Abstract
Conditioned place preference and locomotor sensitization are rodent behavioral models commonly used to investigate the actions of drugs of abuse. However, few studies have examined both paradigms in the same group of animals. We were interested in developing a combined protocol which successfully induced both conditioned place preference and sensitization simultaneously in cocaine-treated Sprague Dawley rats in order to test the hypothesis that the magnitude of these two phenomena would be positively correlated. We used an open-field with a removable place preference insert to assess these measures independently. Cocaine-conditioned animals demonstrated a significant shift in preference for the drug-paired compartment and a sensitized locomotor response which was not observed in saline-conditioned animals challenged with cocaine. There was no significant relationship between locomotor sensitization and conditioned place preference in individual animals. We further examined these results with respect to each rats’ initial response to cocaine, response to a novel environment and central zone entries in an open-field. Locomotor sensitization demonstrated an inverse correlation with the initial cocaine response. In contrast, conditioned place preference demonstrated an inverse correlation with the centre response. These results demonstrate that the combination of the acute cocaine response and the centre response in a novel open field environment can be used to indicate the propensity of a given rat to exhibit either behavioral sensitization or conditioned place preference; however, it seems that sensitization and place preference are not necessarily co-expressed to a similar extent in the same individual animal.
Classification Terms: Section: 7. Cognitive and Behavioral Neuroscience
Keywords: Conditioned place preference1, centre response, cocaine response, locomotor sensitization, individual differences, novelty
1. Introduction
Addiction can be defined as a behavioral syndrome, characterized by compulsive drug seeking with repeated relapses into drug use (Spanagel and Heilig, 2005). In order to understand the underlying neurobiological mechanisms, preclinical animal models have been developed and are increasingly utilized by a wide range of biomedical investigators in the study of addiction. For example, it has been noted “While measures of the reinforcing effects of drugs utilizing the drug self-administration paradigm are used by many as indicators of the development of addiction, even more prevalent in the literature are measures of sensitization and conditioned place preference” (Winger et al., 2005). Sensitization (or reverse tolerance) is a progressively enhanced behavioral response following the repeated administration of many drugs of abuse, including amphetamines, cocaine, opiates, ethanol and nicotine (Robinson and Berridge, 2003). Behavioral sensitization to psychostimulants can be attributed not only to a direct pharmacological action of the drug but also to learned associations with the drug experience (Pierce and Kalivas, 1997). It has been suggested that behavioral sensitization could be involved in the development and maintenance of drug addiction (Tzschentke and Schmidt, 2000) through enhanced incentive salience (Robinson and Berridge, 1993).
Previously sensitized animals exhibit significantly enhanced conditioned place preference (CPP) relative to control animals (Lett, 1989; Shippenberg and Heidbreder, 1995; Simpson and Riley, 2005). Place conditioning typically involves repeatedly pairing a compartment with a specific stimulus while pairing a separate distinct compartment with a neutral stimulus. On the test day, the animal is allowed free access to both compartments in the absence of these stimuli. Increased time spent in the paired compartment indicates a preference for the specific stimulus whereas a decrease in time spent indicates that an aversion (conditioned place aversion) has been induced. Psychostimulants (amphetamine, cocaine), opiates (heroin, morphine), ethanol, nicotine, LSD, caffeine and natural reinforcers (food, novel environment, male/female interaction) all can induce CPP (Tzschentke, 1998, 2007) The use of this method of measuring drug reward has increased significantly, with the number of articles published between 1957 and 1991 (330; Schecter and Calcagnetti, 1993) being equaled in the five-year period following 1991 (Schecter and Calcagnetti, 1998) and is steadily increasing (Tzschentke, 2007). Particularly noteworthy is the rapid increase in the number of reports which utilize an extinction/reinstatement design, based on similar experiments using the self-administration approach (Tzschentke, 2007).
Several recent studies examining gene expression or receptor signaling following exposure to drugs of abuse have investigated CPP and sensitization in separate groups of animals (Ferguson et al., 2006; Valjent et al., 2006; Ogawa et al., 2007; Schroeder et al., 2007). In contrast, few studies have investigated CPP and sensitization in a single group of animals, and mostly they rely on the data from the conditioning trials to quantify the sensitization effect (Cunningham and Noble, 1992; Shimosato and Ohkuda, 2000; Cunningham et al., 2002; Orsini et al., 2005). The obvious advantages of these combined studies over reports examining both phenomena separately include that they are less time consuming and require reduced number of animals. However, utilizing the conditioning trials to assess the locomotor response can result in the measurement of conditioned responses (not sensitization; Martin-Iverson and Reimer, 1996) or even a failure to detect sensitization at all (O’Dell et al., 1996). Furthermore, a significant problem with this approach is that a typical sensitization experiment involves a drug-free period after which a drug challenge is used to demonstrate the sensitized response. Indeed, behavioral sensitization is often more robust long after rather than shortly after the discontinuation of drug treatment (Shuster et al., 1977; Antelman, 1988; Robinson and Berridge, 2001). Thus it is thought that the most appropriate time to measure changes in neural function underlying behavioral sensitization is following a drug challenge administered a week or more after discontinuing the repeated drug administration regimen (Pierce and Kalivas, 1997).
In light of these concerns, and given the ever-increasing utilization of CPP and behavioral sensitization assays, we were interested in developing a combined protocol that incorporated important aspects of both paradigms. There were many reasons why such an approach would be useful. First, the relationship between conditioned place preference and behavioral sensitization had not been rigorously examined in individual animals. If a clearly defined relationship existed, it would help determine whether the expression of these behavioral measures compete with or are mutually beneficial for each other. For example, a highly sensitized animal may not perform well in the place preference task due to an inability to attend to their environment. In this instance, sensitization of the locomotor response would be expected to compete with the establishment of place preference. Second, the co-expression of these phenomena in the same individual might help elucidate aspects of reward and drug effects that would not be evident using each paradigm in separate groups of animals. While many signaling cascades such as the glutamatergic and dopaminergic systems have been demonstrated to underlie both CPP (reviewed in Tzschentke, 1998, 2007) and sensitization (reviewed in Wolf, 1998; Vanderschuren and Kalivas, 2000), there are many other prospective systems that could also be involved in the acquisition and expression of both these behaviors (e.g. growth factors, NF-κB). Finally, a combined behavioral approach would permit potential drug targets and therapies to interact with both conditioned place preference and sensitization in the same individual, allowing a broader analysis of their actions to be determined in less time and with fewer subjects.
The main aim of our study was to test the hypothesis that a positive correlation between the magnitude of sensitization and place preference would be observed in individuals among a cocaine conditioned population of rats. Thus, correlational analyses were performed to determine whether the sensitized response of a given rat to cocaine could predict its performance in the conditioned place preference task. These results were further analyzed in terms of individual differences in the acute response to cocaine administration (cocaine response), in the locomotor response to a novel environment (novelty response) and to an enclosed open field environment (centre response). These parameters were chosen because they have been prominently featured in previous reports of individual differences in responding to drugs of abuse (Piazza et al., 1989; Hooks et al., 1991; Carey and Gui, 1997; Sabeti et al., 2003).
2. Results
This study was done in five cohorts. The first CC15 group showed a consistent sensitization and conditioned place preference and this dose and protocol was used in additional studies involving pharmacological modulators of both behaviours. Thus the extra CC15 cohorts were “control” groups for these other parallel studies. We included these groups to increase the power of our correlational analysis of the various responder subgroups and the co-expression of sensitization and CPP.
Conditioned Place Preference
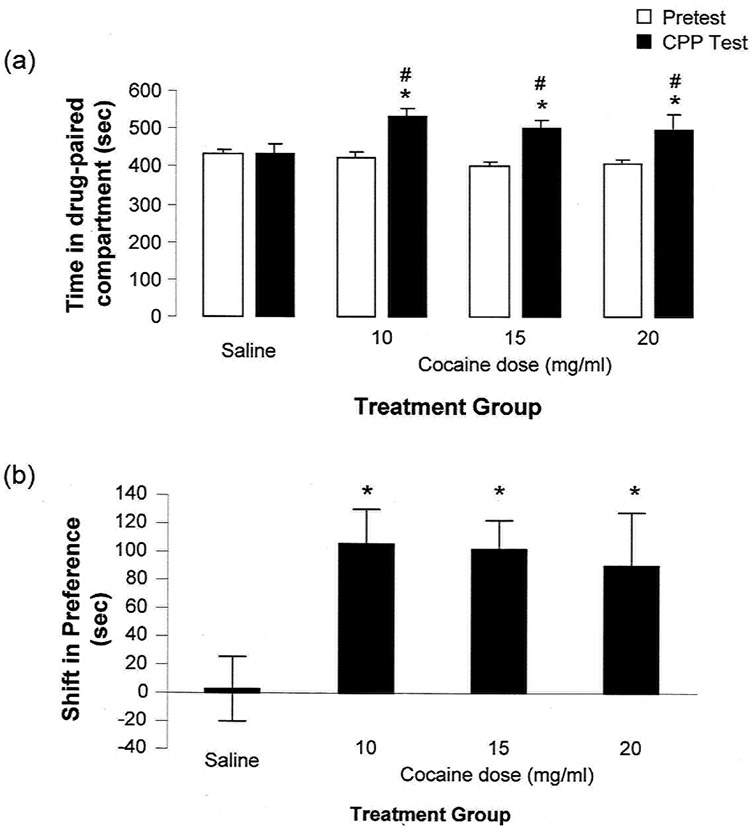
The CPP data collected in this study was analyzed as 10, 15, 20 and 30 min time bins to see if an optimum time period for this combined approach could be ascertained. The data presented here is from the first 15 min as this was the optimum time period for all three cocaine doses. Two-way ANOVA analysis of the time-spent in the drug-paired compartment did not indicate a significant treatment dose effect (F[3,67]=1.07; p=0.368), however, there was a significant effect of test (F[1,67]=24.66, p<0.001) and a significant interaction between treatment and test (F[3,67]=4.44, p=0.007; Fig. 1a). Post-hoc analysis indicated a significant difference between the pre-test and CPP test days (p<0.001, Holm-Sidak post-test), in particular for each of the cocaine doses (Sal: p=0.885, CC10: p=0.009, CC15: p<0.001, CC20: p=0.012). Furthermore, while there was no significant differences between the treatment groups on the pre-test day, all three cocaine doses were significantly different from the saline-conditioned animals on the CPP test day (CC10: p=0.006, CC15: 0.006, CC20: p=0.047) but not from each other (p>0.05). CPP data may also be analyzed by examining the change/shift in preference for the drug-paired compartment when comparing the CPP test to the pre-test session. A significant shift in preference for the drug-paired compartment was observed for the cocaine conditioned animals when compared to the vehicle control animals (Fig. 1b; F[3,67]=4.44, p=0.007, one-way ANOVA) with all three cocaine doses significantly different from vehicle control (CC10: p=0.0228, CC15: p=0.00181, CC20: p=0.0349; Holm-Sidak post-test).
Figure 1. Conditioned place preference following repeated administration of cocaine.
Cocaine treated animals spent significantly more time in the drug-paired compartment after conditioning than their control counterparts. (a) Cocaine conditioned animals spent significantly more time in the drug-paired compartment on the CPP test day compared to the saline-conditioned animals (denoted by an asterisk) and also to the pretest day (denoted by a hash). (b) A significant shift in the preference of the cocaine treated animals was observed following conditioning compared to saline-treated animals (denoted by an asterisk). The shift in preference represents change in time spent in drug-paired compartment at the CPP test and Pre-test (sec). Data are expressed as mean ± SEM with n=29 (Veh), n= 8 (Coc 10 mg/kg), n=24 (Coc 15 mg/kg) and n=10 (Coc 20 mg/kg). Statistical significance was determined with p<0.05.
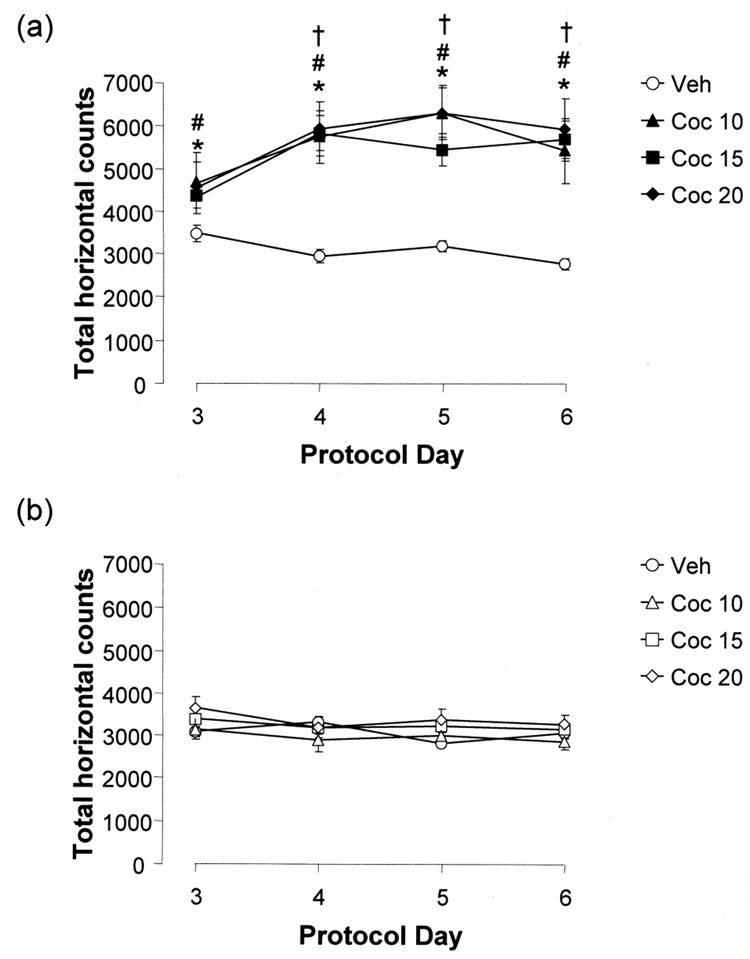
On the conditioning days (Protocol days 3–6), cocaine injected animals were significantly more active than saline injected animals. Statistical analysis by two-way Repeated Measures ANOVA indicated significant effects of treatment (F[3,469]=15.58; p<0.001), session (F[7,469]=42.06; p<0.001) and the interaction between treatment and session (F[21,469]=8.90; p<0.001). The activity of vehicle-treated animals was significantly different from their cocaine-treated counterparts during drug/vehicle-paired sessions on day three (10 mg/kg: p=0.023, 15 mg/kg: p=0.020, 20 mg/kg: p=0.026), day four (all: p<0.001), day five (all: p<0.001) and day six (all: p<0.001, Holm-Sidak post-hoc analysis, Fig 2a). However, during the saline-paired sessions, all treatment groups displayed similar activity (p>0.05 for all four days, Fig 2b). In addition, cocaine conditioned animals were significantly more active during their drug-paired sessions compared to their saline-paired sessions on day three (10 mg/kg: p=0.007, 15 mg/kg: p=0.004), day four (all: p<0.001), day five (all: p<0.001) and day six (all: p<0.001). Saline conditioned animals did not differ significantly in their activity during their drug/vehicle-paired or saline-paired sessions (p>0.05 for all four days). The activity of cocaine conditioned animals was significantly increased on days 4 (10 mg/kg: p=0.046, 15 mg/kg: p<0.001, 20 mg/kg: p=0.005), 5 (10 mg/kg: p=0.003, 15 mg/kg: p<0.001, 20 mg/kg: p<0.001) and 6 (15 mg/kg: p<0.001, 20 mg/kg: p=0.005) compared to day 3. No other drug-paired combinations were significant.
Figure 2. Total photocell counts after injection of saline or cocaine on conditioning days.
Animals were conditioned with either 10 (triangles), 15 (squares) or 20 (diamonds) mg/kg i.p. cocaine or with vehicle (open circles). (a) Cocaine-conditioned animals are significantly more active on drug-paired sessions compared to saline controls. (b) There is no significant difference between any of the groups during the vehicle-paired sessions. Data represent mean ± SEM and statistical significance with p<0.05 is represented by an asterisk for drug/vehicle-paired vs. saline paired, a hash for cocaine vs. saline treatment and a cross for specific day differences compared to Day 3. n=29 (Veh), n= 8 (Coc 10 mg/kg), n=24 (Coc 15 mg/kg) and n=10 (Coc 20 mg/kg)
Behavioral Sensitization
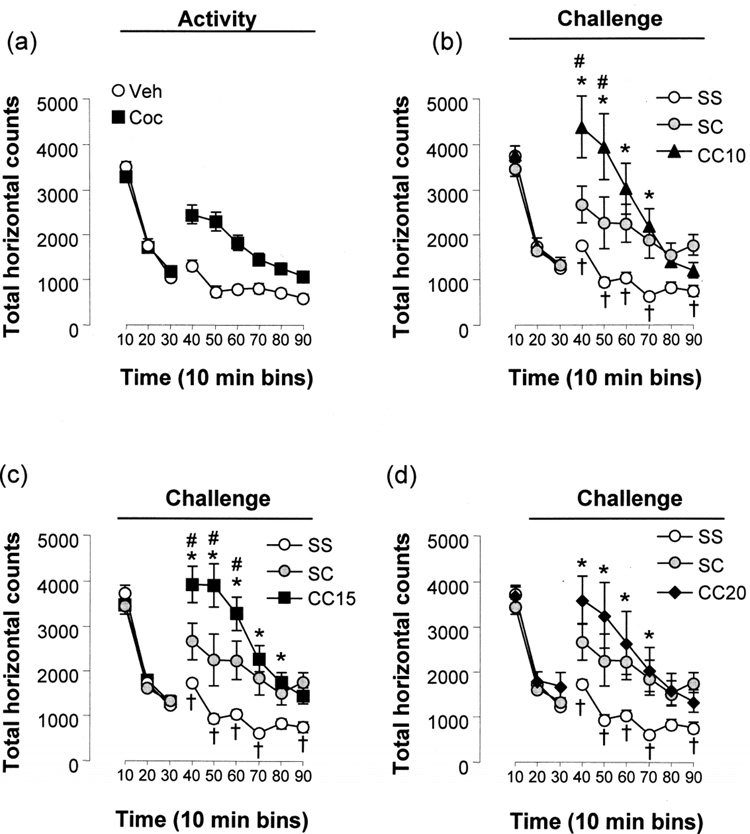
Prior to drug administration in the activity session (Protocol day 2), no difference in the level of activity of the saline or cocaine treatment groups was observed during the 30 min baseline session (Treatment: F[1,138]=0.15, p=0.704, Two-way Repeated Measures ANOVA, Fig. 3a). Both treatment groups showed an initial hyperactivity which significantly decreased over the 30 min (Time: F[2,138]=562.56; p<0.001). Following administration of cocaine (10 mg/kg) or saline, there was a significant effect of treatment (F[1,345]=29.32; p<0.001), time (F[5,345]=28.843; p<0.001) and a treatment × time interaction (F[5,345]=8.85; p<0.001), with significant differences between the two treatment groups at all time bins
Figure 3. Locomotor sensitization to cocaine following repeated administration.
Graphs illustrate the total horizontal counts 30 min prior to and 60 min after an i.p. injection of 10 mg/kg cocaine or saline in 10 min bins. Panel (a) depicts total horizontal counts before and following i.p. injection on the activity day. n= 29 (Veh), n= 42 (Coc). Panels (b–d) depict total horizontal counts before and following i.p. injection on the challenge day. Eleven of the saline-conditioned group received cocaine instead of saline (grey circles). The total horizontal counts for the (b) 10 mg/kg, (c) 15 mg/kg and (d) 20 mg/kg cocaine-conditioned groups are shown with respect to the saline-conditioned group. Significant differences by Holm-Sidak post-hoc test (p<0.05) are indicated by an asterisk (SS vs. CC), by a hash (SC vs. CC), by a cross (SS vs. SC) and by a dollar sign (activity day vs. challenge day). n=18 (SS), n=11 (SC), n=8 (CC10), n=24 (CC15) and n=10 (CC20).
During the challenge session (Protocol day 13), a subset of the original saline treated animals were challenged with cocaine. There was once again no difference between the treatment groups pre-injection (Treatment: F[4,132]=0.69; p=0.601) and a significant reduction in activity over the 30 min session (Time: F[2,132]=348.35; p<0.001, Fig 3b–d). There were significant effects of treatment (F[4,330]=8.22; p<0.001), time (F[5,330]=56.53; p<0.001) and a treatment × time interaction (F[20,335]=4.59; p<0.001) following a challenge injection of cocaine (10 mg/kg) or saline when all groups were compared. All cocaine challenged groups were significantly more active than the SS group (SC: p=0.0142; CC10: p<0.001, Fig 3b; CC15: p<0.001, Fig 3c; CC20: p=0.00175, Fig 3d), in particular over the first 40 min (50 min for the CC15 group). Compared to the SC group, the CC15 treatment group were significantly more active over the first 30 min (10 min: p=0.003, 20 min: p<0.001, 30 min: p=0.020) while the CC10 group were more active over the first 20 min (10 min: p=0.007; 20 min: p=0.008); no other cocaine group comparisons were significant.
The cocaine challenged treatment groups displayed significant increases in activity during the challenge session compared to the activity session following i.p. injection (SS: F[1,225]=2.92; p=0.094, SC: F[1,190]=24.66; p<0.001, CC10: F[1,240]=8.252; p=0.006, CC15: F[1,320]=20.17; p<0.001, CC20: F[1,250]=4.10; p=0.048). The activity of the SC group during their challenge session was not significantly different from that of the cocaine-treated group during the activity session (F[1,255]=1.18; p=0.283), indicating that the additional handling and injection procedure involved in this combined conditioning/sensitization protocol did not in itself induce an increased sensitivity to cocaine.
Correlations between cocaine, novelty and centre responses with sensitization and CPP
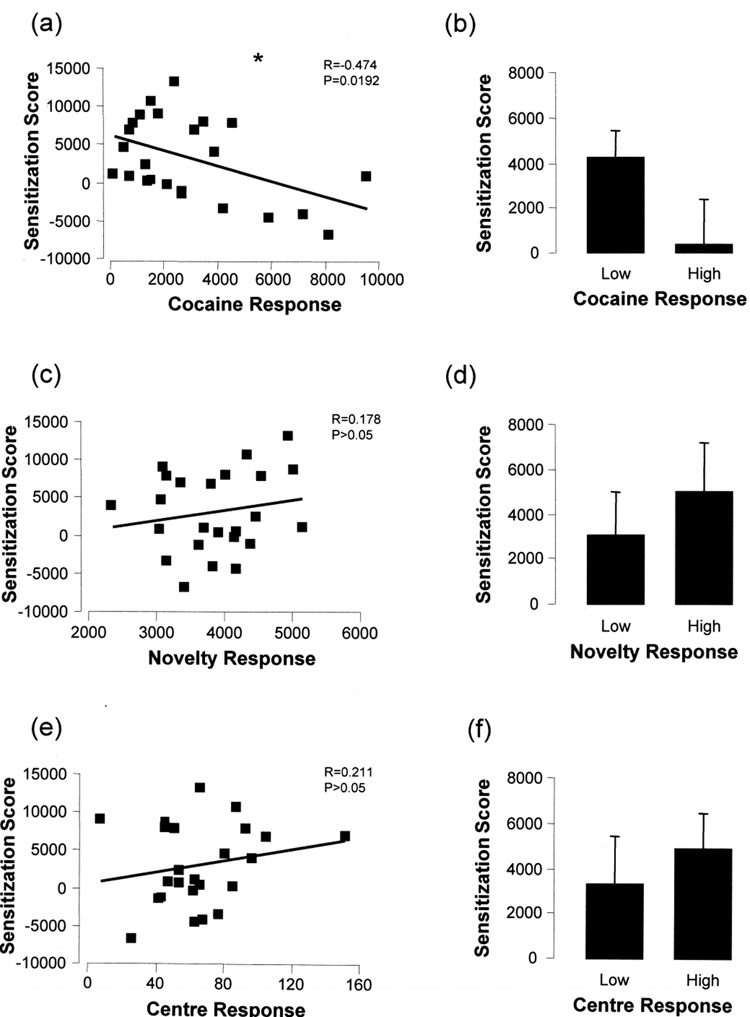
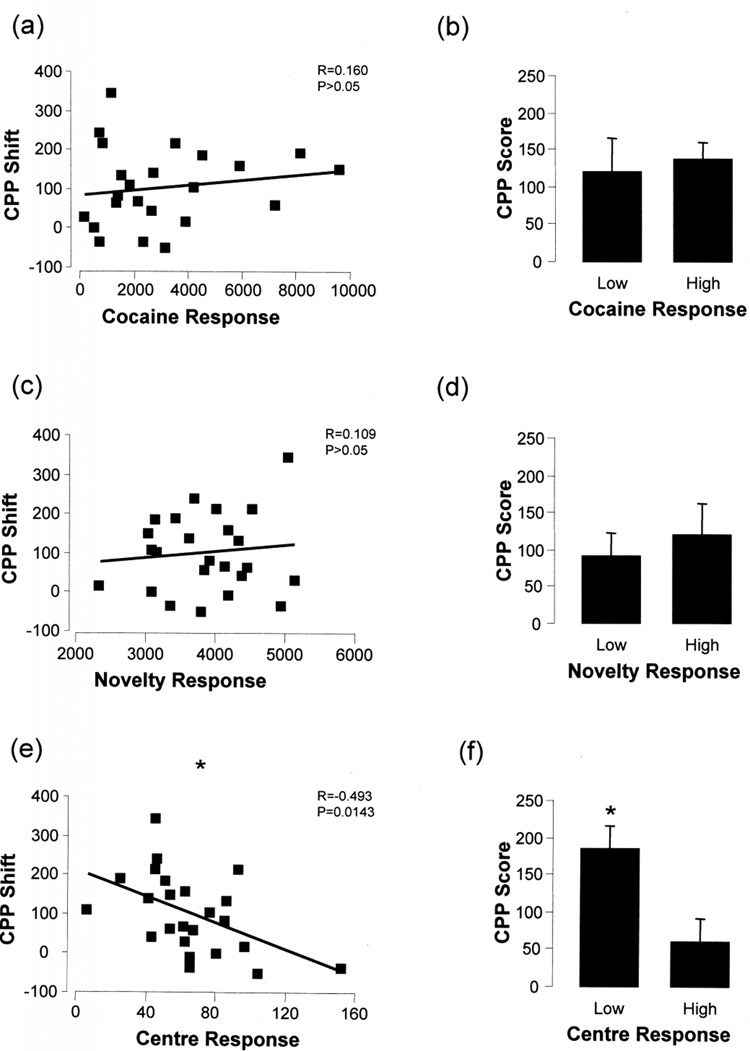
Since the 15 mg/kg cocaine conditioned group demonstrated a consistent shift in preference across the time bins and a significant sensitization in particular against the SC group, we decided to use this group for our correlation analysis. Table 1 illustrates the mean values for the low and high responder groups depicted in Figure 4 and Figure 5. A significant inverse correlation was observed between initial cocaine response (CR) and sensitization score (R=−0.474, p=0.0192; Pearson Correlation, Fig 4a). Division into low (LCR) and high (HCR) cocaine responders was done by splitting the group into three, taking the bottom third as LCR and the top third as HCR. The LCR group had a trend for greater sensitization scores compared to the HCR group, however, due to the large variability in the HCR group this was not significant (p=0.124; Student's t-test, Fig 4b). There was no significant correlation between novelty response and sensitization score (R=0.178, p=0.407; Fig 4c) and division into low (LNR) and high (HNR) responder groups did not yield any significant differences between the two groups (p=0.525, Fig 4d). Also, no significant correlation was detected between the centre response (CtR) and the sensitization score (R=0.211, p=0323; Fig 4e) and no significant differences were found after division into low (LCtR) and high (HCtR) responder groups (p=0.564, Fig 4f). Using a median split for these groups such as is employed by Sabeti et al., 2003 we still observed the same trends (data not shown).
Table 1.
Determination of responder groups by thirds split
| Median | Low | High | |
|---|---|---|---|
| Cocaine (cm) | 2289 | 885 ± 150 | 5906 ± 789 |
| Novelty (cm) | 3884 | 3088 ± 117 | 4633 ± 128 |
| Centre (entries) | 63 | 39 ± 5 | 97 ± 8 |
Data for low and high responders is represented as mean ± SEM (cm or entries).
Figure 4. Relationship between behavioral sensitization and cocaine, novelty or centre response in cocaine-treated animals.
A significant inverse correlation was observed between the sensitization score and the initial cocaine response (a) and the average sensitization score for the low cocaine responders significantly greater than the high cocaine responders (b). No relationship was determined between sensitization score and novelty response (c) and there was no difference between low and high novelty responders in their average sensitization scores (d). Similarly, no significant correlation was detected between the sensitization score and the centre response (e), and there was no significant difference between low and high centre responders in their average sensitization scores (f). Statistical significance is indicated by an asterisk with p<0.05.
Figure 5. Relationship between conditioned place preference and either cocaine or novelty response in cocaine-treated animals.
No significant correlations were observed between the CPP score and the initial cocaine response (a) or novelty response (c). The average CPP score for the cocaine responders (b) or the novelty responders (d) did not show any difference between their responder groups. However, there was a significant inverse relationship detected between the CPP score and the centre response (e), with the average CPP score for the low centre responders significantly higher than the high centre responders (f). Statistical significance is indicated by an asterisk with p<0.05.
We decided to use the CPP shift in preference value for the 0–15 min time bin as the CPP score in these analyses. In contrast to the relationship with sensitization score, there was no significant correlation or trend between the CPP score and CR (R=0.160, p=0.454, Fig. 5a) and further divisions into LCR and HCR subgroups did not produce any significant results (p=0.756, Fig. 5b). There was no significant correlation between novelty response and CPP score (R=0.109, p=0.611, Fig. 5c); and division into LNR and HNR subgroups did not yield any significant difference (p=0.593, Fig 5d). However, there was significant inverse relationship between CPP and the centre response (R=−0.493, p=0.0143, Fig 5e) and the LCtR group had significantly higher CPP scores than the HCtR group (p=0.015, Fig 5f).
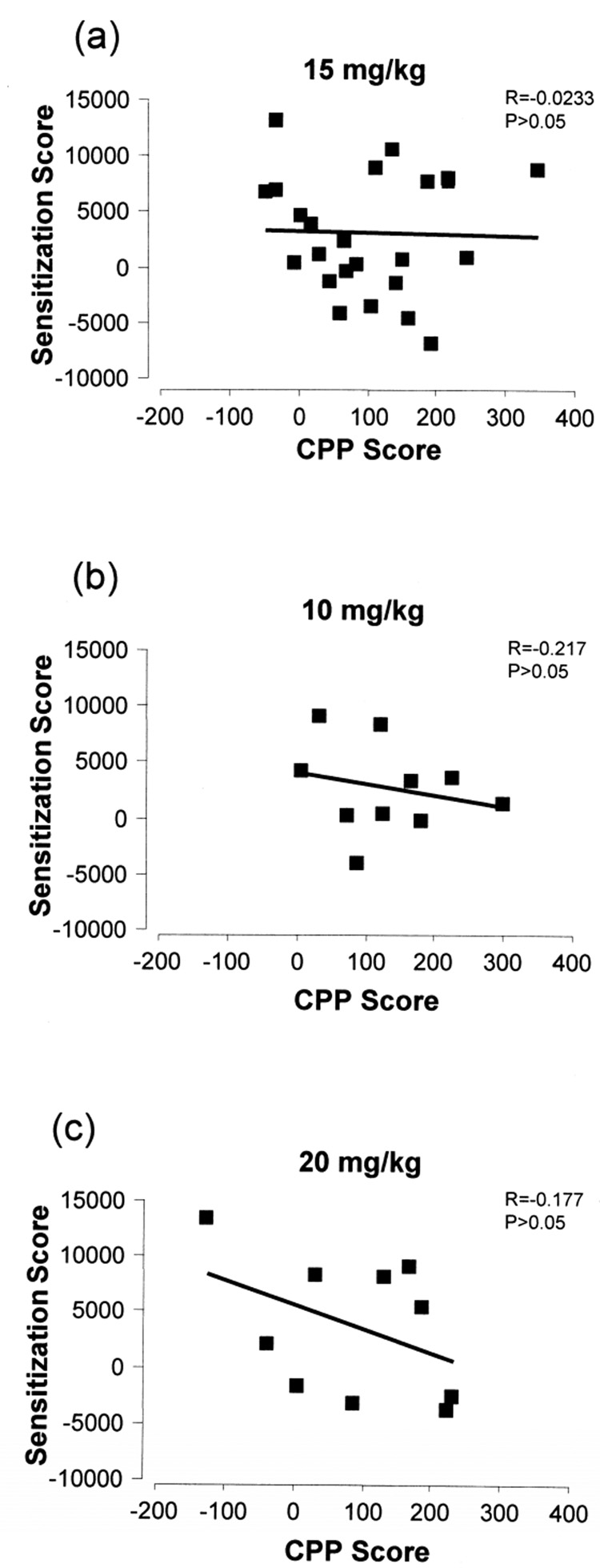
Correlation between CPP and behavioral sensitization
In contrast with our initial expectations, we did not find a significant relationship between sensitization score and CPP score (R=−0.0233, p=0.914, Fig. 6a). This did not change depending on the time bin examined (10 min: R=−0.157, p=0.464; 20 min: R=−0.0964, p=0.654; 30 min: R=0.114, p=0.596; data not shown). Furthermore, neither the 10 mg/kg (R= −0.217, p=0.548, Fig. 6b) nor the 20 mg/kg (R=−0.177, p=0.625, Fig. 6c) cocaine conditioned group demonstrated a significant correlation between sensitization score and CPP score. Thus, an individual animal’s response in one of these measures was not consistently indicative of its response in the other. A criticism of correlational analysis is the lack of consideration for the correlation in the control group (Quertemont et al., 2004). All correlations described here were examined within the saline (SS) group and no significant correlations were observed (data not shown).
Figure 6. Lack of relationship between behavioral sensitization and conditioned place preference.
The sensitization score of each 15 mg/kg cocaine-conditioned animal was plotted against its’ CPP score (a). Furthermore, there was no significant correlation between sensitization and conditioned place preference in animals conditioned with 10 (b) and 20 (c) mg/kg cocaine.
3. Discussion
In the current study, we have described an effective methodology for the study of the behavioral actions of cocaine involving the simultaneous induction of conditioned place preference and behavioral sensitization. This approach has resulted in the unexpected finding that performance in one measure is not suggestive of performance observed in the other for an individual animal. Both sensitization of the locomotor response and the place preference response following repetitive, intermittent conditioning with cocaine have been suggested to reflect behavioral changes that can be related to the addictive properties of the drug (Bardo et al., 1995; Robinson and Berridge, 1993, 2003). The observation here that a given individual rat may exhibit strong sensitization without concomitant place preference (and vice versa) indicates that at a minimum, both behavioral measures cannot be used independently as indicators of the neuroadaptations that underlie the state of addiction.
While behavioral sensitization can be successfully induced in a group of animals, on an individual basis the results can vary enormously (Segal and Kuczenski, 1987; Mayfield et al., 1992; Pierce et al., 1996) as can the susceptibility to the drug itself (Piazza and Le Moal, 1996). Often groups of animals are examined with respect to their acute locomotor response to the drug in order to investigate individual differences. In agreement with other studies (Sabeti et al., 2003), we have demonstrated a significant inverse correlation between sensitization and initial cocaine response, with low cocaine responding animals demonstrating, on average, greater levels of sensitization compared to high cocaine responders. Furthermore, our use of a sensitization score which is based on the difference between horizontal counts on the challenge and activity days avoids the criticism of possible “spurious conclusions” derived from the use of a sensitization score based on the ratio of the horizontal counts on the challenge and activity day (Quertemont et al., 2004). This inverse correlation between sensitization score and initial cocaine response may be due to underlying alterations in dopamine transport, since low and high cocaine responding rats differ in their dopamine uptake (Briegleb et al., 2004) and clearance (Sabeti et al., 2002, 2003) without changes in the level of cocaine or the number and affinity of the dopamine transporter (Gulley et al., 2003).
Another method to distinguish individual differences is to assess the initial locomotor response to a novel environment (Gong et al., 1996; Brabant et al., 2005). While novelty response has been demonstrated to be a predictor of sensitization to amphetamine and cocaine (Piazza et al., 1989; Hooks et al., 1991), morphine (Kalinichev et al., 2004) and methylphenidate (Wooters et al., 2006), we found no differences between our low and high responder rats or a significant correlation between novelty response and sensitization. Similarly, other groups have reported that cocaine sensitization effects were not reliably correlated with an animal’s initial locomotor activity level when the environment was novel (Carey et al., 2003; Sabeti et al., 2003) and locomotor responses to novelty did not significantly correlate with acute response to cocaine or the rate of the development of behavioral sensitization (Djano and Martin-Iverson, 2000).
Since activity in the centre of an enclosed open field is thought to be an indication of anxiety levels (Ennaceur et al., 2006), “anxious” animals will spend more time in the peripheral zones and less in the central one (Clement and Chapouthier, 1998). High centre responders may then be considered to be less anxious than low centre responders. Furthermore, analyzing activity in terms of central zones activity has been used as a method for identifying cocaine effects independent from habituation factors (Carey et al., 2005). We did not observe a significant relationship between the initial centre response on the activity day and sensitization score. This result suggests that anxiety level is not related to the development of sensitization using our approach measuring centre entries. However, it should be noted that if we had used a sensitization score based on the ratio of the beam breaks on the activity and challenge days, a significant positive correlation is observed between sensitization score and centre response (data not shown).
In the case of conditioned place preference, no correlations were apparent with either cocaine or novelty response. Previous studies have also failed to demonstrate a significant relationship between cocaine response and CPP (Kosten and Miserendino, 1998; Brabant et al., 2005). A recent study has demonstrated that division into low and high cocaine responders can predict the development of CPP to cocaine, but only when administration is by the intravenous and not intraperitoneal route (Allen et al. 2007). Thus, we may have observed a different relationship between cocaine response and CPP using an alternative route of cocaine administration. With respect to novelty response, morphine CPP seems to correlate well (Nadal et al., 2005; Pelloux et al., 2006), however results with amphetamine and cocaine vary (Erb and Parker, 1994; Gong et al., 1996; Shimosato and Watanabe, 2003; Pelloux et al., 2004; Brabant et al., 2005; Dietz et al., 2007). It could be argued that the main conditioning dose of cocaine (15 mg/kg) used in our correlation analysis was too high to show a significant relationship with the novelty response, as both Shimosato and Watanabe (2003) and Brabant et al. (2005) observed a significant correlation between CPP score and novelty response with low (4/5 mg/kg) but not with high (8/10, 12/20 mg/kg) doses of cocaine in mice. However, Gong et al. (1996) used three different doses of cocaine (2.5, 5 and 15 mg/kg) and did not find a difference between low and high novelty responders and their degree of preference in rats. Under our conditions, which were developed to induce both sensitization and place preference simultaneously, we similarly found no relationship between novelty response and CPP.
In contrast to our observations with sensitization, conditioned place preference was inversely correlated with centre response, as low centre responders displayed significantly greater CPP scores than high centre responders. Cocaine-associated cues were demonstrated to induce anxiety in the elevated plus-maze, which may inhibit certain behaviors (De Vries and Pert, 1998), and acute administration of cocaine to rats initially classified as non-anxious induces an anxiogenic-like effect in the elevated plus-maze which was not observed in the anxious group (Rogerio and Takahashi, 1992). It may be that high centre responders are less likely to acquire a conditioned place preference due to their heightened anxiety and increasing levels of hyperactivity during the cocaine conditioning trials. This would then prevent these animals from developing an association between environmental cues and the rewarding aspects of cocaine. Thus in contrast to our sensitization results, CPP cannot be correlated with the initial locomotor response to drug, and furthermore it exhibits an inverse relationship with the centre response.
While sensitization and conditioned place preference are generally considered separate phenomena, pre-treated or sensitized animals do show significantly enhanced CPP relative to control animals (Lett, 1989; Shippenberg and Heidbreder, 1995; Simpson and Riley, 2005). Interestingly, we have previously shown pre-treatment with cocaine results in enhanced long-term potentiation (LTP), an affect which could be expected to enhance learned associations such as those involved in CPP (Thompson et al., 2002). Thus, it was somewhat surprising that no apparent relationship between performances in both paradigms was evident in the current study in which sensitization and place preference were induced simultaneously. When combined with the differences noted above among the cocaine and centre responders, these observations suggest that individual performance in the sensitization and place preference behavioral assays cannot both be positively related to performance in a self-administration paradigm. In rats, there is reasonable agreement between CPP and self-administration when comparing the ability of each to detect the rewarding effects of drugs (Bardo and Bevins, 2000) and the conditioned place preference paradigm may model cue-elicited conditioning which induces drug-taking behavior (Bardo et al., 1995). One criticism of the CPP paradigm is that while the preference for the drug-paired environment might be thought of as drug seeking, time in the drug-paired compartment provides no indication of how hard an animal will work to obtain a drug (Olmstead, 2006). Indeed, CPP and self-administration differ in their reinstatement of responses by a conditioned fear stimulus (i.e. footshock); possibly reflecting that each of these paradigms is examining different aspects of behavioral responses (Sanchez and Sorg, 2001). Therefore, the assumption that extinction/reinstatement studies using the CPP assay can be employed as a surrogate for the drug self administration assay must be approached with caution, despite the practical advantages of the former approach (see Bardo and Bevins, 2000 and Tzschentke, 2007 for discussion).
Regardless of their respective strengths and weaknesses, the behavioral sensitization and place preference paradigms likely involve the assessment of different components of the neuroadaptations that are thought to occur in individuals exposed to drugs of abuse. It may also be that these outcomes (i.e. sensitization or CPP) are linked to either incentive salience or motivational reward, and perhaps both of these factors can work separately following drug exposure in a given individual. In this instance, it could be suggested that those having a strong tendency for responding in both aspects would represent the susceptible fraction, and an overall relationship between sensitization and CPP among the entire population may not be evident. Determining the relative importance of these two measures as being predictive of the addictive state is beyond the scope of our current results. If the testing times for either CPP or sensitization were changed, a different association might be observed. However, it does seem clear that the occurrence of either sensitization or conditioned place preference cannot be used to indicate the presence of the other in a given individual, and therefore both cannot be taken as being positive predictors of the susceptibility of that individual to drug abuse.
4. Experimental Procedures
Materials
Cocaine hydrochloride and general lab supplies were obtained from Sigma (St. Louis, MO, USA).
Animal Maintenance
Eighty male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) were housed in pairs in clear plastic cages and maintained on a 12 h light/dark cycle (0700/1900 h). Food and water were available ad libitum except during the behavioral sessions. Animals were allowed to adapt to the lab conditions for a week before behavioral testing began. Each pair of animals was randomly assigned to a treatment group. Behavioral sessions were conducted daily between 0900 and 1700 h. These studies were approved by the University of Georgia Institutional Animal Care and Use Committee and conducted in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Apparatus
The apparatus and measurement of activity have been described in detail elsewhere (Gosnell, 2005). Briefly, activity was measured in four 43.2 × 43.2 cm chambers with clear plastic walls and a solid smooth floor (Med Associates, St. Albans, VT, USA). The chambers were individually housed in sound-attenuating cubicles equipped with a house light (approximately 20 lux) and a ventilation fan. Two banks of 16 infrared photobeams and detectors, mounted at right angles 3.5cm above the floor, detected horizontal activity. Activity Monitor software (Med Associates) was used to count photobeam breaks. This software subdivides total counts into stereotypic and ambulatory counts according to the repetitive (stereotypic) vs. sequential (ambulatory) patterning of the beam-breaks.
For the conditioned place preference (CPP) experiments, a two compartmental insert (Med Associates) was used to modify the chamber. The insert was divided into two equal-sized compartments (42.8 × 21.3 cm) by a black partition that was penetrable by infrared light. The partition contained a guillotine door which could be removed to allow access to both compartments or inserted to confine the animal’s movements to one compartment. The compartments differed in floor type (wire mesh – “grid” vs. steel bars – “rod”) and by ceiling color (transparent vs. black). The compartment with the rod-floor had no light source (black ceiling) since preliminary studies indicated that this would give an equal preference between compartments. The beam heights were adjusted so that they were mounted 3.5cm above the floor of the insert.
The context in which the initial and challenged activity is measured is similar to the context to which the CPP is developed. Each rat was assigned a box and received all sessions in the same activity box, sound attenuating cubicle (thus the lighting and fan were the same) and same room. The only difference between the initial and challenged activity sessions and the CPP sessions is the addition of the CPP insert which alters the floor context, the lighting context and decreases by 50% the amount of floor space available to the animal.
Experimental Design
Animals were concurrently analyzed for conditioned place preference and behavioral sensitization over a period of thirteen days. In the pre-test session, animals were placed in the grid-floored compartment and given free access to either compartment of the CPP insert for 30 min (Protocol day 1). Upon completion of the pre-test session, time spent in each compartment was analyzed to ensure that no animal had a bias for a particular compartment indicated by spending greater than 65% of the total time in that compartment. For each pair of animals regardless of treatment, the animal showing the most bias towards one of the compartments was drug/vehicle-paired with the non-preferred compartments. The second animal was then drug/vehicle-paired with opposite chamber regardless of its preference in order to avoid a biased experimental design (thus, in each treatment group, there were an equal number of animals paired with each compartment; grid vs. rod). Following the pre-test session, the CPP inserts were removed and the beam heights were lowered to the correct height (3.5) above floor level. The next day, animals were placed in the centre of the activity chambers for 30 min to establish a baseline activity for each animal (Protocol day 2). Afterwards they were given an i.p. injection of either 10 mg/kg cocaine (n=42) or saline (n=29) and returned to the chambers for an additional 60 min of monitoring.
During conditioning sessions (Protocol days 3–6), the CPP inserts were replaced and the beams were readjusted. The animals were injected i.p. with either cocaine HCl salt (10n=8/15n=24/20n=10 mg/kg) or 0.9% saline (n=29) and then confined in one of the two compartments (as determined on the pre-test day) for 15 min. Four hours later the animal was injected with either cocaine or saline and confined to the opposite compartment. The order of the injections was rotated daily to prevent the animal developing an association between the drug and the time of day. Activity during the conditioning sessions was measured to monitor the development of behavioral sensitization assessed by progressive increases in activity across the conditioning sessions. The day after the last conditioning session the animals were tested for a place preference on the first drug-free day (Protocol day 7). Animals were placed in their saline-paired chamber and allowed free access to both compartments for 30 min during which the time spent in each was measured. Six days after the place preference test (seven drug-free days), the animals were challenged with cocaine or saline (Protocol day 13). The animals were placed in the activity chambers (without the CPP inserts) for 30 min to establish a baseline activity. Afterwards they were challenged with an i.p. injection of 10 mg/kg cocaine or saline and returned to the chambers for an additional 60 min. Thirteen of the original saline treatment group were challenged with cocaine.
An additional group conditioned with cocaine but challenged with saline is often included in a sensitization protocol to detect conditioned activity effects induced by the environment which are separate from drug-induced effects. Our protocol includes a 30 min habituation period before any drug injections. If there was a significant difference between the treatment groups during this period that would warrant the inclusion of a cocaine-saline group to discriminate between drug and environment conditioned activity. However, there was no difference in the activity levels of any of our groups during the 30 minute period of the challenge day (Fig 3) and thus the changes in activity following i.p. injection are due to drug-induced effects, not environmentally conditioned effects.
Statistical Analysis
All statistical analysis was complied using SigmaStat for windows version 3.11 (SPSS Science, Chicago, IL). Two-way ANOVA was used to analyze the percentage of time spent in the drug-paired compartment (with two factors: treatment × test), while one-way ANOVA was used to analyze the shift in preference for the conditioned place preference results; both with Holm-Sidak post-hoc analysis. Five vehicle (3 SS and 2 SC), two 10 mg/kg and two 15 mg/kg cocaine-treated animals were removed from the study for having a greater than 65% bias for either chamber during the pre-test. Conditioning days (treatment × session) and baseline and post-injection activity (treatment × time) for the behavioral sensitization experiment were analyzed by two-way Repeated Measures ANOVA followed by Holm-Sidak post-hoc analysis. In the sensitization study there were five treatment groups: SS animals (n=18) were injected with saline during the activity, conditioning and challenge sessions, SC animals (n=11) were administered saline during the activity and conditioning sessions but challenged with cocaine, and CC animals (further divided into CC10 (n=8), CC15 (n=24) and CC20 (n=10) groups) which were injected with cocaine during the activity, conditioning and challenge sessions. SS and SC treatment group results for the activity session were pooled as were the results for the CC10, CC15 and CC20 groups since there is no difference in drug treatment before this day. Behavioral sensitization data was analyzed by combining the ambulatory counts and stereotypic counts to get the total horizontal counts (total number of horizontal beam breaks) for each animal.
For correlation analysis, the level of activity of each rat during the CPP pre-test (Protocol day 1) was used as a measure of their novelty response (NR). The cocaine response (CR) was based on the level of activity for each rat during the 30 min following i.p. injection of cocaine in the activity session (Protocol day 2) while the centre response (CtR) was determined using the number of entries into the centre zone (approximately one ninth the area of the open field) during the baseline of the activity session. The sensitization score was defined as the difference between the cocaine-stimulated locomotor activity during the first 30 min after injection in the challenge session and the activity session while the shift in preference value (change in time spent in drug-paired compartment at the CPP test and Pre-test).was used to represent the CPP score. Correlation analysis was analyzed by Pearson Product Moment Correlation. Differences were considered statistically significant at p < 0.05.
Acknowledgements
This work was supported by the National Institute on Drug Abuse grant DA016302.
Footnotes
Abbreviations
CC: cocaine/cocaine treatment, CPP: conditioned place preference, CR: cocaine response, CtR: centre response, H: high, i.p.: intraperitoneal, L: low, NR: novelty response, SC: saline/cocaine treatment, SS: saline/saline treatment.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM. Time-dependent sensitization as the cornerstone for a new approach to pharmacotherapy: drugs as foreign/stressful stimuli. Drug Development Research. 1988;14:1–30. [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Tirelli E. Evidence that the relations between novelty-induced activity, locomotor stimulation and place preference induced by cocaine qualitatively depend upon the dose: a multiple regression analysis in inbred C57BL/6J mice. Behav Brain. Res. 2005;158:201–210. doi: 10.1016/j.bbr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. Individual difference in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: Contribution of the Dopamine Transporter. Neuropsychopharmacology. 2004;29:2168–2179. doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- Carey R, Gui J. A simple reliable method for the positive identification of pavlovian conditioned cocaine effects in open-field behavior. Journal of Neuroscience Methods. 1997;73:1–8. doi: 10.1016/s0165-0270(96)02203-0. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Response to novelty as a predictor of cocaine sensitization and conditioning in rats: a correlational analysis. Psychopharmacology. 2003;168:245–252. doi: 10.1007/s00213-003-1443-9. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Acute and chronic cocaine behavioral effects in novel versus familiar environments: open-field familiarity differentiates cocaine locomotor stimulant effects from cocaine emotional behavioral effects. Behav Brain Res. 2005;158:321–330. doi: 10.1016/j.bbr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Clement Y, Chapouthier G. Biological bases of anxiety. Neurosci Biobehav Rev. 1998;22:623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Nobel D. Conditioned activation induced by ethanol: Role in sensitization and conditioned place preference. Pharm Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect but no change in rewarding effect. Psychopharmacology. 2002;160:414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotrophin-releasing factor. Psychopharmacology. 1998;137:333–340. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- Dietz D, Wang H, Kabbaj M. Corticosterone fails to produce conditioned place preference or conditioned place aversion in rats. Behav Brain Res. 2007;181:287–291. doi: 10.1016/j.bbr.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djano S, Martin-Iverson MT. Does locomotor response to novelty in rats predict susceptibility to develop sensitization to cocaine and PHNO? Behav Pharmacol. 2000;11:455–470. doi: 10.1097/00008877-200009000-00003. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: Responses of rats to noelty in an open space and an enclosed space. Behav Brain Res. 2006;171:26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharm Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharm Biochem Behav. 1996;53:191–196. [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: Behavioral characteristics, cocaine pharmacokinetics and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharm Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, White DA, Holtzman SG. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. I. Expression of morphine-induced locomotor sensitization. Psychopharmacology. 2004;177:61–67. doi: 10.1007/s00213-004-1990-8. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD. Dissociation of novelty- and cocaine-induced conditioned locomotor activity from cocaine place conditioning. Pharm Biochem Behav. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Reimer AR. Classically conditioned motor effects do not occur with cocaine in an unbiased conditioned place preference procedure. Behav Pharmacol. 1996;7:303–314. doi: 10.1097/00008877-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Larson G, Zahniser NR. Cocaine-induced behavioral sensitization and D1 dopamine receptor function in rat nucleus accumbens and striatum. Brain Res. 1992;573:331–335. doi: 10.1016/0006-8993(92)90783-6. [DOI] [PubMed] [Google Scholar]
- Nadal R, Rotllant D, Márquez C, Armario A. Perseverance of exploration in novel environments predicts morphine place conditioning in rats. Behav Brain Res. 2005;165:72–79. doi: 10.1016/j.bbr.2005.06.039. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology. 1996;123:144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyakawa T, Nakamura K, Kitano J, Furushima K, Kiyonari H, Nakayama R, Nakao K, Moriyoshi K, Nakanishi S. Altered sensitivities to morphine and cocaine in scaffold protein tamalin knockout mice. PNAS. 2007;104:14789–14794. doi: 10.1073/pnas.0706945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC. Animal models of drug addiction: Where do we go from here? Q J Exp Psychol. 2006;59:625–653. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 in bred strains. Psychopharmacology. 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Constentin J, Duterte-Boucher D. Differential effects of novelty exposure on place preference conditioning to amphetamine and its oral consumption. Psychopharmacology. 2004;171:277–285. doi: 10.1007/s00213-003-1584-x. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Novelty preference predicts place preference conditioning to morphine and its oral consumption in rats. Pharm Biochem Behav. 2006;84:43–50. doi: 10.1016/j.pbb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Demininere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Brabant C, Tirelli E. Response to novelty as a predictor for drug effects: the pitfalls of some correlational studies. Psychopharmacology. 2004;173:221–224. doi: 10.1007/s00213-004-1796-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus maze. Pharm Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters acumbens and striatal dopamine clearance in low and high cocaine locomotor responders: Behavioral and electrochemical recordings in freely moving rats. JPET. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. JPET. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Sorg BA. Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res. 2001;908:86–92. doi: 10.1016/s0006-8993(01)02638-5. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Trends in place preference conditioning with a cross-indexed bibliography; 1957–1991. Neurosci Biobehav Rev. 1993;17:21–41. doi: 10.1016/s0149-7634(05)80228-3. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Continued trends in the conditioned place preference literature from 1992 to 1996, inclusive, with a cross-indexed bibliography. Neurosci Biobehav Rev. 1998;22:827–846. doi: 10.1016/s0149-7634(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Uterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology. 2007;195:265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristic and neurochemical correlates. JPET. 1987;242:917–926. [PubMed] [Google Scholar]
- Shimosato K, Ohkuda S. Simultaneous monitoring of conditioned place preference and locomotor sensitization following repeated administration of cocaine and methamphetamine. Pharmacol Biochem Behav. 2000;66:285–292. doi: 10.1016/s0091-3057(00)00185-4. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Watanabe S. Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. J Neurosci Methods. 2003;128:103–110. doi: 10.1016/s0165-0270(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: Pharmacological and temporal characteristics. JPET. 1995;273:808–815. [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology. 1977;54:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharm Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Heilig M. Addiction and its brain science. Addiction. 2005;100:1813–1822. doi: 10.1111/j.1360-0443.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology. 2002;42:1039–1042. doi: 10.1016/s0028-3908(02)00059-x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Blockade of behavioral sensitization by MK-801: fact or artifact? Psychopharmacology. 2000;151:142–151. doi: 10.1007/s002130000395. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbillé A-G, Bertram-Gonzalez J, Hervé D, Girault J-A. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. PNAS. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH, Galuska CM, Wade-Galuska T. Behavioral perspectives on the neuroscience of drug addiction. Journal of the Experimental Analysis of Behavior. 2005;84:667–681. doi: 10.1901/jeab.2005.101-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology. 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]