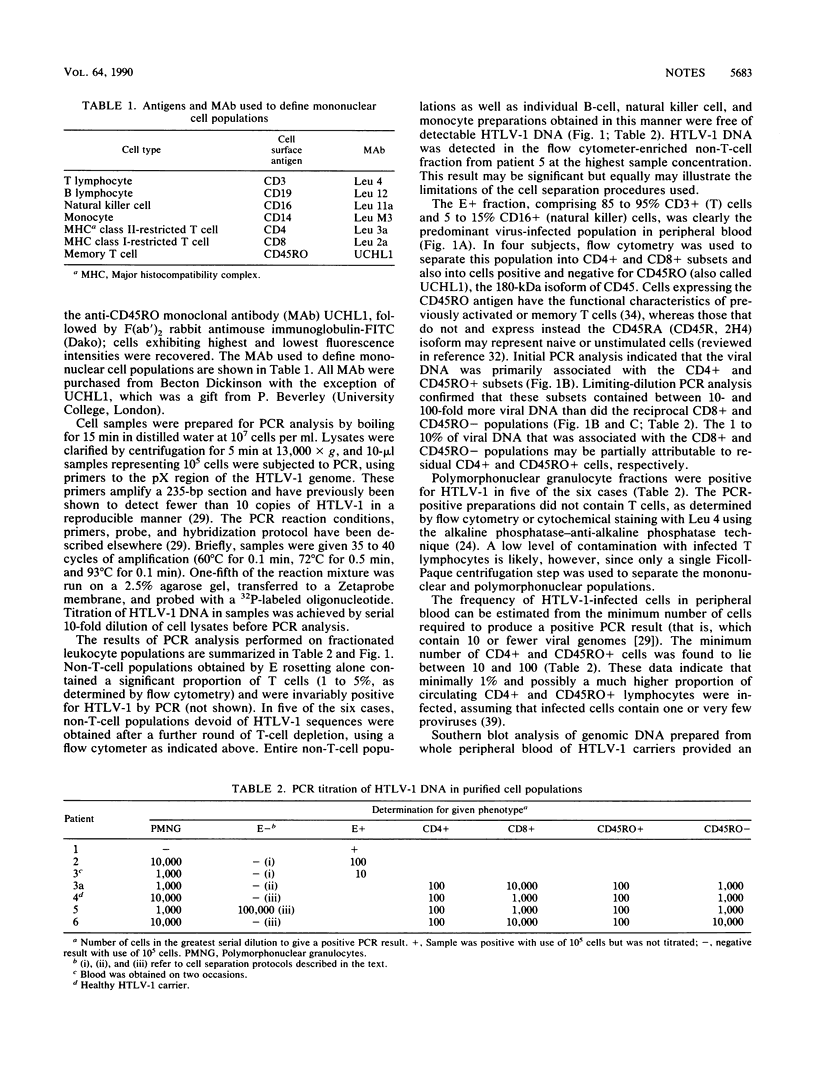
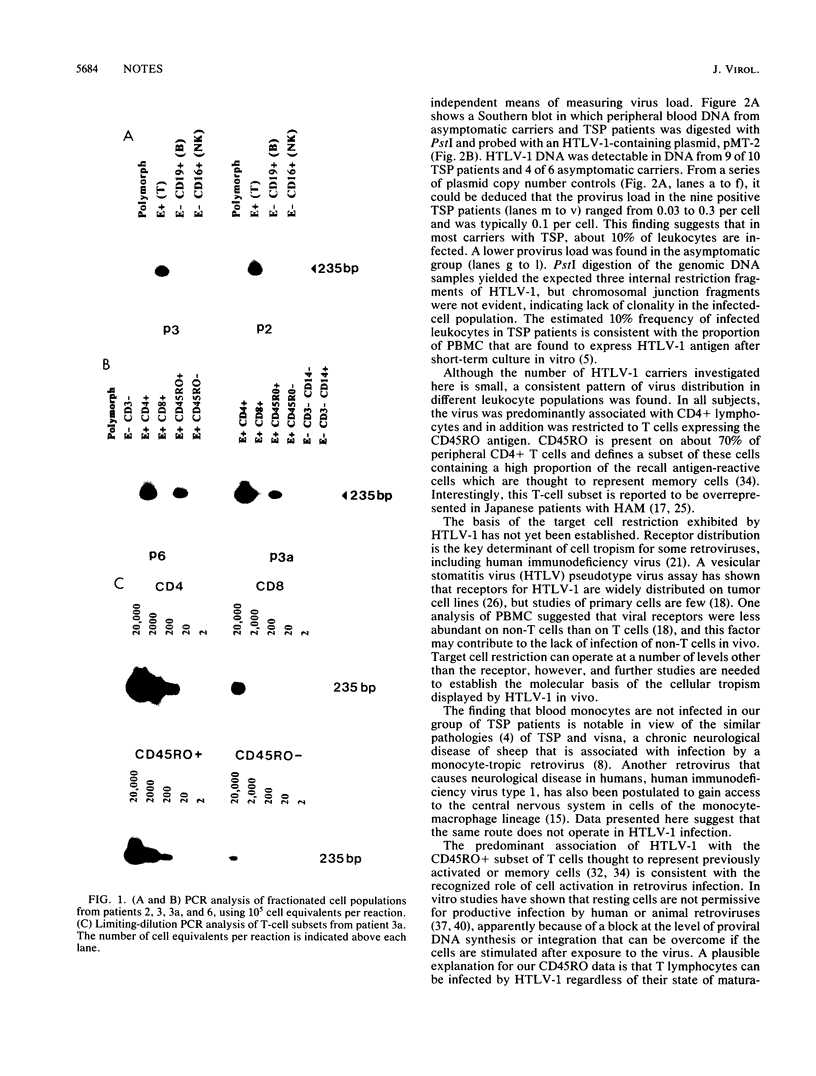
To establish the phenotype of human T-cell leukemia virus type 1 (HTLV-1)-infected cells in peripheral blood, the polymerase chain reaction was used to detect and quantitate viral DNA in subpopulations of leukocytes obtained from patients with tropical spastic paraparesis and asymptomatic carriers. HTLV-1 could not be detected in peripheral blood mononuclear cells thoroughly depleted of T lymphocytes (E- CD3-), nor could it be detected in highly enriched populations of B lymphocytes (E- CD19+), monocytes (E- CD14+), or natural killer cells (E- CD16+). T lymphocytes were strongly positive for HTLV-1, and fractionation of this population revealed that 90 to 99% of the HTLV-1 DNA segregated with the CD4+ CD8- and CD45RO+ subsets. No difference between the cell type distribution of HTLV-1 in the asymptomatic carrier and the subjects with tropical spastic paraparesis was evident. Southern hybridization of genomic DNA prepared from the peripheral blood of HTLV-1 carriers indicated that up to 10% of circulating leukocytes may carry the HTLV-1 provirus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catovsky D., Greaves M. F., Rose M., Galton D. A., Goolden A. W., McCluskey D. R., White J. M., Lampert I., Bourikas G., Ireland R. Adult T-cell lymphoma-leukaemia in Blacks from the West Indies. Lancet. 1982 Mar 20;1(8273):639–643. doi: 10.1016/s0140-6736(82)92200-0. [DOI] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Cruickshank J. K., Rudge P., Dalgleish A. G., Newton M., McLean B. N., Barnard R. O., Kendall B. E., Miller D. H. Tropical spastic paraparesis and human T cell lymphotropic virus type 1 in the United Kingdom. Brain. 1989 Aug;112(Pt 4):1057–1090. doi: 10.1093/brain/112.4.1057. [DOI] [PubMed] [Google Scholar]
- Dalgleish A., Richardson J., Matutes E., Cruickshank K., Newell A., Sinclair A., Thorpe R., Brasher M., Weber J., Catovsky D. HTLV-1 infection in tropical spastic paraparesis: lymphocyte culture and serologic response. AIDS Res Hum Retroviruses. 1988 Dec;4(6):475–485. doi: 10.1089/aid.1988.4.475. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Crimmins M. A., Mentzer S. J. Human T-cell leukemia virus type I infection of CD4+ or CD8+ cytotoxic T-cell clones results in immortalization with retention of antigen specificity. J Virol. 1988 Aug;62(8):2942–2950. doi: 10.1128/jvi.62.8.2942-2950.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo L., Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987 Apr 16;326(6114):714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Gessain A., Saal F., Giron M. L., Lasneret J., Lagaye S., Gout O., De Thé G., Sigaux F., Peries J. Cell surface phenotype and human T lymphotropic virus type 1 antigen expression in 12 T cell lines derived from peripheral blood and cerebrospinal fluid of West Indian, Guyanese and African patients with tropical spastic paraparesis. J Gen Virol. 1990 Feb;71(Pt 2):333–341. doi: 10.1099/0022-1317-71-2-333. [DOI] [PubMed] [Google Scholar]
- Gessain A., Saal F., Gout O., Daniel M. T., Flandrin G., de The G., Peries J., Sigaux F. High human T-cell lymphotropic virus type I proviral DNA load with polyclonal integration in peripheral blood mononuclear cells of French West Indian, Guianese, and African patients with tropical spastic paraparesis. Blood. 1990 Jan 15;75(2):428–433. [PubMed] [Google Scholar]
- Hattori T., Uchiyama T., Toibana T., Takatsuki K., Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981 Sep;58(3):645–647. [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K., Masuda M., Yoshikura H. Mode of transmission of human T-cell leukemia virus type I (HTLV I) in a human promyelocytic leukemia HL60 cell. Int J Cancer. 1986 Apr 15;37(4):601–606. doi: 10.1002/ijc.2910370420. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Pomerantz R. J., Kaplan J. C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987 Jul 30;317(5):278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Matthews D. M., Cines D. B. Infection of human endothelial cells by human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7591–7595. doi: 10.1073/pnas.81.23.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama Y., Minato S., Kira J., Goto I., Sato H., Okochi K., Yamamoto N. Altered subsets of peripheral blood lymphocytes in patients with HTLV-I associated myelopathy (HAM). Neurology. 1988 May;38(5):816–818. doi: 10.1212/wnl.38.5.816. [DOI] [PubMed] [Google Scholar]
- Krichbaum-Stenger K., Poiesz B. J., Keller P., Ehrlich G., Gavalchin J., Davis B. H., Moore J. L. Specific adsorption of HTLV-I to various target human and animal cells. Blood. 1987 Nov;70(5):1303–1311. [PubMed] [Google Scholar]
- Longo D. L., Gelmann E. P., Cossman J., Young R. A., Gallo R. C., O'Brien S. J., Matis L. A. Isolation of HTLV-transformed B-lymphocyte clone from a patient with HTLV-associated adult T-cell leukaemia. Nature. 1984 Aug 9;310(5977):505–506. doi: 10.1038/310505a0. [DOI] [PubMed] [Google Scholar]
- Macchi B., Popovic M., Allavena P., Ortaldo J., Rossi P., Gallo R. C., Bonmassar E. In vitro susceptibility of different human T-cell subpopulations and resistance of large granular lymphocytes to HTLV-I infection. Int J Cancer. 1987 Jul 15;40(1):1–6. doi: 10.1002/ijc.2910400102. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Kalyanaraman V. S., Popovic M., Sarin P., Gallo R. C. Infection and transformation of fresh human umbilical cord blood cells by multiple sources of human T-cell leukemia-lymphoma virus (HTLV). Int J Cancer. 1983 Apr 15;31(4):413–420. doi: 10.1002/ijc.2910310404. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- Mori M., Kinoshita K., Ban N., Yamada Y., Shiku H. Activated T-lymphocytes with polyclonal gammopathy in patients with human T-lymphotropic virus type I--associated myelopathy. Ann Neurol. 1988 Aug;24(2):280–282. doi: 10.1002/ana.410240220. [DOI] [PubMed] [Google Scholar]
- PCR analysis of DNA from multiple sclerosis patients for the presence of HTLV-I. Science. 1989 Nov 10;246(4931):821–824. [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Psallidopoulos M. C., Schnittman S. M., Thompson L. M., 3rd, Baseler M., Fauci A. S., Lane H. C., Salzman N. P. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989 Nov;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Robert-Guroff M., Ceccherini-Nelli L., Minowada J., Popovic M., Gallo R. C. Persistent in vitro infection by human T-cell leukemia-lymphoma virus (HTLV) of normal human T-lymphocytes from blood relatives of patients with HTLV-associated mature T-cell neoplasms. Int J Cancer. 1983 Feb 15;31(2):171–180. doi: 10.1002/ijc.2910310207. [DOI] [PubMed] [Google Scholar]
- Saida T., Saida K., Funauchi M., Nishiguchi E., Nakajima M., Matsuda S., Ohta M., Ohta K., Nishitani H., Hatanaka M. HTLV-I myelitis: isolation of virus, genomic analysis, and infection in neural cell cultures. Ann N Y Acad Sci. 1988;540:636–638. doi: 10.1111/j.1749-6632.1988.tb27196.x. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Kobayashi N., Sakitani M., Hatanaka M., Hinuma Y. Retrovirus-induced expression of interleukin 2 receptors on cells of human B-cell lineage. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7441–7445. doi: 10.1073/pnas.81.23.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K., Sakitani M., Hinuma Y. Microplate method for retrovirus-induced transformation of normal human T-cells. J Immunol Methods. 1984 Oct 26;73(2):379–385. doi: 10.1016/0022-1759(84)90413-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Matsumoto T., Koyanagi Y., Tanaka Y., Hinuma Y. Unique cell lines harbouring both Epstein-Barr virus and adult T-cell leukaemia virus, established from leukaemia patients. Nature. 1982 Sep 23;299(5881):367–369. doi: 10.1038/299367a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Seiki M., Yamaguchi K., Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]