Abstract
Methanothermobacter thermautotrophicus contains a multi-aminoacyl-tRNA synthetase complex (MSC) of LysRS, LeuRS and ProRS. Elongation factor (EF) 1A also associates to the MSC, with LeuRS possibly acting as a core protein. Analysis of the MSC revealed that LysRS and ProRS specifically interact with the idiosyncratic N- and C- termini of LeuRS, respectively. EF-1A instead interacts with the inserted CP1 proofreading domain, consistent with models for post-transfer editing by class I synthetases such as LeuRS. Together with previous genetic data, these findings show that LeuRS plays a central role in mediating interactions within the archaeal MSC by acting as a core scaffolding protein.
Keywords: aminoacyl-tRNA synthetase, translation, amino acid
1. Introduction
Protein translation based on a nucleic acid code relies on the correct pairing of amino acid and tRNA molecules and subsequent delivery to the ribosome. Aminoacyl-tRNA synthetases (aaRSs) play an essential role in protein synthesis by attaching the correct amino acid onto the cognate tRNA. Once synthesized, aa-tRNAs are bound by EF-1A as a ternary complex with GTP and ushered to the ribosome. AaRSs are commonly found in higher order complexes in eukaryotes and archaea. For example, a multi-aaRS complex (MSC) composed of nine aaRS activities (aminoacylating cognate lysine, leucine, proline, isoleucine, methionine, glutamine, glutamic acid, asparagine, and aspartic acid) is found in mammalian cells. Three auxiliary proteins (p38, p43, and p18) complete the complex, promoting the binding of tRNA and contributing to the stability of the complex. The association of aaRSs and auxiliary proteins in the macromolecular MSC requires a network of protein-protein interactions within the complex. Genetic and biophysical data suggest that most, if not all, aaRSs within the complex associate with p38 and/or p43, enhancing the stability of the complex. Several interactions within the MSC are known to be mediated via N- and C-terminal appended domains of the associated eukaryotic aaRSs, which are generally absent from their bacterial and archaeal counterparts [1]; for example, LeuRS associates with the mammalian MSC via N- and C-terminal extensions [2]. These appendages to the cores of aaRSs are non-catalytic and instead function to mediate protein-protein interactions or act as general RNA binding domains [3-5]. Conversely several aaRSs, such as LysRS and AspRS, associate with the MSC through their catalytic cores, since deletions of the appended domains have no effect on complex formation [6;7].
Methanothermobacter thermautotrophicus contains a smaller MSC composed of LysRS, LeuRS, and ProRS [8]. EF-1A also associates with LeuRS [9], which could act as a core protein to mediate stable complex formation of the archaeal MSC, since no homologues of the eukaryotic auxiliary proteins have been found in archaea. M. thermautotrophicus LeuRS possesses a C-terminal extension beyond the catalytic core; although shorter than its eukaryotic counterpart, this suggests that comparable regions of the structurally distinct C-terminus of archaeal LeuRS might also mediate interactions with other proteins within the MSC. This led us to more closely investigate the potential role of LeuRS in the archaeal MSC, which revealed an essential role for this aaRS as a core scaffolding protein.
2. Materials and methods
Protein and tRNA production and purification
His6 fusion derivatives of N- and C-terminally truncated LeuRS, ΔCP1 LeuRS, LysRS, ProRS, EF-Tu and EF-1A were prepared as described, as were preparation and aminoacylation of tRNA and activation of elongation factors [9]. Both the full-length and truncation proteins were relatively labile during purification, presumably as a result of a significant level of misfolding, typically displaying 15-20% active fractions (Table 1).
Table 1.
Activities of LeuRS variants.
| LeuRS variant | Total protein concentration
(BioRad assay) |
Active LeuRS concentration
(active site titration) |
Active percentage of LeuRS variants |
|---|---|---|---|
| Full-length | 110 μM | 20 μM | 18 % |
| ΔN1 | 260 μM | 55 μM | 21 % |
| ΔN2 | 200 μM | 1 μM | < 1% |
| ΔC1 | 170 μM | 20 μM | 12 % |
| ΔC2 | 100 μM | 17 μM | 17 % |
| ΔC3 | 5 μM | < 0.01 μM | < 1 % |
| ΔCP1 | 190 μM | 30 μM | 16 % |
Fluorescence anisotropy
Equilibrium dissociation constants were determined by measuring the fluorescence anisotropy of labeled proteins as a function of increasing concentrations of unlabeled proteins as previously described [9] with the following exceptions. Each of the LeuRS variants and EF-1A were labeled with Alexa Fluor (AF) 488 tetrafluorophenyl ester (Molecular Probes, Eugene, OR). LeuRS and EF-1A were chosen due to the significantly higher stability they displayed over LysRS and ProRS during labeling. LeuRS variants and EF-1A were labeled with AF-488 at a molar ratio of 1:15 enzyme:fluorophore as previously described [9]. Each fluorescently labeled LeuRS variant and EF-1A sample were visualized on a 10 % SDS-polyacrylamide gel, which confirmed that the final labeled product contained little or no free fluorophore. Prior to use in fluorescence anisotropy measurements, the activity of the labeled LeuRS variants and EF-1A were verified by aminoacylation and GDP-exchange assays, respectively, and protein concentrations determined by active site titration and dye binding (BioRad) (data not shown; [10]). Equilibrium dissociation constants were determined by measuring the fluorescence anisotropy of labeled EF-1A or LeuRS variants (50 nM each) as a function of increasing concentrations of unlabeled protein using a Fluorolog-3 spectrofluorimeter (Horiba Jobin Yvon) as previously described [9]. The following concentration ranges of unlabeled proteins were used: 0-2000 nM LysRS, and 0-2400 nM ProRS, full-length LeuRS, ΔCP1 LeuRS, LeuRS C-terminal deletion variants ΔC1 and ΔC2, and LeuRS N-terminal deletion variant ΔN1. All measurements were carried out at least three times. Titration curves were fitted assuming a 1:1 binding stoichiometry as previously described [11].
Surface plasmon resonance
Surface plasmon resonance (SPR) experiments were performed utilizing a Biacore T100 instrument (GE Healthcare). 2000 resonance units (RU) of EF-1A in 100 mM sodium acetate (pH 4.5) were immobilized to a carboxymethyl dextran chip according to the manufacturer's instructions. As a control, a flow cell was activated and blocked in the absence of protein, which was then used to subtract RU resulting from non-specific interactions and the bulk refractive index. Experiments were performed at 25 °C in HBS-EP+ buffer (0.01 M Na-Hepes (pH 7.4), 150 mM NaCl, 3 mM EDTA, 0.05% v/v Surfactant P20), 10 mM MgCl2, 50 mM KCl and 100 μM GDP at a flow rate of 10 μl/min. The chip was regenerated by flowing 10 mM glycine-HCl (pH 2.5) over it for 30 sec at a flow rate of 25 μl/min. Each cycle consisted of injection of 80 μl of analyte (full-length LeuRS or variant ΔC2) over the immobilized protein (EF-1A) and the control flow cell, 660 sec dissociation time in HBS-EP+ buffer, and regeneration prior to the next injection. RU values were determined following subtraction of the control from the protein of interest immobilized to the cell. Rmax values for interactions between the analyte and immobilized protein were calculated according to the manufacturer's protocol. Dissociation constants were calculated using the average RU under steady-state conditions and data were fitted using the Binding Analysis Biacore T100 Evaluation Software.
ATP consumption editing assay
As a measure of cis-editing, LeuRS catalyzed ATP consumption was monitored in the presence of non-cognate amino acids. The reaction, carried out at 37 °C, contained 2 U/ml pyrophosphatase (Roche), 2 -10 mM leucine, methionine, valine, isoleucine, or norvaline, 5 μM tRNALeu, 2 mM [γ-32P]ATP (5 cpm/pmol), 0.1 M Na-Hepes (pH 7.2), 30 mM KCl, 10 mM MgCl2, and 1 μM ΔCP1 LeuRS or 1 μM LeuRS in the presence or absence of 5 μM EF-1A or EF-Tu. Aliquots were removed at various times and quenched in glacial acetic acid. Remaining [γ-32P]ATP and [γ-32P]Pi formed during the reaction were separated by TLC on a PEI cellulose plate (Sigma) prewashed with water. The TLC was subsequently developed in 0.7 M potassium phosphate (pH 3.5) and labeled products visualized and quantified on a Storm phosphorimager (Amersham Biosciences). Pi (mM) formed during the time-course reaction was estimated by multiplying the measured Pi/ATP ratio by the initial concentration of ATP (2 mM).
3. Results
Defining the roles of LeuRS N- and C-termini on complex formation
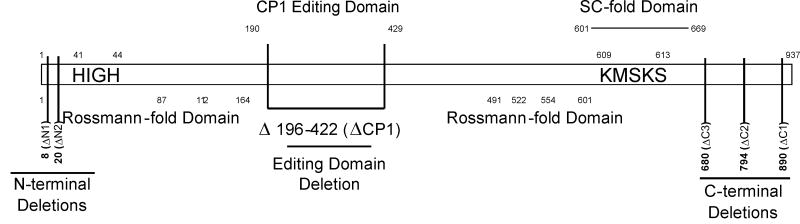
The LeuRS active site is composed of a Rossmann-fold domain containing the conserved sequence motifs HIGH and KMSKS near the N- and C-terminus (Fig. 1). To probe the role of LeuRS in the archaeal MSC five variants were created, truncated from the N-terminus to HIGH (ΔN1 and ΔN2) and from the C-terminus to KMSKS (ΔC1, ΔC2, and ΔC3; Fig. 1, Table 1). Variants adjacent to the HIGH and KMSKS motifs (ΔN2 and ΔC3) were inactive in aminoacylation and leucine activation (data not shown). LeuRS variants ΔN1, ΔC1 and ΔC2 were all able to bind and activate Leu, but only ΔN1 also retained aminoacylation activity. These three truncation variants were then utilized to investigate the domains of LeuRS responsible for mediating protein-protein interactions within the archaeal MSC. Full-length LeuRS bound LysRS, ProRS, and EF-1A with comparable affinities over a range of KDs between 80-170 nM (Table 2). Deletion of eight amino acid residues from the N-terminus of LeuRS (ΔN1 variant) had little effect on the binding of ProRS and EF-1A (KDs approximately 110 nM). Deletion of the LeuRS N-terminus increased affinity for LysRS three-fold, suggesting this region may modulate the LysRS:LeuRS interaction, in agreement with previous yeast two-hybrid analyses [8]. Attempts to further investigate LysRS:LeuRS binding in more detail were hindered by the lack of viable LeuRS variants further truncated from the N-terminus. LysRS and EF-1A each bound C-terminal truncated LeuRS variants (ΔC1 and ΔC2) with affinities comparable to full-length LeuRS (Table 2). Truncation of the LeuRS C-terminus severely impaired ProRS binding (Table 2), indicating complex formation between these two aaRSs is at least partly mediated by the C-terminal extension of LeuRS, again consistent with previous yeast-two hybrid data [8].
Figure 1. M. thermautotrophicus LeuRS variants.
Table 2.
Binding affinities between components of the archaeal MSC determined by fluorescence anisotropy.
| Unlabeled protein | Fluorescently labeled protein | KD (nM) |
|---|---|---|
| LysRS | LeuRS | 170±40 |
| ProRS | LeuRS | 80±10 |
| LeuRS | EF-1A | 90±10 |
| LysRS | ΔN1 LeuRS | 50±10 |
| ProRS | ΔN1 LeuRS | 110±20 |
| ΔN1 LeuRS | EF-1A | 110±30 |
| LysRS | ΔCP1 LeuRS | 40±10 |
| ProRS | ΔCP1 LeuRS | 110±10 |
| ΔCP1 LeuRS | EF-1A | >2000 |
| LysRS | ΔC2 LeuRS | 160±30 |
| ProRS | ΔC1 LeuRS | >2000 |
| ProRS | ΔC2 LeuRS | >2000 |
| ΔC1 LeuRS | EF-1A | 150±30 |
| ΔC2 LeuRS | EF-1A | 110±20 |
Effects of the LeuRS CP1 editing domain on complex formation
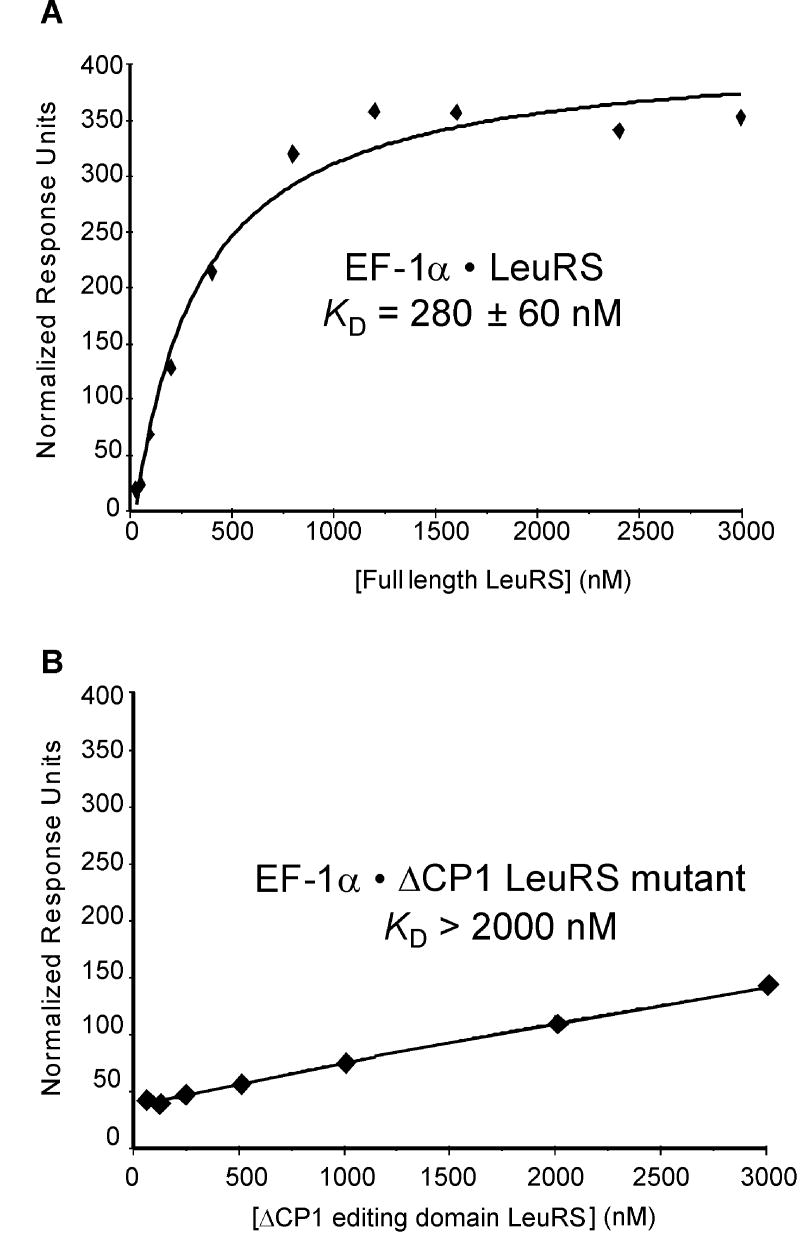
In addition to N- and C-terminal truncations of LeuRS, the CP1 domain was deleted to investigate its role in complex formation. Located between the HIGH and KMSKS motifs, CP1 is an inserted editing domain which recognizes and hydrolyzes tRNAs misacylated with non-cognate amino acids. The ΔCP1 variant was active in leucine activation (Table1), but inactive in editing of non-cognate amino acids (see below) and in aminoacylation (data not shown). LysRS and ProRS bound the ΔCP1 LeuRS variant with affinities comparable to full-length LeuRS suggesting that the editing domain is not essential for complex formation (Table 2). Deletion of CP1 had a pronounced effect on EF-1A binding to LeuRS, suggesting that the editing domain is involved in mediating protein-protein interactions (Table 2). To further investigate the binding of EF-1A to LeuRS via the CP1 domain, SPR experiments were performed. Deletion of the LeuRS CP1 domain significantly impaired interactions with EF-1A (Fig. 2) again indicating that the CP1 editing domain specifically associates with EF-1A.
Figure 2. Surface plasmon resonance.
EF-1A binding to (A) full-length LeuRS or (B) ΔCP1 variant.
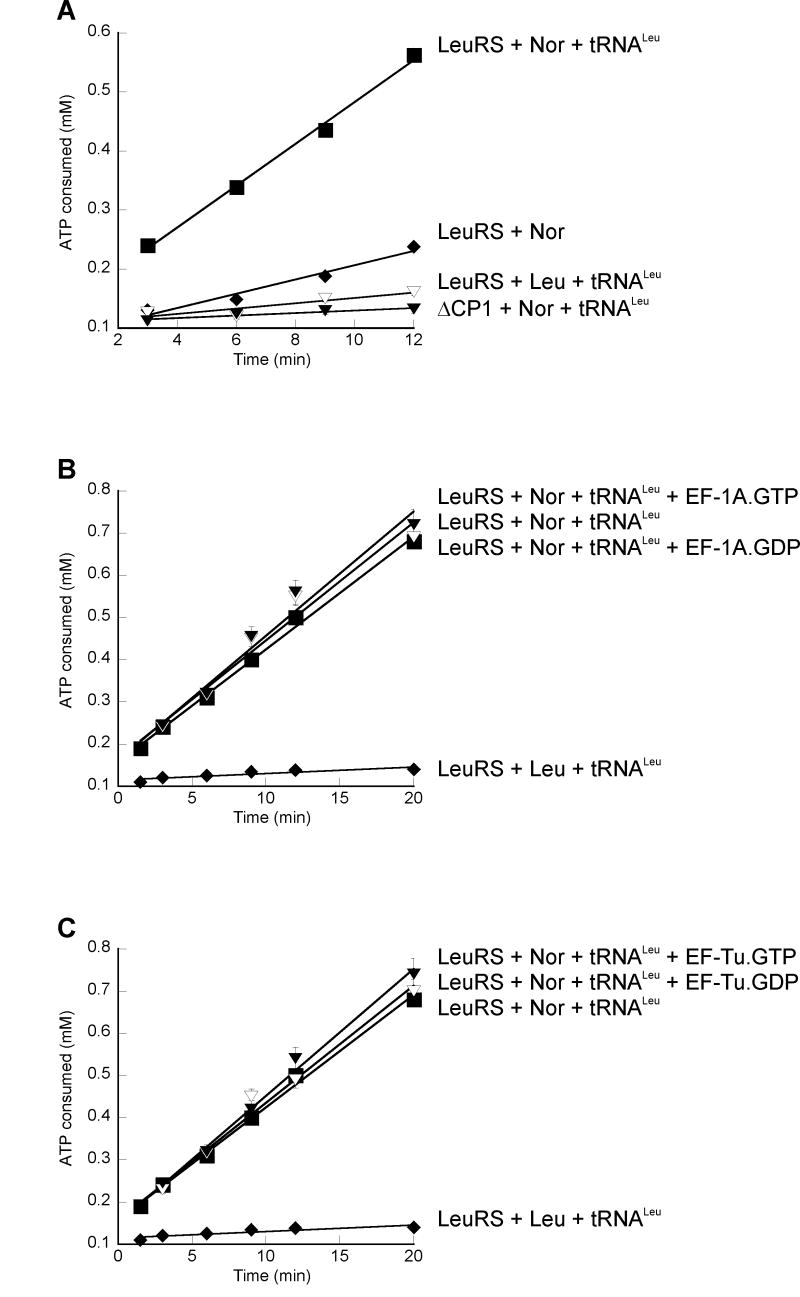
The effects of complex formation with EF-1A on the editing activities of LeuRS were monitored for the non-cognate substrates methionine, valine, isoleucine, and norvaline in the absence and presence of tRNALeu. Methionine, valine, and isoleucine were not editing substrates (data not shown), while wild-type LeuRS, but not the ΔCP1 variant, edited norvaline in a tRNA-dependent manner (Fig. 3A). Since EF-1A is known to adopt alternative conformations, the overall editing reaction was compared in the presence of EF-1A·GTP (required for aa-tRNA binding) and EF-1A·GDP. Regardless of the bound guanine nucleotide, LeuRS in complex with EF-1A displayed editing activities comparable to LeuRS alone (Fig. 3B), indicating that EF-1A does not effect the hydrolysis of non-cognate norvaline. Similarly, bacterial EF-Tu had no effect on LeuRS editing regardless of the GTP- or GDP-bound states (Fig. 3C), consistent with previous data that EF-Tu does not form specific interactions with M. thermautotrophicus LeuRS [9].
Figure 3. LeuRS editing.
ATP consumption was monitored without elongation factor (A) or in the presence of EF-1A (B) or EF-Tu (C).
4. Discussion
LeuRS acts as the core protein of the archaeal MSC
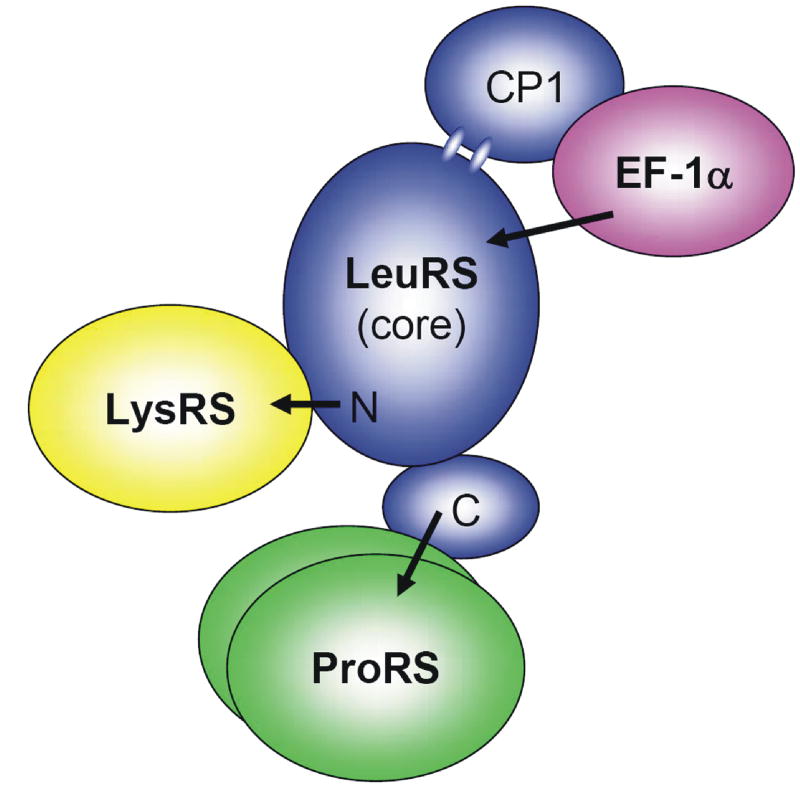
The eukaryotic counterparts of LysRS, LeuRS, and ProRS associate into a higher order complex with six additional aaRS activities and three auxiliary proteins, homologues of which have not been observed in archaea. M. thermautotrophicus EF-1A associates with the archaeal MSC, through specific interactions with LeuRS, suggesting a central role of LeuRS as a platform for complex formation and assembly in archaea. Towards structural mapping of protein interactions within the MSC, variants of archaeal LeuRS were constructed including truncation mutants from the N- and C-terminus and deletion of the modular CP1 editing domain. Deletion of the N-terminus of LeuRS had a modest effect on LysRS binding, suggesting that the N-terminal region modulates the LysRS:LeuRS interaction, in agreement with previous yeast two-hybrid analyses. When examining the domains of LeuRS that mediate interactions with ProRS, it was discovered that truncation of the LeuRS C-terminus significantly decreased binding to ProRS, also consistent with yeast two-hybrid data.
Deletion of the LeuRS CP1 editing domain resulted in decreased binding by EF-1A compared to full-length or LeuRS truncation variants as assessed by both fluorescence and SPR experiments. Determination of binding kinetics was not possible under these experimental conditions, likely due to the instability of proteins over the lengthy association and dissociation phases required for measurements of kinetic parameters in real time (data not shown). Overall, these data suggest that LysRS associates with the N-terminus of LeuRS, which in turn serves as a platform for the association of ProRS and EF-1A (Fig. 4). This potentially open structure is reminiscent of the extended V-shaped structure proposed for the far larger mammalian MSC [12], and would facilitate access of tRNA to the archaeal MSC consistent with the enhanced aminoacylation of all the aaRSs associated with the complex.
Figure 4. Schematic representation of the structural and functional association of the archaeal MSC.
Functional interactions with EF-1A in the archaeal MSC
It was suggested that steric clashes with CP1 prevent class I aaRSs such as LeuRS from stably associating with EF-Tu ([13;14]). In the present study, however, we found that archaeal EF-1A makes specific contacts with the LeuRS CP1 editing domain. This may be explained by the fact that CP1 is a distinct globular domain flexibly linked to the catalytic body (Fig. 5). Consequently, the position of CP1 has been observed to depend on the orientation of the tRNA CCA-acceptor end as it enters the editing domain during post-transfer editing, rotating approximately 20° as observed in crystal structures of P. horikoshii and up to 35° in T. thermophilus LeuRS [15-17]. Given that the domain orientations of EF-1A and EF-Tu also differ (Fig. 5) reorientation of the CP1 domain may permit LeuRS association with EF-1A, resulting in enhanced synthesis of Leu-tRNALeu. No concomitant alteration in editing was detected as a result of the LeuRS:EF-1A interaction. This indicates that EF-1A has no effect on the rate-limiting step of editing, which is likely to be translocation of the misactivated or misacylated amino acid from the active site to the editing site [18]. These data indicate that EF-1A cannot directly abstract aa-tRNALeu from LeuRS, consistent with previous data which indicated that enhanced synthesis of Leu-tRNALeu could be specifically attributed to complex formation with EF-1A, since bacterial EF-Tu displayed no effect on aminoacylation despite its high affinity for Leu-tRNALeu. This supports the notion that EF-1A forms specific interactions with LeuRS which do not affect the overall editing reaction, but instead enhance the rate of Leu-tRNALeu synthesis, possibly by inducing conformational changes in the catalytic core of LeuRS.
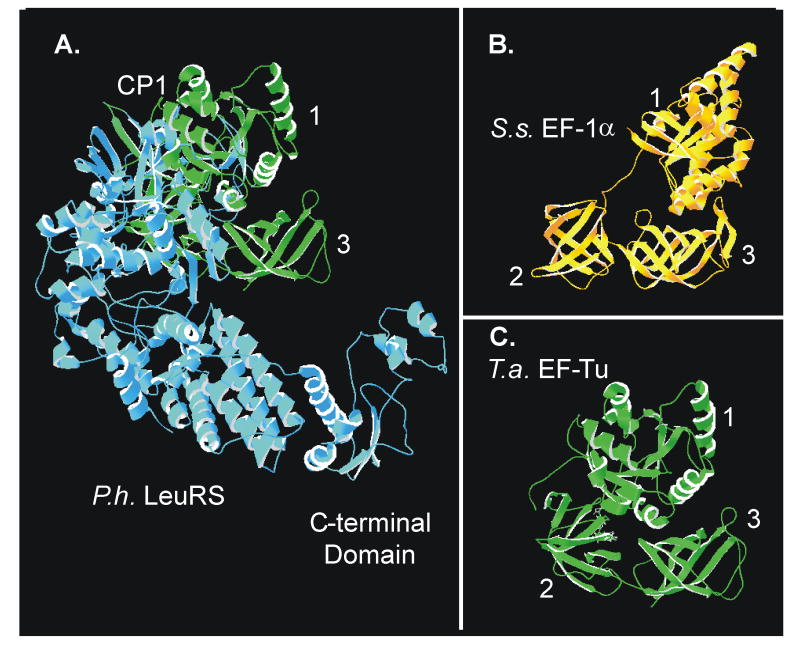
Figure 5. Structural modeling of a P. horikoshii LeuRS:T. aquifex EF-Tu complex.
(A) Structural modeling of a LeuRS:EF-Tu complex was performed by aligning the tRNA backbones (not shown for effective protein visualization) from the structural models of P. horikoshii LeuRS:tRNALeu and T. aquifex EF-Tu·GDPNP:tRNAPhe. (B) Crystal structure of Sulfolobus solfataricus EF-1A·GDP and (C) T. aquifex EF-Tu.
Acknowledgments
We thank A. Katz and J. Ling for critical reading of the manuscript. This work was supported by Grant GM 65183 from the National Institutes of Health.
Abbreviations
- aaRS
aminoacyl-tRNA synthetase
Footnotes
Structured summary: MINT-6551032: EF1A (uniprotkb:O27132) physically interacts (MI:0218) with LeuRS (uniprotkb:O27552) by surface plasmon resonance (MI:0107)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mirande M. Aminoacyl-tRNA synthetase family from prokaryotes and eukaryotes: structural domains and their implications. Prog Nucleic Acid Res Mol Biol. 1991;40:95–142. doi: 10.1016/s0079-6603(08)60840-5. [DOI] [PubMed] [Google Scholar]
- 2.Ling C, Yao YN, Zheng YG, Wei H, Wang L, Wu XF, Wang ED. The C-terminal appended domain of human cytosolic leucyl-tRNA synthetase is indispensable in its interaction with arginyl-tRNA synthetase in the multi-tRNA synthetase complex. J Biol Chem. 2005;280:34755–34763. doi: 10.1074/jbc.M413511200. [DOI] [PubMed] [Google Scholar]
- 3.Cahuzac B, Berthonneau E, Birlirakis N, Guittet E, Mirande M. A recurrent RNA-binding domain is appended to eukaryotic aminoacyl-tRNA synthetases. EMBO J. 2000;19:445–452. doi: 10.1093/emboj/19.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guigou L, Shalak V, Mirande M. The tRNA-interacting factor p43 associates with mammalian arginyl-tRNA synthetase but does not modify its tRNA aminoacylation properties. Biochemistry. 2004;43:4592–4600. doi: 10.1021/bi036150e. [DOI] [PubMed] [Google Scholar]
- 5.Robinson JC, Kerjan P, Mirande M. Macromolecular assemblage of aminoacyl-tRNA synthetases: quantitative analysis of protein-protein interactions and mechanism of complex assembly. J Mol Biol. 2000;304:983–994. doi: 10.1006/jmbi.2000.4242. [DOI] [PubMed] [Google Scholar]
- 6.Agou F, Mirande M. Aspartyl-tRNA synthetase from rat: in vitro functional analysis of its assembly into the multisynthetase complex. Eur J Biochem. 1997;243:259–267. doi: 10.1111/j.1432-1033.1997.0259a.x. [DOI] [PubMed] [Google Scholar]
- 7.Francin M, Kaminska M, Kerjan P, Mirande M. The N-terminal domain of mammalian lysyl-tRNA synthetase is a functional tRNA-binding domain. J Biol Chem. 2002;277:1762–1769. doi: 10.1074/jbc.M109759200. [DOI] [PubMed] [Google Scholar]
- 8.Praetorius-Ibba M, Hausmann CD, Paras M, Rogers TE, Ibba M. Functional association between three archaeal aminoacyl-tRNA synthetases. J Biol Chem. 2007;282:3680–3687. doi: 10.1074/jbc.M609988200. [DOI] [PubMed] [Google Scholar]
- 9.Hausmann CD, Praetorius-Ibba M, Ibba M. An aminoacyl-tRNA synthetase:elongation factor complex for substrate channeling in archaeal translation. Nucleic Acids Res. 2007;35:6094–6102. doi: 10.1093/nar/gkm534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fersht AR, Ashford JS, Bruton CJ, Jakes R, Koch GL, Hartley BS. Active site titration and aminoacyl adenylate binding stoichiometry of aminoacyl-tRNA synthetases. Biochemistry. 1975;14:1–4. doi: 10.1021/bi00672a001. [DOI] [PubMed] [Google Scholar]
- 11.An S, Musier-Forsyth K. Cys-tRNAPro editing by Haemophilus influenzae YbaK via a novel synthetase. YbaK.tRNA ternary complex. J Biol Chem. 2005;280:34465–34472. doi: 10.1074/jbc.M507550200. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe CL, Warrington JA, Treadwell L, Norcum MT. A three-dimensional working model of the multienzyme complex of aminoacyl-tRNA synthetases based on electron microscopic placements of tRNA and proteins. J Biol Chem. 2005;280:38870–38878. doi: 10.1074/jbc.M502759200. [DOI] [PubMed] [Google Scholar]
- 13.Negrutskii BS, Shalak VF, Kerjan P, El'skaya AV, Mirande M. Functional interaction of mammalian valyl-tRNA synthetase with elongation factor EF-1alpha in the complex with EF-1H. J Biol Chem. 1999;274:4545–4550. doi: 10.1074/jbc.274.8.4545. [DOI] [PubMed] [Google Scholar]
- 14.Zhang CM, Perona JJ, Ryu K, Francklyn C, Hou YM. Distinct kinetic mechanisms of the two classes of Aminoacyl-tRNA synthetases. J Mol Biol. 2006;361:300–311. doi: 10.1016/j.jmb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Tukalo M, Yaremchuk A, Fukunaga R, Yokoyama S, Cusack S. The crystal structure of leucyl-tRNA synthetase complexed with tRNALeu in the post-transfer-editing conformation. Nat Struct Mol Biol. 2005;12:923–930. doi: 10.1038/nsmb986. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga R, Yokoyama S. Aminoacylation complex structures of leucyl-tRNA synthetase and tRNALeu reveal two modes of discriminator-base recognition. Nat Struct Mol Biol. 2005;12:915–922. doi: 10.1038/nsmb985. [DOI] [PubMed] [Google Scholar]
- 17.Fukunaga R, Yokoyama S. The C-terminal domain of the archaeal leucyl-tRNA synthetase prevents misediting of isoleucyl-tRNAIle. Biochemistry. 2007;46:4985–4996. doi: 10.1021/bi6024935. [DOI] [PubMed] [Google Scholar]
- 18.Nomanbhoy TK, Hendrickson TL, Schimmel P. Transfer RNA-dependent translocation of misactivated amino acids to prevent errors in protein synthesis. Mol Cell. 1999;4:519–528. doi: 10.1016/s1097-2765(00)80203-8. [DOI] [PubMed] [Google Scholar]