Abstract
The discovery that the CC chemokines RANTES, MIP-1α and MIP-1β act as potent natural inhibitors of HIV-1, the causative agent of AIDS, and the subsequent identification of CCR5 as a major virus coreceptor have triggered a wealth of basic and applied research approaches aimed at developing safe and effective viral entry inhibitors. Some of these efforts have focused on RANTES engineering with the goal of enhancing the antiviral activity of the native molecule while reducing or abrogating its inflammatory properties. The wavefront generated a decade ago is still on its course, with a flow of promising leads constantly emerging and being evaluated in preclinical studies. Here, we present an overview of this rapidly evolving field, highlighting the most important features of RANTES molecular architecture and structure-function relationships.
1. Introduction
Human immunodeficiency virus type 1 (HIV-1), the causative agent of the acquired immunodeficiency syndrome (AIDS), is among the most studied living organisms, with enormous financial and scientific efforts profused over the past three decades. This notwithstanding, sadly, AIDS still represents a devastating disease worldwide. Although potent drugs have been developed, which in combination effectively suppress HIV-1 replication for prolonged periods of time in most treated patients, large areas of the planet have limited, if any, access to such drugs due to their high cost and the need for continuous medical and laboratory monitoring. While there is little doubt that the most desirable measure for the control of the AIDS pandemic would be a protective vaccine, which could be implemented on large populations at relatively low cost, it is still uncertain whether the development of such a vaccine will ever be possible. An alternative type of low-cost prophylaxis, by far more attainable than a protective vaccine, is represented by topical microbicides, capable of blocking HIV-1 infection at mucosal sites where initial virus transmission occurs in the majority of the cases. Recently, both vaccine and microbicide strategies have witnessed major setbacks, with the unconditional failure of a few seemingly promising experimental clinical trials [1-4].
While the vaccine approach has been a long-lasting endeavor in HIV research, microbicide development is a relatively young field, with many different strategies currently being explored [5–7]. One of the most important approaches is the topical use of HIV-1 entry inhibitors, a novel class of antiviral agents prototyped by the gp41-derived peptide T20/Enfuvirtide [8]. HIV enters its target cells following the binding of its envelope to a cell-surface receptor complex via a sequence of molecular events involving stepwise conformational changes on both membrane sides [9]. The HIV-1 gp120 trimer docks onto the N-terminal domain of the primary viral receptor, the CD4 glycoprotein, undergoing a profound conformational change whose details have been evinced from the structure of the CD4-bound and unbound gp120 of HIV-1 and simian immunodeficiency virus (SIV), respectively [10,11]. Consequently, gp120 exposes the binding site for the coreceptor, CCR5 or CXCR4 [9], the former being the most widely used and the one almost exclusively involved in viral transmission [9,12]. Virus-entry information is then transmitted through the virus envelope protein to the gp41 trimer moiety that undergoes a series of conformational changes eventually leading to type I virus-cell membrane fusion [13].
CC chemokines come into action during the HIV-1 entry process as CCR5 ligands with natural antiviral activity [14]. Among these chemokines, RANTES is the most powerful HIV-1 blocker [14]. Given the central role of RANTES and CCR5 in HIV-1 pathology, much of the research in the field of HIV-entry inhibitors has been generated focusing on these two molecules. However, the three-dimensional structure of CCR5 is still unsolved, essentially due to its seven-transmembrane-domain structure, and therefore the fine structural details of the RANTES-CCR5 interaction remain unknown. On the contrary, structural data on CC chemokines are abundant, including nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography studies on wild-type molecules, mutants and chemically-modified variants, as well as the characterization of their interaction with glycosaminoglycans (GAGs) [15–20]. These studies have built fundamental knowledge to drive the rational engineering of chemokines with improved antiviral activity and pharmacological properties.
In this review, we summarize the accomplishments achieved in this rapidly evolving field with particular focus on the RANTES-CCR5 interaction, as well as RANTES-engineering strategies for the development of novel HIV-1 entry inhibitors. Although the complex role of the chemokine system in the regulation of immune functions is of fundamental importance, a discussion of the immunologic role of RANTES is beyond the scope of the present review and has been extensively reviewed elsewhere [21].
2. Molecular architecture of RANTES
Similar to other chemokines, RANTES is a small globular protein with a very stable fold, which represents an invaluable advantage for successful protein engineering. Its three-dimensional structure, solved by NMR, showed that the protein is present in solution predominantly as a dimer [16,17]. In the dimer context, each monomer presents a partially disordered N-terminal region, followed by a short β-strand (β0) leading to the signature two-cysteine (CC) motif, an extended region (N-loop) ending with a 310 turn, three anti-parallel β-strands (β1-3) connected by loops, and a C-terminal α-helix (Fig. 1) [16,17]. RANTES dimerization and oligomerization, as well as the role of its different domains, are described below, particularly for their differential contribution to the anti-HIV activity.
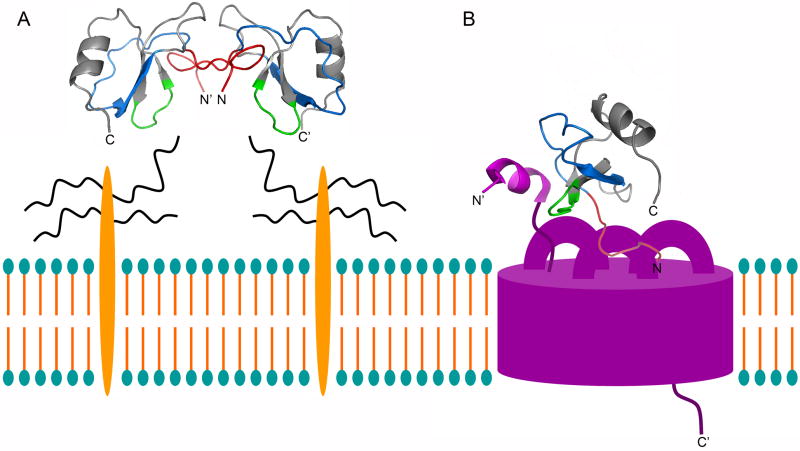
Figure 1.
Schematic representation of RANTES binding to GAGs and CCR5 on the cell surface. A, Ribbon side view representation of the RANTES dimer (PDB entry 1HRJ) and its interaction with cell surface glycosaminoglycans (the protein core is represented in orange; the carbohydrate chains in black). The N-terminus (aa 1-10) is colored in red; the N-loop/β1-strand (aa 11-29) in blue; the GAG-binding 40’s loop (aa 43-48) in green. N and C denote the N- and C-terminus, respectively (N′ and C′, the termini of the second RANTES monomer within the dimer). B, Side view of the RANTES monomer (color code as in A) in a proposed complex with CCR5 (pink). The RANTES monomer is shown with a 45° anti-clockwise rotation compared to A, and is slightly tilted towards the observer. The α-helix conformation adopted by the CCR5 N-terminal peptide, spanning residues 7-15, corresponds to that illustrated in PDB entry 2RLL. As previously suggested [48], the RANTES N-terminus is likely to be embedded within transmembrane CCR5 helices, while the extracellular CCR5 loops, particularly ECL2, should surround RANTES. N and C denote RANTES N- and C-terminus, respectively (N′ and C′, denote the CCR5 termini). In both A and B, RANTES orientation towards GAGs, CCR5 and the cell membrane have been chosen arbitrarily.
2.1 Monomeric versus dimeric interaction of RANTES with CCR5 and other complexities
RANTES, MIP-1α and MIP-1β typically present a monomer-dimer equilibrium that, under physiologic conditions, is shifted towards dimer stabilization. Conclusive evidence for the existence of RANTES dimers was provided by the three-dimensional structure solution of this chemokine obtained by NMR [16,17]. Understanding whether RANTES interacts with CCR5 in its dimeric or monomeric conformation has become a primary goal immediately after the elucidation of its quaternary structure. Two recent reports have provided strong evidence in favor of monomeric binding of RANTES to CCR5 [22,23]. Duma and colleagues [22] studied the interaction of RANTES with a sulfated N-terminal peptide of CCR5, providing a detailed mapping of the RANTES residues involved. In the presence of the CCR5 peptide, many NMR chemical shifts indicative of binding were detected for residues otherwise involved in the monomer-monomer interface, providing clear evidence for monomeric RANTES recognition of the CCR5 N-terminus [22]. Moreover, analyzing the distinct resonance sets of monomer and dimer RANTES forms, it was clear that the shifts involved in the CCR5 peptide binding belonged exclusively to the monomeric form [22]. Interestingly, RANTES binding to the non-sulfated version of the CCR5 peptide was dramatically reduced [22]. Strikingly, tyrosine sulfation at CCR5 N-terminus was earlier shown to be critical also for gp120 binding [24], opening the possibility to translate key molecular features to RANTES-based therapeutics (see below). Using a different approach, Jin and colleagues [23] provided evidence against the binding of the MIP-1β dimer to CCR5. A covalent MIP-1β dimer was created by engineering an intermolecular disulfide bridge at the chemokine N-terminus (A10C mutation), which was shown to be unable to bind CCR5. The MIP-1β A10C mutant appeared to be properly folded as attested by NMR spectroscopy and by its ability to bind GAGs [23]. Notably, by reducing the intermolecular disulfide bridge, CCR5-binding activity was restored [23].
In spite of this recent evidence, the overall picture is more complex than a mere 1:1 chemokine:receptor recognition. The CCR5 membrane environment, particularly the simultaneous expression of other chemokine receptors, may significantly modify ligand interactions via homo-and hetero-dimerization [25]. Likewise, chemokine-receptor homo- and hetero-dimerization appear to play a role in HIV infection, although there is controversy on whether this may result in inhibition of coreceptor function [25]. Particularly interesting for HIV physiology is the lateral association between CCR5 and CD4. This conformational state could be catalyzed or reinforced by the sequential interactions of gp120 with CD4 and CCR5 [26]. Conversely, pre-existing CCR5-CD4 heterodimers may constitute the exclusive fusion-permissive binding partners for HIV Env [27]. Conclusive evidence for pre-existing or gp120-promoted CD4-CCR5 complexes in relation to HIV entry has yet to be achieved, although the results of immunoprecipitation and fluorescence resonance energy transfer studies are suggestive of CD4-CCR5 lateral association [26–28]. This complexity extends to RANTES where several regions are believed to interact with CCR5, as depicted in Fig. 1A. Although not directly related to CCR5 binding, the presence of GAGs on the cellular surface determines an increase in membrane-proximal chemokine concentration, promoting RANTES accessibility to CCR5 [29]. This occurs primarily through a GAG-binding motif present on the 40’s loop of CC chemokines [30,31]. Reportedly, GAGs are recognized by dimeric CC chemokines and chemokine dimers increase their stability upon GAG binding [32]. The possibility that RANTES may be presented to CCR5 in a GAG-complexed form has also been postulated [33,34]. This would imply a two-step process whereby dimeric RANTES would bind to GAGs via the 40’s loop, which in turn would prime the chemokine for CCR5 binding, a process that might involve monomerization. Monomeric RANTES-CCR5 would then be the favored endpoint, given the higher affinity of RANTES for CCR5 compared to GAGs.
Besides RANTES monomers and dimers, which are the prevalent forms in solution, higher-order oligomers have been observed for CC chemokines [18]. GAG binding has also been characterized as a chemokine aggregation-promoting process occurring at the cellular surface [35]. GAG-induced aggregation plays an important role in chemokine physiology, and disruption of this process is under investigation for devising potential therapeutic interventions [36,37].
In conclusion, accumulating evidence suggests that CC chemokines need to be in a monomeric conformation to functionally interact with CCR5. The N-terminal portion of RANTES, including the first β-strand, is extensively involved in the dimerization interface, hence it appears intuitive that the two N-termini of dimeric RANTES should dissociate into monomers to allow full CCR5 binding and activation.
2.2 N-terminal modifications
Potent anti-HIV derivatives of RANTES have been obtained by modification of the N-terminal domain, including mutagenesis, aminoacid deletions and substitutions with chemical groups (Table 1). Certain hydrophobic moieties added at the RANTES N-terminus dramatically enhanced the rate of CCR5 internalization, resulting in a prolonged disappearance of the receptor from the cell surface. The first attempts were prompted by the fortuitous observation that E. coli-based RANTES expression systems maintain the N-terminal methionine, yielding a Met-RANTES analogue, the first described derivative with receptor-antagonistic activity [38]. Subsequently, various chemical groups were successfully added at the RANTES N-terminus, yielding AOP-RANTES [aminooxypentane oxime of (glyoxylyl1) 2-68 RANTES], NNY-RANTES (n-nonanoyl 2-68 RANTES) and PSC-RANTES [N-nonanoyl, des-Ser1(L-thioproline2, L-cyclohexylglycine3) 2-68 RANTES] [39–42]. The latter is the most powerful anti-HIV RANTES derivative developed to date. A strong anti-HIV activity was also obtained with N-terminal deletions, as seen with 3-68 RANTES [43] while a more extensive truncation (9-68 RANTES) showed a considerably reduced activity [44]. Most of these variants exert their action by sequestering CCR5 from the cellular surface via agonistic activation followed by internalization of the receptor. Altogether, these observations have reinforced the concept that the RANTES N-terminus is the CCR5-activating domain.
Table 1.
RANTES derivatives developed as HIV-1 inhibitors
| RANTES derivatives | Anti-HIV activitya | CCR5 agonist/antagonist | CCR5 affinitya | References |
|---|---|---|---|---|
| Full length analogs | ||||
|
| ||||
| N-terminal truncation | ||||
| 3-68 RANTES | = | agonist | = | [43,63,64] |
| 4-68 RANTES | < | agonist | = | [65] |
| 9-68 RANTES | < | antagonist | = | [44,66] |
| N-terminal chemical modification | ||||
| AOP-RANTES | > | agonist | ≤ | [39,67] |
| NNY-RANTES | > | agonist | = | [40] |
| PSC-RANTES | > | agonist | = | [41] |
| N-terminal mutagenesis and elongation | ||||
| Met-RANTES | ≤ | antagonist | = | [38] |
| L-RANTES | ≥ | antagonist | > | [45] |
| C1-RANTES | < | agonist | N.D. | [45] |
| A3-RANTES | < | agonist | N.D. | [45] |
| R6-RANTES | < | agonist | N.D. | [45] |
| LSPVSSQSSA-(RANTES 10-68) | > | agonist | ≥ | [56] |
| FSPVSSQSSA-(RANTES 10-68) | > | agonist | ≥ | [56] |
| C1C5-RANTES | > | antagonist | > | [45] |
|
| ||||
| Peptides | ||||
|
| ||||
| N-terminus | ||||
| 1-14(A10A11) | < | agonist | N.D. | [68-70] |
| 3-14(A10A11) | < | agonist | N.D. | [68-70] |
| N-loop/β1-strand | ||||
| 11-29 | < | antagonist | N.D. | [46,53] |
| 11-29 derivatives | ≤ | antagonist | N.D. | (unpublished |
|
| ||||
| Fusion proteinsb | ||||
|
| ||||
| Anti-CD4 scFv-RANTES | > | agonist | = | [71] |
| RANTES-Anti-CD4 scFv | > | agonist | = | [71] |
Anti-HIV activity and CCR5 affinity are indicated in comparison to wild-type full-length RANTES.
Not discussed in the text.
In contrast to all the analogues indicated above, C1C5-RANTES, a serine-to-cysteine double mutant (at both position 1 and 5), presents peculiar features originating from the intramolecular loop created by a C1C5 disulfide bridge (Table 1) [45]. The uniqueness of C1C5-RANTES is that it fails to elicit CCR5 activation and internalization, acting as a receptor antagonist; thus, its anti-HIV activity is to be ascribed entirely to interference with gp120 for CCR5 binding. The molecular basis for the antagonistic properties of C1C5-RANTES is most likely the profound modification of the chemokine N-terminal domain induced by the C1C5 constraint.
2.3 N-loop and β1-strand derivative molecules
A thorough analysis of the RANTES regions involved in anti-HIV activity has been conducted by mutagenesis and overlapping peptide scanning, leading to the identification of the N-loop/β1-strand region as the core determinants for CCR5 binding as well as a crucial moiety participating in the anti-HIV activity [46]. Further analysis, by means of chemokine and CCR5 mutants and chimeras, confirmed the N-loop/β1-strand-including RANTES core region as the binding determinant to extracellular CCR5 domains, i.e., CCR5 N-terminus and the second extracellular loop (ECL2) [47], and the RANTES N-terminus as a moiety embedding into CCR5 transmembrane helices [48]. Interestingly, peptides encompassing the N-loop/β1-strand region (aa 11-29) are sufficient to achieve HIV-blocking activity in an antagonistic manner (Table 1) [46]. Such activity is in line with the concept that the RANTES N-terminus acts as the CCR5-activating moiety, while the N-loop/β1-strand-including core is the portion responsible for initial receptor docking, providing the necessary affinity for the interaction [48]. The two-site organization model for G protein-coupled receptor (GPCR) binding and activation [49], along with the evidence that the GPCR ECL2 can be modulated between active and silent conformations [50,51], point to a partitioned receptor-ligand molecular organization. Indeed, GPCRs appear to possess this conformational complexity [52].
Considering the molecular architecture of RANTES and its interactions with CCR5, it should be possible to selectively improve (either by natural evolution or by human engineering) the region mainly responsible for the receptor binding affinity, uncoupling it from the receptor activation moieties. In line with this hypothesis, refined investigation of the RANTES aa 11-29 region, by the use of short derivative peptides, suggested a critical role of the two hydrophobic stretches derived from the N-loop (aa 11-16) and β1-strand (aa 27-29), respectively [53]. Further rational modifications of this region have resulted in peptides with progressively increasing antagonistic activity (unpublished data).
An important step forward in the pursuit of the molecular mechanisms leading to HIV-1 entry has recently been added with the demonstration that a sulfated CCR5 N-terminal peptide adopts a well-defined α-helical conformation upon binding to HIV-1 gp120, [24], further confirming the fundamental importance of tyrosine sulfation at the N-terminus of HIV-1 coreceptors [54]. As previously discussed, tyrosine sulfation is also critical for RANTES recognition of N-terminal CCR5 peptides [22]. Interestingly, a parallel role of tyrosine sulfation has been reported for SDF-1 recognition of CXCR4 [55]. The CCR5 N-terminus is commonly viewed as the non-activating domain of the receptor, which should interact with the RANTES core where the N-loop/β1-strand region is the major determinant [46,53]. In this respect, it is possible to postulate a RANTES binding switch from sulfated GAG moieties to the sulfated CCR5 N-terminus, as previously hypothesized [54]. A model for the possible orientation of monomeric RANTES for binding to the CCR5 N-terminal α-helix is shown in Fig. 1B.
3. CCR5 internalization/activation versus antagonistic competitive binding
Powerful N-terminally modified RANTES derivatives have been proposed as HIV therapeutics or microbicides, but their clinical use is potentially hampered by their CCR5 agonistic activity. In fact, persistent CCR5 activation is likely to elicit undesirable pro-inflammatory effects. Hence, despite the superior antiviral activity exerted by the potent agonist PSC-RANTES, a CCR5 antagonist such as C1C5-RANTES might represent a preferential candidate for therapeutic or prophylactic interventions. An antagonistic interaction with CCR5 implies that the anti-HIV activity is based purely on gp120-binding competition. A further advantage of using an antagonistic inhibitor is its potential anti-inflammatory effect in vivo, which is highly desirable in AIDS, a disease associated with a sustained and deleterious immune activation. In this perspective, RANTES-derived peptides evolved from the N-loop/β1-strand scaffold represent a valid alternative to a full-length RANTES derivative, as they combine a high CCR5 affinity with a convenient small molecular weight. A third route to RANTES engineering has been proposed, in which the mutants would be capable of combining a rapid induction of CCR5 internalization with antagonistic properties [56]. Although intriguing, this hypothesis implies the possibility of achieving CCR5 internalization in the absence of receptor-mediated signalling, which seems unlikely since receptor activation and internalization are generally believed to be tightly interconnected phenomena.
4. Toward a clinical development
The availability of safe and effective engineered RANTES derivatives will provide valuable new assets in the expanding armamentarium of HIV-1 entry inhibitors. RANTES derivatives could be employed both as components of systemic treatment protocols and as topical microbicides for the prophylaxis of sexual transmission. The clinical development of peptidic inhibitors, pioneered by the experience with the gp41-derived peptide T20/Enfuvirtide [8], poses a series of unique challenges related to manufacturing, administration routes and long-term tolerability, whose discussion goes beyond the purpose of the present review [57]. It is obvious that the continuous systemic administration of a peptide drug carries a considerably heavier load of specific issues compared to its intermittent topical application before sexual intercourse, as would be the case with microbicides. Nevertheless, the question of manufacturing costs and the potential immunogenicity of modified natural molecules represent significant hurdles for both strategies.
4.1 Therapeutic uses: Systemic administration
Several multi-drug therapeutic protocols are currently in use for the treatment of HIV-infected subjects. In most cases, these protocols are capable of reducing the level of viral replication by several orders of magnitude, as inferred by the measurement of viral RNA copies circulating in plasma. However, low levels of viral replication can still be detected using ultrasensitive methods [58] and, most important, complete virus eradication has never so far been achieved. These shortcomings emphasize the need for boosting the power of current treatment protocols through the addition of other drugs belonging to different classes of HIV inhibitors. Engineered RANTES derivatives represent potential candidates for inclusion in multi-drug protocols as they target a highly conserved cellular structure, CCR5. An important advantage in this strategy is represented by the evidence that CCR5 genetic impairment is not associated with any obvious clinical phenotype, as seen in people bearing the homozygous crippling deletion, CCR5-Δ32 [9].
The main limitations related to the systemic use of peptidic drugs include the administration route, which is forcefully by needle injection, and the potential induction of a specific antibody response. However, it should be emphasized that an antibody response is more likely to occur with full-length RANTES molecules bearing significant alterations, whilst it is less likely with short peptides. Finally, one has to consider the possible emergence of viral escape mutants that can use CCR5 despite the presence of a receptor-bound inhibitor, as already documented with small-molecule CCR5-targeted inhibitors [59].
All the above consideration should be attentively evaluated before moving forward with the systemic use of engineered RANTES derivatives. However, once data on the clinical efficacy of such molecules will be available, it should be possible to calculate the risk-benefit balance for a long-term utilization. Alternatively, the use of such molecules should be limited to critical situations in which a short-term administration is warranted, like post-exposure prophylaxis or acute phase treatment, thus avoiding some of the potential drawbacks listed above.
4.2 Prophylactic uses: Topical application
Compared to long-term systemic administration, the topical application of engineered RANTES derivatives as microbicides for the prevention of HIV sexual transmission appears more realistic and achievable in a relatively short time span. In fact, the local application of peptidic inhibitors within the female genital tract does not pose specific problems of administration and emergence of escape mutants, although a broad and sustained use in a large population group may eventually select for inhibitor-resistant viruses. Another major concern with microbicides is proinflammatory activity, which has played a main role in the clinical failure of several candidate compounds. Indeed, inflammation of the genital tract represents a significant risk factor increasing HIV transmission. In this respect, the use of antagonistic CCR5 inhibitors is undoubtedly preferable over agonists, regardless of their in vitro potency as HIV-1 inhibitors. Despite its potent agonistic effect, PSC-RANTES has been proven to protect monkeys from vaginal infection with a chimeric SHIV virus, providing a strong proof-of-principle that inhibiting CCR5 can completely prevent HIV transmission through the genital tract [42]. However, it should be emphasized that extremely high doses were required for protection, several orders of magnitude higher than the doses effective in vitro [42].
RANTES-based HIV microbicides could be administered exogenously, formulated in a gel vehicle in order to maintain protective concentrations for a prolonged period of time. Alternatively, recombinant RANTES derivatives could be engineered into bacterial systems capable of producing them endogenously upon colonization of the female genital tract (live microbicides). Lactobacillus spp., which constitute the most abundant component of the vaginal commensal flora, represent an obvious candidate for the generation of an engineered live microbicide. Moreover, unlike other prokaryotes, lactobacilli show an unusual ability to secrete proteins. Considerable progress has recently been made in this field, with the successful expression of various HIV inhibitors in L. jensenii [60–62]. Likewise, both wild-type full-length RANTES and C1C5-RANTES have been successfully expressed and secreted by L. jensenii (unpublished data).
In conclusion, the field of microbicides could be the primary beneficiary from the availability of safe and effective RANTES derivatives capable of blocking HIV-1 transmission to intraepithelial CD4+ T cells and mononuclear phagocytic cells in the genital tract. The proof-of-principle recently obtained in macaques [42] provides a strong rationale for the pursue of novel, safer and more effective RANTES derivatives and test their efficacy in preclinical and clinical trials.
5. Conclusion
The CCR5-ligand chemokine RANTES provides a suitable molecular scaffold for the generation of potent HIV inhibitors. A “mixed” approach combining structure-guided design with empirical trials is driving the continuous evolution of selected molecular leads. This approach has already provided excellent HIV-entry inhibitors, reducing the gap toward a future clinical applicability.
Acknowledgments
This work was supported by grants from NIH (U19 AI60615) and the EU (EMPRO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen J. AIDS RESEARCH: Promising AIDS Vaccine’s Failure Leaves Field Reeling. Science. 2007;318:28–29. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 2.Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. doi: 10.1038/446012a. [DOI] [PubMed] [Google Scholar]
- 3.Abdool Karim SS, Abdool Karim Q. Diverse approaches useful for microbicide trials. Nature. 2007;449(7158):24. doi: 10.1038/449024c. [DOI] [PubMed] [Google Scholar]
- 4.Ramjee G, Govinden R, Morar NS, Mbewu A. South Africa’s experience of the closure of the cellulose sulphate microbicide trial. PloS Med. 2007;4(7):e235. doi: 10.1371/journal.pmed.0040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klasse PJ, Shattock R, Moore JP. Antiretroviral Drug-Based Microbicides to Prevent HIV-1 Sexual Transmission. Annu Rev Med. 2008;59:291–307. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 6.Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369(9563):787–97. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein G. Anti-HIV microbicides: don’t forget basic immunology. Lancet. 2007;369(9571):1429. doi: 10.1016/S0140-6736(07)60665-5. [DOI] [PubMed] [Google Scholar]
- 8.Kilby JM, Hopkins S, Venetta TM, DiMassimo B, Cloud GA, Lee JY, et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4(11):1302–7. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 9.Lusso P. HIV and the chemokine system: 10 years later. EMBO J. 2006;25(3):447–56. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310(5750):1025–8. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433(7028):834–41. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- 12.Margolis L, Shattock R. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nat Rev Microbiol. 2006;4(4):312–7. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]
- 13.Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4(4):309–19. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Lodi PJ, Garrett DS, Kuszewski J, Tsang ML, Weatherbee JA, Leonard WJ, et al. High-resolution solution structure of the beta chemokine hMIP-1 beta by multidimensional NMR. Science. 1994;263(5154):1762–7. doi: 10.1126/science.8134838. [DOI] [PubMed] [Google Scholar]
- 16.Skelton NJ, Aspiras F, Ogez J, Schall TJ. Proton NMR assignments and solution conformation of RANTES, a chemokine of the C-C type. Biochemistry. 1995;34(16):5329–42. doi: 10.1021/bi00016a004. [DOI] [PubMed] [Google Scholar]
- 17.Chung CW, Cooke RM, Proudfoot AE, Wells TN. The three-dimensional solution structure of RANTES. Biochemistry. 1995;34(29):9307–14. doi: 10.1021/bi00029a005. [DOI] [PubMed] [Google Scholar]
- 18.Czaplewski LG, McKeating J, Craven CJ, Higgins LD, Appay V, Brown A, et al. Identification of amino acid residues critical for aggregation of human CC chemokines macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES. Characterization of active disaggregated chemokine variants. J Biol Chem. 1999;274(23):16077–84. doi: 10.1074/jbc.274.23.16077. [DOI] [PubMed] [Google Scholar]
- 19.Wilken J, Hoover D, Thompson DA, Barlow PN, McSparron H, Picard L, et al. Total chemical synthesis and high-resolution crystal structure of the potent anti-HIV protein AOP-RANTES. Chem Biol. 1999;6(1):43–51. doi: 10.1016/S1074-5521(99)80019-2. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JP, Johnson Z, Borlat F, Zwahlen C, Kungl A, Roulin K, et al. The X-ray structure of RANTES: heparin-derived disaccharides allows the rational design of chemokine inhibitors. Structure. 2004;12(11):2081–93. doi: 10.1016/j.str.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 22.Duma L, Haussinger D, Rogowski M, Lusso P, Grzesiek S. Recognition of RANTES by extracellular parts of the CCR5 receptor. J Mol Biol. 2007;365(4):1063–75. doi: 10.1016/j.jmb.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Jin H, Shen X, Baggett BR, Kong X, LiWang PJ. The human CC chemokine MIP-1beta dimer is not competent to bind to the CCR5 receptor. J Biol Chem. 2007;282(38):27976–83. doi: 10.1074/jbc.M702654200. [DOI] [PubMed] [Google Scholar]
- 24.Huang CC, Lam SN, Acharya P, Tang M, Xiang SH, Hussan SS, et al. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317(5846):1930–4. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springael JY, Urizar E, Parmentier M. Dimerization of chemokine receptors and its functional consequences. Cytokine Growth Factor Rev. 2005;16(6):611–23. doi: 10.1016/j.cytogfr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Baker AM, Sauliere A, Gaibelet G, Lagane B, Mazeres S, Fourage M, et al. CD4 interacts constitutively with multiple CCR5 at the plasma membrane of living cells: A vrFRAP approach. J Biol Chem. 2007 Sep 13; doi: 10.1074/jbc.M705617200. [Epub ahead of print] http://www.jbc.org/cgi/doi/10.1074/jbc.M705617200. [DOI] [PubMed]
- 27.Xiao X, Wu L, Stantchev TS, Feng YR, Ugolini S, Chen H, et al. Constitutive cell surface association between CD4 and CCR5. Proc Natl Acad Sci U S A. 1999;96(13):7496–501. doi: 10.1073/pnas.96.13.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaibelet G, Planchenault T, Mazeres S, Dumas F, Arenzana-Seisdedos F, Lopez A, et al. CD4 and CCR5 constitutively interact at the plasma membrane of living cells: a confocal fluorescence resonance energy transfer-based approach. J Biol Chem. 2006;281(49):37921–9. doi: 10.1074/jbc.M607103200. [DOI] [PubMed] [Google Scholar]
- 29.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 30.Proudfoot AE, Fritchley S, Borlat F, Shaw JP, Vilbois F, Zwahlen C, et al. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J Biol Chem. 2001;276(14):10620–6. doi: 10.1074/jbc.M010867200. [DOI] [PubMed] [Google Scholar]
- 31.Martin L, Blanpain C, Garnier P, Wittamer V, Parmentier M, Vita C. Structural and functional analysis of the RANTES-glycosaminoglycans interactions. Biochemistry. 2001;40(21):6303–18. doi: 10.1021/bi002670n. [DOI] [PubMed] [Google Scholar]
- 32.McCornack MA, Boren DM, LiWang PJ. Glycosaminoglycan disaccharide alters the dimer dissociation constant of the chemokine MIP-1 beta. Biochemistry. 2004;43(31):10090–101. doi: 10.1021/bi049751u. [DOI] [PubMed] [Google Scholar]
- 33.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100(4):1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paavola CD, Hemmerich S, Grunberger D, Polsky I, Bloom A, Freedman R, et al. Monomeric monocyte chemoattractant protein-1 (MCP-1) binds and activates the MCP-1 receptor CCR2B. J Biol Chem. 1998;273(50):33157–65. doi: 10.1074/jbc.273.50.33157. [DOI] [PubMed] [Google Scholar]
- 35.Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, et al. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36(44):13570–8. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 36.Johnson Z, Kosco-Vilbois MH, Herren S, Cirillo R, Muzio V, Zaratin P, et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J Immunol. 2004;173(9):5776–85. doi: 10.4049/jimmunol.173.9.5776. [DOI] [PubMed] [Google Scholar]
- 37.Proudfoot AE. The biological relevance of chemokine-proteoglycan interactions. Biochem Soc Trans. 2006;34(Pt 3):422–6. doi: 10.1042/BST0340422. [DOI] [PubMed] [Google Scholar]
- 38.Proudfoot AE, Power CA, Hoogewerf AJ, Montjovent MO, Borlat F, Offord RE, et al. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem. 1996;271(5):2599–603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 39.Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, et al. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276(5310):276–9. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 40.Mosier DE, Picchio GR, Gulizia RJ, Sabbe R, Poignard P, Picard L, et al. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J Virol. 1999;73(5):3544–50. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartley O, Gaertner H, Wilken J, Thompson D, Fish R, Ramos A, et al. Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc Natl Acad Sci U S A. 2004;101(47):16460–5. doi: 10.1073/pnas.0404802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306(5695):485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 43.Oravecz T, Pall M, Roderiquez G, Gorrell MD, Ditto M, Nguyen NY, et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186(11):1865–72. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arenzana-Seisdedos F, Virelizier JL, Rousset D, Clark-Lewis I, Loetscher P, Moser B, et al. HIV blocked by chemokine antagonist. Nature. 1996;383(6599):400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 45.Polo S, Nardese V, De Santis C, Arcelloni C, Paroni R, Sironi F, et al. Enhancement of the HIV-1 inhibitory activity of RANTES by modification of the N-terminal region: dissociation from CCR5 activation. Eur J Immunol. 2000;30(11):3190–8. doi: 10.1002/1521-4141(200011)30:11<3190::AID-IMMU3190>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 46.Nardese V, Longhi R, Polo S, Sironi F, Arcelloni C, Paroni R, et al. Structural determinants of CCR5 recognition and HIV-1 blockade in RANTES. Nat Struct Biol. 2001;8(7):611–5. doi: 10.1038/89653. [DOI] [PubMed] [Google Scholar]
- 47.Samson M, LaRosa G, Libert F, Paindavoine P, Detheux M, Vassart G, et al. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J Biol Chem. 1997;272(40):24934–41. doi: 10.1074/jbc.272.40.24934. [DOI] [PubMed] [Google Scholar]
- 48.Blanpain C, Doranz BJ, Bondue A, Govaerts C, De Leener A, Vassart G, et al. The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J Biol Chem. 2003;278(7):5179–87. doi: 10.1074/jbc.M205684200. [DOI] [PubMed] [Google Scholar]
- 49.Siciliano SJ, Rollins TE, DeMartino J, Konteatis Z, Malkowitz L, Van Riper G, et al. Two-site binding of C5a by its receptor: an alternative binding paradigm for G protein-coupled receptors. Proc Natl Acad Sci U S A. 1994;91(4):1214–8. doi: 10.1073/pnas.91.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klco JM, Wiegand CB, Narzinski K, Baranski TJ. Essential role for the second extracellular loop in C5a receptor activation. Nat Struct Mol Biol. 2005;12(4):320–6. doi: 10.1038/nsmb913. [DOI] [PubMed] [Google Scholar]
- 51.Massotte D, Kieffer BL. The second extracellular loop: a damper for G protein-coupled receptors? Nat Struct Mol Biol. 2005;12(4):287–8. doi: 10.1038/nsmb0405-287. [DOI] [PubMed] [Google Scholar]
- 52.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28(8):397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Vangelista L, Longhi R, Sironi F, Pavone V, Lusso P. Critical role of the N-loop and beta1-strand hydrophobic clusters of RANTES-derived peptides in anti-HIV activity. Biochem Biophys Res Commun. 2006;351(3):664–8. doi: 10.1016/j.bbrc.2006.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96(5):667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 55.Veldkamp CT, Seibert C, Peterson FC, Sakmar TP, Volkman BF. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) J Mol Biol. 2006;359(5):1400–9. doi: 10.1016/j.jmb.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartley O, Dorgham K, Perez-Bercoff D, Cerini F, Heimann A, Gaertner H, et al. Human immunodeficiency virus type 1 entry inhibitors selected on living cells from a library of phage chemokines. J Virol. 2003;77(12):6637–44. doi: 10.1128/JVI.77.12.6637-6644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Este JA, Telenti A. HIV entry inhibitors. Lancet. 2007;370(9581):81–8. doi: 10.1016/S0140-6736(07)61052-6. [DOI] [PubMed] [Google Scholar]
- 58.Persaud D, Siberry GK, Ahonkhai A, Kajdas J, Monie D, Hutton N, et al. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol. 2004;78(2):968–79. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marozsan AJ, Kuhmann SE, Morgan T, Herrera C, Rivera-Troche E, Xu S, et al. Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D) Virology. 2005;338(1):182–99. doi: 10.1016/j.virol.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 60.Chancey CJ, Khanna KV, Seegers JF, Zhang GW, Hildreth J, Langan A, et al. Lactobacilli-expressed single-chain variable fragment (scFv) specific for intercellular adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1 transmission across a cervical epithelial monolayer. J Immunol. 2006;176(9):5627–36. doi: 10.4049/jimmunol.176.9.5627. [DOI] [PubMed] [Google Scholar]
- 61.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, et al. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci U S A. 2003;100(20):11672–7. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, et al. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother. 2006;50(10):3250–9. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proost P, De Meester I, Schols D, Struyf S, Lambeir AM, Wuyts A, et al. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J Biol Chem. 1998;273(13):7222–7. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 64.Schols D, Proost P, Struyf S, Wuyts A, De Meester I, Scharpe S, et al. CD26-processed RANTES(3-68), but not intact RANTES, has potent anti-HIV-1 activity. Antiviral Res. 1998;39(3):175–87. doi: 10.1016/s0166-3542(98)00039-4. Erratum in: Antiviral Res 1999 Jan;40(3):189–90. [DOI] [PubMed] [Google Scholar]
- 65.Lim JK, Lu W, Hartley O, DeVico AL. N-terminal proteolytic processing by cathepsin G converts RANTES/CCL5 and related analogs into a truncated 4-68 variant. J Leukoc Biol. 2006;80(6):1395–404. doi: 10.1189/jlb.0406290. [DOI] [PubMed] [Google Scholar]
- 66.Ylisastigui L, Vizzavona J, Drakopoulou E, Paindavoine P, Calvo CF, Parmentier M, et al. Synthetic full-length and truncated RANTES inhibit HIV-1 infection of primary macrophages. AIDS. 1998;12(9):977–84. [PubMed] [Google Scholar]
- 67.Mack M, Luckow B, Nelson PJ, Cihak J, Simmons G, Clapham PR, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187(8):1215–24. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wells TN, Guye-Coulin F, Bacon KB. Peptides from the amino-terminus of RANTES cause chemotaxis of human T-lymphocytes. Biochem Biophys Res Commun. 1995;211(1):100–5. doi: 10.1006/bbrc.1995.1783. [DOI] [PubMed] [Google Scholar]
- 69.Nishiyama Y, Murakami T, Shikama S, Kurita K, Yamamoto N. Anti-HIV-1 peptides derived from partial amino acid sequences of CC-chemokine RANTES. Regulated upon activation, normal T-cell expressed and secreted. Bioorg Med Chem. 2002;10(12):4113–7. doi: 10.1016/s0968-0896(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 70.Ramnarine EJ, Devico AL, Vigil-Cruz SC. Analogues of N-terminal truncated synthetic peptide fragments derived from RANTES inhibit HIV-1 infectivity. Bioorg Med Chem Lett. 2006;16(1):93–5. doi: 10.1016/j.bmcl.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 71.Mack M, Pfirstinger J, Haas J, Nelson PJ, Kufer P, Riethmuller G, et al. Preferential targeting of CD4-CCR5 complexes with bifunctional inhibitors: a novel approach to block HIV-1 infection. J Immunol. 2005;175(11):7586–93. doi: 10.4049/jimmunol.175.11.7586. [DOI] [PubMed] [Google Scholar]