Abstract
To examine whether age influences taste solution preferences, we measured taste preferences of C57BL/6J and 129X1/SvJ mice given a series of 48-h 2-bottle tests with a choice between water and one of the following taste solutions: 2mM saccharin, 5mM citric acid, 30 μM quinine hydrochloride, 75 mM sodium chloride (NaCl), 10 mM inosine monophosphate (IMP), 50 mM calcium chloride (CaCl2), and 10% ethanol. We tested separate groups of male mice fed Teklad 8604 chow at ages 4, 6, 9, 12, 15, 20, 25, 30, 40, and 50 weeks and retested some of these mice at 54, 75, and 100 weeks and again at 125 weeks. Female mice fed chow were tested at ages 4, 12, 25, and 50 weeks and retested at 54, 75, 100, and 125 weeks. Male mice fed AIN-93G semisynthetic diet were tested at ages 4, 12, 25, and 50 weeks and retested at 54, 75, and 100 weeks. Concentration-response functions for each taste solution were collected from male and female mice fed chow aged 8 or 125 weeks. In general, the results showed that age had little effect on taste preferences. Exceptions included 1) a small increase in quinine hydrochloride preference between 54 and 125 weeks in mice of both strains and sexes, 2) a marked increase in NaCl preference between 4 and 12 weeks in female B6 mice, 3) a gradual decrease in IMP preference between 4 and 125 weeks in male and female 129 mice, 4) a marked decrease in CaCl2 preference between 54 and 125 weeks in male and female 129 mice, and 5) a marked reduction in ethanol preference between 4 and 12 weeks in male B6 mice fed AIN-93G diet but not chow. These results show that over a wide range and with the exceptions noted, age contributes little to the variation in taste preferences observed in C57BL/6J and 129X1/SvJ mice.
Keywords: AIN-93G diet, bitter, calcium, ethanol, salty, sour, sweet, umami
Introduction
Rodents have often been used to study the development of taste perception, but there has been comparatively little interest in taste-related behavior at older ages. For the purposes of this article on laboratory mice, we consider “older ages” to include adolescence (~4 weeks old), puberty (~6 weeks old), early adulthood (6–50 weeks), and old age (50–150+ weeks). Anatomical, electrophysiological, and behavioral evidence suggests that throughout this period, the rodent taste system changes little except perhaps during extreme old age (Bloomquist and Candland 1965; Mistretta 1984, 1989; Mistretta and Baum 1984;Mistretta and Oakley 1986; Thaw 1996; Osada et al. 2003). However, even if taste transduction mechanisms do not change, there are several reasons to expect age-related changes in taste preferences. First, some “taste” solutions have trigeminal and olfactory components (Rhinehart-Doty et al. 1994; Capaldi et al. 2004), and sensitivity to these declines with age (Mistretta 1984; Nakayasu et al. 2000; Laska 2001; Frasnelli and Hummel 2003; Wysocki et al. 2003). Second, there may be neurochemical changes with age that affect the perceived value of rewards (e.g., Shram et al. 2006), which could influence the hedonic value of taste solutions. Third, increases in circulating steroid hormones associated with puberty could influence taste preferences (e.g., Wade and Zucker 1969; Krecek 1973; Chow et al. 1992; Bushong and Mann 1994). Fourth, taste preferences reflect postingestive status (e.g., Richter 1942–1943), and there are pronounced changes in the postingestive milieu with age (e.g., Thompson et al. 1988; Morita et al. 1994; Smriga et al. 2000). Unfortunately, the literature on taste preferences during aging is incomplete and often contradictory. The few data that are available cannot easily be synthesized into a coherent picture because they involve different rodent strains and species tested at different ages with different taste compounds (see Discussion).
The present study was part of a project that is designed to evaluate some of the methodological factors that influence the results of the 2-bottle taste preference test (Tordoff and Bachmanov 2001b). The overall goal is to improve the sensitivity of the test for use in gene discovery experiments, which involve accurately phenotyping many thousands of mice (e.g., Belknap 1998; Schimenti and Bucan 1998; Tordoff and Bachmanov 2001b). The major focus of the study presented here was to determine whether there was an optimal age range for testing mice in 2-bottle choice tests. We also investigated whether taste solution preferences were influenced by sex, diet, and extreme old age.
Materials and methods
Subjects and maintenance
The experiment involved 288 C57BL/6J (B6) and 288 129X1/SvJ (129) mice. The mice were purchased at the age of 21 days from The Jackson Laboratory (Bar Harbor, ME). They were shipped by truck to the Monell Center where they were housed in a vivarium maintained at 23 °C with a 12:12 h light/dark cycle (lights off at 7 PM).
Each mouse was housed alone in a plastic “tub” cage with a stainless steel wire lid and wood shavings scattered on the floor. The lid included space for pelleted food and a water bottle (see Tordoff and Bachmanov 2001b for details). All the mice had deionized water to drink and pellets of either Teklad 8604 chow or AIN-93G diet to eat. Teklad 8604 is a cereal-based “chow” diet produced by Harlan (Madison, WI). It contains roughly 245 g protein, 466 g carbohydrate, 44 g fat, and 37 g fiber per kilogram and has an energy content of ~3.1 kcal/g. AIN-93G is a semisynthetic diet with rigorously controlled ingredients (see Reeves et al. 1993). It contains 200 g protein (casein), 629 g carbohydrate (cornstarch and sucrose), 70 g fat (soybean oil), and 50 g fiber (cellulose) per kilogram and has an energy content of~3.8 kcal/g. It was produced and pelleted by Dyets Inc. (Bethlehem, PA; catalog no. 110700).
The mice were transferred to clean cages with fresh bedding once every week while waiting to be tested and once every 6 or 8 days during tests (so as not to disturb them during a 2-day test).
Design
Part 1
Shipping of the mice was arranged so that they all could be tested simultaneously in 2 identical replications offset by 1 day. All mice were fed the appropriate diet from the time they arrived at our institution (at age 23 days) until they were tested. Testing began when the mice were nominally the following ages: 4, 6, 9, 12, 15, 20, 25, 30, 40, or 50 weeks old. However, due to supply problems, some mice were not their nominal age; the 50-week old females were actually only 49 weeks 5 days old, some of the 25-week old group were actually only 24 weeks old, and the 4-week-old mice were actually 3 weeks 5 days old. The 4-week-old mice arrived 5 days before tests began.
Each of the 10 age cohorts consisted of 16 male mice of each strain fed Teklad 8604 chow. The cohorts at 4, 12, 25, and 50 weeks also included groups of 16 female mice of each strain fed Teklad 8604 chow and 16 male mice of each strain fed AIN-93G diet.
Part 2
After the tests in Part 1, all the mice in the 4-, 25-, and 50- week-old groups were maintained undisturbed for 50 weeks. When these mice (i.e., groups of both the males and females fed chow and males fed AIN-93G diet) were nominally 54, 75, and 100 weeks old, they were retested using the same procedures as in Part 1.
Part 3
This part involved a comparison of groups fed Teklad 8604 that were nominally 125 weeks old and 8 weeks old. The 125 week olds were formed from the 75- and 100-week-old groups tested in Part 2. The 100-week-old groups fed Teklad 8604 chow were maintained undisturbed until 125 weeks old, and the 75-week-old groups fed Teklad 8604 chow were maintained undisturbed until 120 weeks old. The latter groups were tested 5 weeks earlier than the former because of the high death rate of the former during subsequent tests (after age 125 weeks). AIN-93G-fed mice were not tested because few of them survived to 120 weeks (see Mortality, below). Each of the nominal 125-week groups was accompanied by experimentally naive “young” controls, consisting of 8-week-old mice that were shipped from the vendor 10–14 days before tests began and maintained under the same conditions as the 125-week-old mice. All these mice were tested using the same procedures as in Part 1.
Part 4
This part involved a more detailed characterization of the mice tested in Part 3. The mice continued to be fed Teklad 8604 chow while they received several series of various concentrations of 6 taste solutions, with the old group beginning at age ~127 weeks and ending at ~135 weeks and the young group beginning at age ~10 weeks and ending at ~18 weeks.
Test procedures
Body weight and food intake
Body weights of all mice were recorded at the beginning and end of the 16-day taste preference test series (see below). Mice in Parts 1 and 2 of the experiment also had their food intakes measured: After the end of the 16-day series, they received a single bottle of deionized water for 3 days. Then, the entire lid of the cage, including food but excluding the water bottle, was weighed, and 4 days later, it was reweighed (accuracy ± 0.1 g). Any spilled food found in bedding material was also weighed and accounted for. Daily food intakes were calculated by dividing the spillage-adjusted intakes by 4. Food intakes were converted to energy intakes (kcal) by multiplying Teklad 8604 chow weights by 3.1 and AIN-93G weights by 3.8.
Two-bottle test series
The following test procedure was used in Parts 1–3 of the experiment. The mice received a series of eight 2-bottle choice tests. Each test was 2 days long, so the entire test series lasted 16 days. The first test was a choice between 2 bottles of deionized water. Subsequent tests were a choice between deionized water and 2 mM sodium saccharin, 5 mM citric acid, 30 μM quinine hydrochloride (QHCl), 75 mM NaCl, 10 mM inosine monophosphate (IMP), 50 mM CaCl2, and 10% ethanol (v/v). The tests were always conducted in the order listed. The first 5 compounds were chosen to represent the 5 primary taste qualities (sweet, sour, bitter, salty, and umami). Calcium and ethanol were included because these are of particular interest to our group. The concentrations of each solution were chosen based on previous work (e.g., Bachmanov, Schlager, et al. 1998; Bachmanov, Tordoff, and Beauchamp 2001; Tordoff and Bachmanov 2001b, 2002a; Tordoff et al. 2002; Tordoff, Bachmanov, and Reed in press; Tordoff, Reed, and Bachmanov in press). All compounds were purchased from Sigma Chemical Corp. (St Louis, MO) except for the ethanol, which was purchased from Pharmco (Brookfield, CT). The solute for all compounds was deionized water. Solutions were made freshly before each test, but a few hundred milliliters were saved in plastic bottles to refill drinking tubes if needed.
During tests, each mouse was presented with 2 drinking tubes, according to Materials and Methods described in detail elsewhere (Tordoff and Bachmanov 2001b). Initially, the taste solution was placed on the left (from the mouse's viewpoint), but its position was switched after 24 h of each 48-h test to control for the left spout side preference of the 129 strain (Tordoff and Bachmanov 2001a; Bachmanov et al. 2002).
Concentration-response series
In Part 4 of the experiment, mice were given several consecutive series of 2-bottle tests. They received 3 concentrations of each of the 7 compounds used in the initial 2-bottle test series. The tests involved 1, 3.16, and 10mM saccharin; 3.16, 10, and 31.6mM citric acid; 0.01, 0.1, and 1.0mM QHCl; 75, 150, and 300 mM NaCl; 1, 10, and 100 mM IMP; 3%, 10%, and 32% ethanol; and 7.5, 25, and 75 mM CaCl2. They were given in the order listed, with each concentration series preceded by a choice between 2 bottles of deionized water. Thus, the entire test series took 56 days. Other methods were similar to those used in the initial 2-bottle test series.
Data analyses and presentation
To reduce confusion, all groups are referred to by their nominal age when testing began. Daily intakes of taste solution and water for each test were calculated by dividing intakes obtained during each 2-day test by 2. Total fluid intakes were considered to be the sum of daily taste solution and daily water intakes. Taste solution preference scores were calculated according to the formula, preference = daily taste solution intake/daily total fluid intake × 100.
We treated data involving body weight, food intake, water intake, and preferences for each of the 7 taste solutions separately. The responses of the 20 groups of male mice fed Teklad 8604 chow in Part 1 were compared using 2-way analyses of variance (ANOVAs) with Strain and Age as between subjects factors. The responses of male and female mice aged 4, 12, 25, and 50 weeks were compared using 3-way ANOVAs with Strain, Sex and Age as between-subjects factors, and the responses of male mice aged 4, 12, 25 and 50 weeks and fed Teklad 8604 chow or AIN-93G diet were compared using 3-way ANOVAs with Strain, Diet and Age as between-subjects factors. The same factors were used to analyze the results of Parts 2 and 3 but with different ages as levels in the Age factor. The analysis of results from Part 4 involved an additional factor of Solution Concentration.
In addition to the omnibus analyses, we conducted planned comparisons based on 1-way ANOVAs for each strain and condition (male fed chow, female fed chow, male fed AIN-93G) to identify changes in taste solution preferences related to age. Tukey's post hoc tests were used to isolate the source of interactions in the omnibus ANOVAs and to determine homogenous groups. Because of the large number of analyses conducted, the criterion for statistical significance of all tests was set at P < 0.01.
Results
For brevity, here we present the results related to mortality, body weight, food intake, total water intake, and taste solution preferences. Analyses of taste solution intakes, water intakes during tests with taste solutions available, and total fluid intakes during tests with taste solutions available are available on line (Tordoff and Bachmanov 2001b).
Mortality
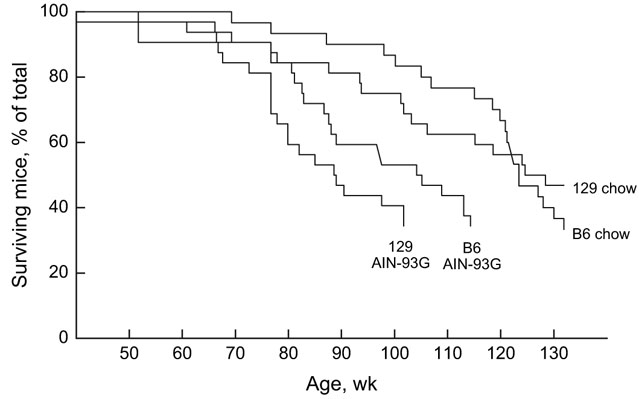
Several mice died during the course of this study. We did not conduct a formal statistical analysis of mortality rates because of the complexity involved with different numbers of mice given each treatment that were retired at different times. However, survival functions (Figure 1) show that 1) mice fed Teklad 8604 chow generally outlived those fed AIN-93G diet, 2) male mice fed Teklad 8604 chow generally lived slightly longer than did female mice fed Teklad 8604 chow, and 3) overall, B6 and 129 mice died at about the same age. The age at which half the mice in each group died was as follows: B6-male-chow = 123 weeks, 129-male-chow = 125 weeks, B6-female-chow = 116 weeks, 129-female-chow = 105 weeks, B6-male-AIN-93G = 104 weeks, and 129-male-AIN-93G = 99 weeks.
Figure 1.
Survival functions for male C57BL/6J (B6) and 129X1/SvJ (129) mice fed either Teklad 8604 chow or AIN-93G diet. Each of the 4 groups began with 32 mice. Females (data not shown) fed chow survived about as long as did the males fed chow.
For all analyses except those involving concentratio-nresponse functions, mice that died during a test series were discounted from the entire series. Fifteen of the 44 125-week-old mice died during the 56-day concentration-response series of tests in Part 4. These animals were discounted from the series they did not complete but not earlier series with other taste compounds. In no case when a mouse died during tests did we observe unusually low intakes (that might indicate illness) on the day before.
Part 1: mice aged 4, 6, 9, 12, 15, 20, 25, 30, 40, and 50 weeks
Male 4- to 50-week-old mice fed Teklad 8604 chow
As expected, the 2 strains gained weight progressively with age (Table 1). At most ages, the B6 strain weighed slightly more than did the 129 strain, but this was significant only at 40 weeks (Tables 1 and 2). Food intakes were fairly constant. They did not change with age, except that the oldest (50 weeks) mice ate more than some of the younger groups. Water intakes did not change with age, except that the youngest (4 weeks old) B6 mice drank significantly more than some of the older groups of this strain. Over all 20 groups combined, the 129 strain ate significantly more chow and drank significantly less water than did the B6 strain (Tables 1 and 2).
Table 1.
Part 1—number of mice in each age group, body weights, food intakes, and water intakes
| Condition and age (weeks) |
Group size |
Body weight (g) |
Food intake (kcal/d) |
Water intake (ml/day) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B6 | 129 | C57BL/6J | 129X1/SvJ | C57BL/6J | 129X1/SvJ | C57BL/6J | 129X1/SvJ | |||
| Males fed Teklad 8604 | ||||||||||
| 4 | 16 | 16 | 13.3 ± 0.3a | 10.9 ± 0.5a | 11.0 ± 0.2a | 12.1 ± 0.2a | 6.0 ± 0.2d | 5.1 ± 0.2a | ||
| 6 | 16 | 16 | 21.3 ± 0.4b | 21.1 ± 0.4b | 11.3 ± 0.3a,b | 11.9 ± 0.2a | 5.3 ± 0.1a–d | 5.2 ± 0.1a | ||
| 9 | 16 | 16 | 24.9 ± 0.2c | 23.8 ± 0.8b,c | 11.8 ± 0.3a–c | 12.1 ± 0.3a | 5.2 ± 0.2a–d | 4.3 ± 0.2a | ||
| 12 | 16 | 16 | 27.7 ± 0.4c | 26.8 ± 0.7c,d | 11.9 ± 0.2a–c | 12.9 ± 0.3a,b | 4.6 ± 0.2a–c | 4.8 ± 0.2a | ||
| 15 | 16 | 16 | 28.0 ± 0.5c,d | 27.2 ± 0.4c,d | 11.7 ± 0.3a–c | 12.4 ± 0.2a | 4.7 ± 0.1a–c | 4.4 ± 0.1a | ||
| 20 | 16 | 15 | 30.6 ± 0.7c,d | 28.4 ± 0.8d,e | 11.4 ± 0.2a | 12.1 ± 0.3a | 4.6 ± 0.2a–c | 4.4 ± 0.3a | ||
| 25 | 16 | 16 | 30.7 ± 1.0d | 28.2 ± 0.5d,e | 11.5 ± 0.3a,b | 12.3 ± 0.3a | 4.4 ± 0.2a,b | 4.4 ± 0.2a | ||
| 30 | 16 | 16 | 32.2 ± 0.4d | 30.9 ± 0.7e,f | 11.0 ± 0.4a | 12.1 ± 0.3a | 5.7 ± 0.2c,d | 4.7 ± 0.4a | ||
| 40 | 16 | 15 | 37.4 ± 0.7e | 32.4 ± 1.0f,* | 12.9 ± 0.3c | 12.8 ± 0.3a,b | 4.7 ± 0.2a–c | 4.0 ± 0.3a | ||
| 50 | 16 | 16 | 36.2 ± 0.9e | 33.9 ± 0.8f | 12.9 ± 0.3b,c | 13.9 ± 0.4b | 4.7 ± 0.2a–c | 3.9 ± 0.2a | ||
| Females fed Teklad 8604 | ||||||||||
| 4 | 16 | 16 | 11.5 ± 0.2a | 10.0 ± 0.4a | 9.8 ± 0.2a | 10.4 ± 0.3a | 5.2 ± 0.1a | 4.5 ± 0.2a | ||
| 12 | 16 | 16 | 21.8 ± 0.2b,† | 21.4 ± 0.5b,† | 11.8 ± 0.3b | 10.8 ± 0.3a,b | 5.3 ± 0.3a | 4.6 ± 0.3a | ||
| 25 | 16 | 16 | 24.7 ± 0.3c,† | 21.6 ± 0.3b,† | 10.9 ± 0.4a,b | 9.8 ± 0.3a,b | 6.2 ± 0.3a,*,** | 3.7 ± 0.2a | ||
| 50 | 16 | 16 | 26.4 ± 0.5d,† | 24.1 ± 0.5c,† | 11.7 ± 0.4b | 11.5 ± 0.4b | 5.9 ± 0.3a,*,** | 4.4 ± 0.3a | ||
| Males fed AIN-93G | ||||||||||
| 4 | 16 | 15 | 13.8 ± 0.4a | 10.1 ± 0.5a,* | 11.2 ± 0.1a | 11.9 ± 0.2a | 3.9 ± 0.1a | 3.2 ± 0.2a,*,** | ||
| 12 | 16 | 14 | 27.4 ± 0.4b | 28.7 ± 0.5b | 16.0 ± 0.4c | 15.7 ± 0.2a | 3.4 ± 0.3a | 3.2 ± 0.3a,† | ||
| 25 | 16 | 14 | 38.0 ± 0.7c,† | 33.3 ± 1.5b,c,*,** | 13.2 ± 0.3b | 11.9 ± 0.4a | 4.0 ± 0.3a | 4.2 ± 0.7a | ||
| 50 | 16 | 14 | 46.5 ± 0.7d,† | 35.7 ± 1.2a,* | 13.6 ± 0.4b | 11.9 ± 0.4a | 3.9 ± 0.3a | 3.7 ± 0.6a | ||
Letter superscripts show homogenous groups of mice according to strain and dependent variable. For each of the 3 sections of each column, groups with the same letter do not differ significantly from each other, according to post hoc Tukey's tests (P < 0.01).
P < 0.01, comparison between strains at the same age;
P < 0.01, comparison with males fed Teklad 8604 chow of the same strain and age.
Table 2.
Results of Part 1 ANOVAs comparing male B6 and 129 mice fed chow at 10 different ages
| Measure | Strain (1,293) |
Age (9,293) |
Strain × Age (9,293) |
|
|---|---|---|---|---|
| Body weight | 18.4* | 230.4* | 9.95* | |
| Food intake | 34.0* | 10.4* | 1.07 | |
| Water intake | 31.9* | 8.84* | 2.37 | |
| Preferences | ||||
| Saccharin | 1009.9* | 1.71 | 2.06 | |
| Citric acid | 0.05 | 2.73* | 2.27 | |
| QHCl | 4.90 | 5.16* | 1.43 | |
| NaCl | 23.9* | 1.50 | 3.27* | |
| IMP | 169.0* | 4.59* | 1.15 | |
| CaCl2 | 201.0* | 1.19 | 1.27 | |
| Ethanol | 469.7* | 1.84 | 3.54* | |
Values in parentheses are degrees of freedom. Levels: strain = B6 or 129; age = 4, 6, 9, 12, 15, 20, 25, 30, 40, or 50 weeks.
P < 0.01.
There were Strain × Age interactions influencing NaCl and ethanol preference and main effects of Age influencing citric acid, QHCl, and IMP preference (Table 2). Relative to some of the older groups, B6 mice aged 4 weeks had significantly lower preferences for citric acid and higher preferences for IMP, and 129 mice aged 4 weeks had significantly lower preferences for ethanol and higher preferences for IMP (Table 3). Once mice were 6 weeks old, there were no progressive changes with age in preference for any taste solution.
Table 3.
Part 1—differences in solution preferences among various groups of mice
| Preference | Strain | Age (weeks) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males fed Teklad chow |
Females fed chow |
Males fed AIN-93G |
|||||||||||||||||
| 4 | 6 | 9 | 12 | 15 | 20 | 25 | 30 | 40 | 50 | 4 | 12 | 25 | 50 | 4 | 12 | 25 | 50 | ||
| Saccharin | B6 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| 129 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| Citric acid | B6 | a | ab | b | b | ab | b | ab | b | ab | b | a | a | a | a | a | ab | a | b |
| 129 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| QHCl | B6 | ab | ab | ab | ab | a | b | ab | ab | b | ab | a | a | a | a | a | a | a | a |
| 129 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| NaCl | B6 | ab | b | ab | ab | a | ab | ab | ab | ab | ab | a | b | b | b | a | a | a | a |
| 129 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| IMP | B6 | b | ab | ab | ab | b | ab | ab | ab | ab | a | a | a | a | a | a | a | a | a |
| 129 | b | ab | ab | ab | ab | ab | ab | ab | a | a | b | ab | ab | a | c | bc | ab | a | |
| CaCl2 | B6 | ab | b | ab | ab | ab | ab | ab | a | ab | ab | a | a | a | a | a | a | a | a |
| 129 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| Ethanol | B6 | ab | ab | ab | ab | ab | b | ab | ab | ab | a | a | a | a | a | b | a | a | a |
| 129 | a | ab | ab | ab | ab | ab | ab | ab | ab | b | a | a | a | a | a | a | a | a | |
Letters show homogenous groups of mice according to strain and dependent variable. For each row and for each of the 3 conditions, groups with the same letter do not differ significantly from each other according to post hoc Tukey's tests (P < 0.01). The groups are arranged from low (a) to high (b or c) mean values.
Relative to 129 mice, B6 mice had significantly higher preferences for saccharin, NaCl, IMP, and ethanol and significantly lower preferences for CaCl2. The 2 strains did not differ in preferences for citric acid or QHCl.
Male and female mice aged 4, 12, 25, or 50 weeks fed Teklad 8604 chow
The body weights of male and female mice of both strains did not differ significantly at 4 weeks but males weighed significantly more than did females at older ages (Tables 1 and 4). Food intake of both strains was influenced by age, with 12- and 50-week-old mice eating significantly more than 4- or 25-week-old mice. Over all ages combined, there was no difference between B6 and 129 females in food intake. However, B6 males ate significantly more than 129 males, and for both strains, males ate more than did females.
Table 4.
Results of Part 1 ANOVAs comparing male and female B6 and 129 mice fed chow at 4 different ages
| Measure | Strain (1,233) |
Age (3,233) |
Sex (1,233) |
Strain × Age (3,233) |
Strain × Sex (1,233) |
Age × Sex (3,233) |
Strain × Age × Sex (3,233) |
|
|---|---|---|---|---|---|---|---|---|
| Body weight | 46.2* | 876.2* | 431.4* | 2.45 | 0.07 | 37.9* | 0.44 | |
| Food intake | 3.35 | 25.2* | 98.7* | 2.55 | 22.4* | 0.87 | 1.12 | |
| Water intake | 60.3* | 4.37* | 3.67 | 3.79 | 17.9* | 8.64* | 6.58* | |
| Preference | ||||||||
| Saccharin | 673.0* | 2.68 | 4.42 | 0.42 | 1.46 | 0.23 | 1.69 | |
| Citric acid | 12.8* | 8.17* | 0.11 | 3.63 | 7.20* | 0.83 | 0.77 | |
| QHCl | 4.67 | 6.62* | 4.04 | 0.78 | 0.06 | 2.20 | 0.03 | |
| NaCl | 0.43 | 5.20* | 0.34 | 9.87* | 3.41 | 3.56* | 2.83* | |
| IMP | 88.3* | 16.5* | 2.04 | 2.79 | 0.26 | 0.28 | 0.33 | |
| CaCl2 | 159.7* | 1.15 | 4.86 | 1.26 | 0.78 | 1.27 | 0.45 | |
| Ethanol | 270.6* | 2.23 | 0.18 | 7.32* | 0.00 | 0.91 | 2.24 | |
Values in parentheses are degrees of freedom. Levels: Strain = B6 or 129; Sex = male or female; Age = 4, 12, 25, and 50 weeks.
P < 0.01
B6 females aged 25 and 50 weeks drank significantly more water than did the corresponding groups of B6 males or 129 females. The high water intakes of these 2 groups were responsible for Strain × Age × Sex, Strain × Sex, and Age × Sex interactions (Tables 1 and 4). There were no differences in water intake between B6 females and males at younger ages or between 129 females and males at any age. Over all groups combined, B6 mice drank more water than did 129 mice.
Age-related sex differences influenced preferences for NaCl but not other taste solutions. Female 129 mice aged 4 weeks had significantly lower NaCl preferences than did older females or males of any age, whereas female B6 mice aged 4 weeks did not differ from other B6 groups (Figure 1, Table 4). Sex had a strain (but not age)-dependent effect on citric acid preferences: Female 129 mice had higher citric acid preferences than did female B6 mice or male 129 mice (Figure 1). Other significant effects were similar to those observed in the males-only analyses, with the exception that the analyses of both sexes combined revealed that B6 mice had higher citric acid preferences than did 129 mice and the strains did not differ in NaCl preferences (Table 4). Sex did not influence preferences for saccharin, QHCl, IMP, CaCl2, or ethanol.
Male mice aged 4, 12, 25, or 50 weeks fed Teklad 8604 chow or AIN-93G diet
Relative to mice fed Teklad 8604 chow, mice of both strains fed AIN-93G diet gained more weight, ate more food, and drank less water. Feeding AIN-93G diet increased body weight of B6 mice more than 129 mice (Tables 1 and 5), with significant differences from the equivalent chow-fed groups present at 25 weeks (both strains) and 50 weeks (B6 only). The 4-week-old 129 mice fed AIN-93G diet had significantly lower water intakes than did the 4-week-old B6 mice fed AIN-93G diet.
Table 5.
Results of Part 1 ANOVAs comparing male B6 and 129 mice fed Teklad 8604 chow or AIN-93G diet at 4 different ages
| Measure | Strain (1,229) |
Age (3,229) |
Diet (1,229) |
Strain × Age (3,229) |
Strain × Diet (1,229) |
Age × Diet (3,229) |
Strain × Age × Diet (3,229) |
|
|---|---|---|---|---|---|---|---|---|
| Body weight | 75.2* | 923.0* | 77.8* | 13.2* | 11.7* | 21.5* | 10.0* | |
| Food intake | 1.75 | 2.28 | 6.83* | 2.33 | 0.24 | 2.73 | 1.96 | |
| Water intake | 4.71 | 2.71 | 52.4* | 2.31 | 0.29 | 7.32* | 0.70 | |
| Preference | ||||||||
| Saccharin | 418.6* | 3.87 | 3.74 | 2.87 | 4.56 | 0.75 | 3.28 | |
| Citric acid | 5.23 | 13.7* | 0.53 | 4.46* | 10.4* | 3.93* | 1.05 | |
| QHCl | 0.01 | 7.32* | 2.04 | 1.22 | 6.32 | 0.96 | 0.36 | |
| NaCl | 48.3* | 0.75 | 0.32 | 3.15 | 21.9* | 0.59 | 0.58 | |
| IMP | 63.6* | 26.3* | 15.6* | 6.98* | 1.38 | 3.56 | 2.53 | |
| CaCl2 | 110.1* | 0.85 | 3.47 | 0.29 | 5.96 | 0.43 | 0.42 | |
| Ethanol | 163.8* | 1.92 | 15.0* | 12.9* | 17.7* | 1.58 | 2.96 | |
Values in parentheses are degrees of freedom. Levels: Strain = B6 or 129; Diet = Teklad chow or AIN-93G diet; Age = 4, 12, 25, and 50 weeks.
P < 0.01.
The effects of diet on preferences can be summarized as follows: 1) Citric acid preferences of 129 mice fed AIN-93G were significantly lower than those of 129 mice fed Teklad chow or B6 mice fed AIN-93G diet. 2) Citric acid preferences of both strains combined were significantly higher at 50 weeks than at 4 and 25 weeks in mice fed AIN-93G diet but not in mice fed Teklad 8604 chow (Figure 1, Table 5). 3) NaCl preferences of B6 mice fed AIN-93G diet were significantly higher than those of B6 mice fed Teklad chow, whereas NaCl preferences of 129 mice fed Teklad chow were significantly higher than those fed AIN-93G diet. 4) IMP preferences were significantly higher in mice fed chow than mice fed AIN-93G, irrespective of strain. (5) Ethanol preferences of B6 mice fed AIN-93G diet were significantly lower than those of B6 mice fed Teklad chow, whereas ethanol preferences of 129 mice fed the 2 diets did not differ. Ethanol preferences of B6 mice fed AIN-93G diet declined between age 4 and 12 weeks and remained low relative to equivalent groups fed chow aged 25 and 50 weeks. Diet had no effect on preferences for saccharin, QHCl, or CaCl2.
Part 2: mice aged 54, 75, and 100 weeks
Male and female mice aged 54, 75, or 100 weeks fed Teklad 8604 chow
Body weights, food intakes, and water intakes of male mice of both strains and female 129 mice were stable at 54, 75, and 100 weeks (Tables 6 and 7). Female B6 mice weighed significantly more at 75 weeks than at 54 weeks. They also ate more food and drank more water at 100 weeks than at 54 weeks (Tables 6 and 7).
Table 6.
Part 2—number of mice in each age group, body weights, food intakes, and water intakes
| Condition and age (weeks) |
Group size |
Body weight (g) |
Food intake (kcal/d) |
Water intake (ml/day) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B6 | 129 | C57BL/6J | 129X1/SvJ | C57BL/6J | 129X1/SvJ | C57BL/6J | 129X1/SvJ | |||
| Males fed Teklad 8604 | ||||||||||
| 54 | 15 | 15 | 35.2 ± 0.7a | 31.5 ± 1.0a | 10.1 ± 0.2a | 9.7 ± 0.4a | 5.3 ± 0.2a | 4.4 ± 0.1a | ||
| 75 | 12 | 15 | 37.4 ± 1.9a | 28.6 ± 0.8a | 10.0 ± 0.7a | 9.9 ± 0.4a | 5.2 ± 0.2a | 4.5 ± 0.2a | ||
| 100 | 15 | 12 | 36.8 ± 1.4a | 30.5 ± 0.7a | 9.9 ± 0.2a | 11.7 ± 0.6a | 4.6 ± 0.3a | 4.2 ± 0.3a | ||
| Females fed Teklad 8604 | ||||||||||
| 54 | 14 | 15 | 27.3 ± 0.6a | 24.1 ± 0.6a | 8.8 ± 0.4a | 7.4 ± 0.4a | 4.9 ± 0.2a | 3.9 ± 0.3a | ||
| 75 | 12 | 13 | 33.9 ± 1.2b | 22.3 ± 0.5a | 11.8 ± 0.5a,b | 7.4 ± 0.5a | 5.9 ± 0.2a,b | 3.6 ± 0.3a | ||
| 100 | 14 | 9 | 32.4 ± 1.1b | 24.9 ± 1.2a | 11.9 ± 1.0b | 9.0 ± 0.8a | 6.4 ± 0.3b | 4.7 ± 0.4a | ||
| Males fed AIN-93G | ||||||||||
| 54 | 16 | 15 | 48.4 ± 0.9a | 36.5 ± 2.0a | 12.4 ± 0.6a | 11.2 ± 0.5a | 4.5 ± 0.2a | 4.6 ± 0.4a | ||
| 75 | 14 | 13 | 52.0 ± 0.8a | 35.0 ± 2.1a | 15.2 ± 0.7b | 10.0 ± 0.5a | 4.8 ± 0.4a | 5.2 ± 0.4a | ||
| 100 | 9 | 8 | 51.8 ± 2.6a | 29.3 ± 1.7a | 13.3 ± 0.8ab | 8.7 ± 0.8a | 4.4 ± 0.5a | 5.3 ± 1.6a | ||
Letter superscripts show homogenous groups of mice according to strain and dependent variable. For each of the 3 conditions in each column, groups with the same letter do not differ significantly from each other, according to post hoc Tukey's tests (P < 0.01).
Table 7.
Results of Part 2 ANOVAs comparing male and female B6 and 129 mice fed chow at 54, 75, and 100 weeks old
| Measure | Strain (1,149) |
Age (2,149) |
Sex (1,149) |
Strain × Age (2,149) |
Strain × Sex (1,149) |
Age × Sex (2,149) |
Strain × Age × Sex (2,149) |
|
|---|---|---|---|---|---|---|---|---|
| Body weight | 126.0* | 2.44 | 90.3* | 10.9* | 0.98 | 2.27 | 0.61 | |
| Food intake | 17.6* | 10.3* | 8.07* | 3.16 | 31.2* | 3.06 | 4.28 | |
| Water intake | 54.4* | 1.51 | 2.01 | 1.31 | 12.0* | 9.35* | 2.53 | |
| Preference | ||||||||
| Saccharin | 469.3* | 0.65 | 6.13 | 1.99 | 0.49 | 0.37 | 0.43 | |
| Citric acid | 0.28 | 0.04 | 0.92 | 3.06 | 1.93 | 2.29 | 0.44 | |
| QHCl | 7.35* | 7.22* | 1.44 | 0.20 | 0.41 | 0.08 | 0.40 | |
| NaCl | 29.4* | 2.41 | 1.21 | 0.01 | 6.50 | 2.93 | 0.15 | |
| IMP | 93.7* | 3.58 | 3.40 | 0.27 | 6.06 | 0.17 | 0.34 | |
| CaCl2 | 56.5* | 7.27* | 6.17 | 8.84* | 0.13 | 0.89 | 0.17 | |
| Ethanol | 333.5* | 1.01 | 0.83 | 1.96 | 0.09 | 1.47 | 0.99 | |
Values in parentheses are degrees of freedom. Levels: Strain = B6 or 129; Sex = male or female; Age = 54, 75, and 100 weeks.
P < 0.01.
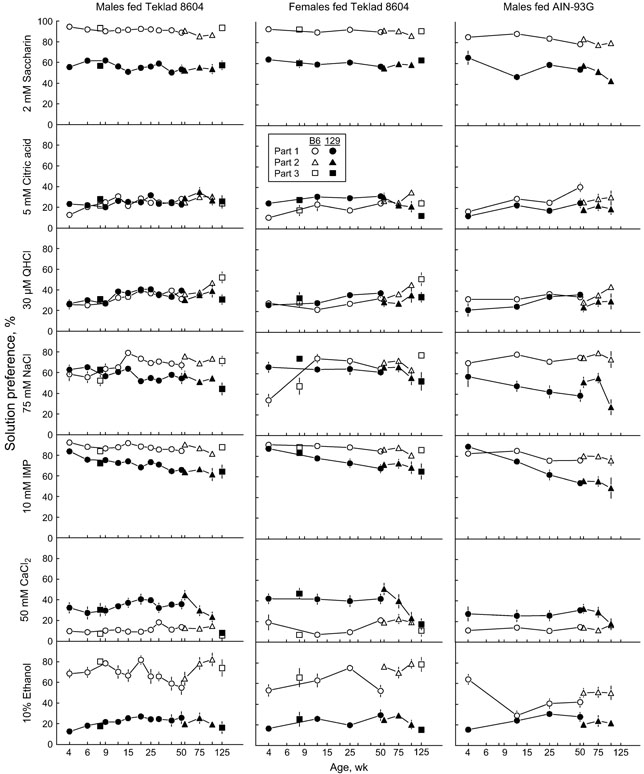
Preferences for saccharin, QHCl, NaCl, IMP, CaCl2, and ethanol displayed the same strain differences as those observed in Part 1 (Tables 7 and 8). None of the solution preferences were influenced by Sex (or its interactions), and the only age-related effects on preferences involved QHCl and CaCl2. For QHCl, this was because the preferences of all groups combined were higher at 100 weeks than at 54 or 75 weeks. For CaCl2, this was because the preferences of 129 mice were lower at 100 weeks than at 54 or 75 weeks; there were no differences in CaCl2 preferences of B6 mice.
Table 8.
Results of Part 2 ANOVAs comparing male B6 and 129 mice at 54, 75, and 100 weeks old fed Teklad 8604 chow or AIN-93G diet
| Measure | Strain (1,148) |
Age (2,148) |
Diet (1,148) |
Strain × Age (2,148) |
Strain × Diet (1,148) |
Age × Diet (2,148) |
Strain × Age × Diet (2,148) |
|
|---|---|---|---|---|---|---|---|---|
| Body weight | 192.2* | 0.55 | 109.6* | 6.11* | 41.4* | 1.34 | 2.22 | |
| Food intake | 23.2* | 0.58 | 5.33 | 3.49 | 40.5* | 5.25* | 8.13* | |
| Water intake | 0.23 | 2.35 | 0.02 | 0.52 | 13.8* | 4.50 | 0.68 | |
| Preference | ||||||||
| Saccharin | 352.3* | 4.15 | 6.85* | 1.42 | 1.98 | 1.37 | 1.76 | |
| Citric acid | 2.03 | 1.53 | 4.44 | 0.73 | 4.93 | 0.15 | 0.06 | |
| QHCl | 8.40* | 6.67* | 5.56 | 0.53 | 0.72 | 0.20 | 0.33 | |
| NaCl | 106.9* | 3.99 | 1.37 | 1.73 | 8.77* | 5.91* | 2.88 | |
| IMP | 129.7* | 3.01 | 15.5* | 0.23 | 0.16 | 0.02 | 0.25 | |
| CaCl2 | 53.0* | 4.47 | 1.59 | 6.44* | 2.99 | 0.47 | 0.90 | |
| Ethanol | 185.2* | 1.61 | 13.3* | 0.48 | 14.7* | 0.79 | 0.78 | |
Values in parentheses are degrees of freedom. Levels: Strain = B6 or 129; Diet = Teklad chow or AIN-93G diet; Age = 54, 75, and 100 weeks.
P < 0.01.
Male B6 and 129 mice aged 54, 75, or 100 weeks old and fed Teklad 8604 chow or AIN-93G diet
Like the males fed Teklad 8604, the males fed AIN-93G diet had stable body weights, food intakes, and water intakes at 54, 75, and 100 weeks old, with the exception that the 75-week old B6 mice ate more than the 54-week-old B6 mice (Tables 6 and 8). B6 mice fed the AIN-93G diet ate significantly more energy and were significantly heavier than B6 mice fed chow or 129 mice fed either diet. B6 mice fed Teklad 8604 chow drank significantly more water than B6 mice fed AIN-93G or 129 mice fed either diet.
There were only 3 effects of age on preferences. First, similar to the comparison of males and females, QHCl preferences of all groups (irrespective of strain and diet) were significantly higher at 75 and 100 weeks than at 54 weeks. Second, NaCl preferences of 100-week-old mice (of both strains) fed AIN-93G diet were significantly lower than those of mice of the same age fed chow or mice aged 75 weeks fed AIN-93G diet. Third, CaCl2 preferences of 129 mice (fed either diet) but not B6 mice were significantly lower at 100 weeks than at 54 weeks (Figure 2, Table 8).
Figure 2.
Influence of age on the preference for 7 taste solutions by male mice fed Teklad 8604 (left column), female mice fed Teklad 8604 (middle column), and male mice fed AIN-93G. Symbols represent means; vertical lines are standard errors (usually smaller than the symbols). Note that the x axis scale is logarithmic.
Over all 3 ages combined, both strains had stronger preferences for IMP if fed Teklad 8604 chow than AIN-93G diet. Ethanol preferences of B6 mice fed AIN-93G were significantly lower than those of B6 mice fed Teklad 8604 chow; diet had no effect on ethanol preferences of 129 mice.
Part 3: mice aged 8 and 125 weeks
Mice aged 125 weeks were significantly heavier than mice aged 8 weeks, and this difference was more pronounced in the B6 than in the 129 strain (Tables 9 and 10). At 8 weeks,129 females weighed significantly more than B6 females, and males of the 2 strains weighed the same. At 125 weeks, B6 males and females weighed significantly more than the corresponding groups of 129 mice. The 125-week-old B6 females drank significantly more water than any other group. The 125-week-old 129 females drank significantly less water than 125-week-old 129 males. Water intakes of the 4 groups of males did not differ.
Table 9.
Part 3—number of mice in each age group, body weights, and water intakes
| Sex and age (weeks) | Group size |
Body weight (g) |
Water intake (ml/day) |
||||
|---|---|---|---|---|---|---|---|
| B6 | 129 | C57BL/6J | 129X1/SvJ | C57BL/6J | 129X1/SvJ | ||
| Males fed Teklad 8604 | |||||||
| 8 | 12 | 12 | 21.5 ± 0.7 | 22.6 ± 0.7 | 5.5 ± 0.4 | 5.0 ± 0.4 | |
| 125 | 12 | 15 | 33.9 ± 0.7 | 26.3 ± 0.7* | 4.6 ± 0.4 | 5.4 ± 0.3 | |
| Females fed Teklad 8604 | |||||||
| 8 | 12 | 8 | 16.7 ± 0.7† | 19.2 ± 0.9 | 4.9 ± 0.4 | 4.4 ± 0.5 | |
| 125 | 10 | 9 | 30.1 ± 0.8 | 23.3 ± 0.9*,** | 6.2 ± 0.4† | 4.4 ± 0.4*,** | |
P < 0.01, comparison between strains at the same age and same sex;
P < 0.01, comparison with males of the same strain and age.
Table 10.
Results of Part 3 ANOVAs comparing male and female B6 and 129 mice at 8 and 125 weeks old fed Teklad 8604 chow
| Measure | Strain (1,81) |
Age (1,81) |
Sex (1,81) |
Strain × Age (1,81) |
Strain × Sex (1,81) |
Age × Sex (1,81) |
Strain × Age × Sex (1,81) |
|
|---|---|---|---|---|---|---|---|---|
| Body weight | 23.9* | 216.5* | 44.9* | 65.3* | 0.79 | 0.25 | 0.12 | |
| Water intake | 5.02 | 0.10 | 0.82 | 0.12 | 7.06* | 1.85 | 6.56* | |
| Preference | ||||||||
| Saccharin | 277.8* | 0.09 | 1.52 | 0.93 | 3.38 | 0.13 | 0.93 | |
| Citric acid | 0.15 | 0.01 | 1.03 | 4.94 | 0.62 | 0.10 | 2.42 | |
| QHCl | 4.20 | 11.7* | 0.16 | 9.85* | 0.45 | 0.13 | 0.02 | |
| NaCl | 0.76 | 0.23 | 4.33 | 27.7* | 2.35 | 0.53 | 0.02 | |
| IMP | 20.9* | 3.06 | 2.99 | 3.70 | 2.00 | 0.60 | 0.08 | |
| CaCl2 | 87.4* | 48.2* | 15.8* | 61.7* | 11.0* | 1.41 | 3.54 | |
| Ethanol | 168.0* | 0.43 | 0.03 | 0.74 | 0.60 | 0.05 | 1.96 | |
Values in parentheses are degrees of freedom. Levels: Strain = B6 or 129; Sex = male or female; Age = 8 or 125 weeks.
P < 0.01.
Age significantly influenced preferences for QHCl, NaCl, and CaCl2 but not for the other taste solutions tested. QHCl and NaCl preferences of 125-week-old B6 mice were significantly higher than those of 8-week-old B6 mice or 129 mice of either age. CaCl2 preferences of 8-week-old 129 mice were significantly higher than those of 8-week-old B6 mice and 125-week-old mice of either strain.
The only influence of Sex on taste solution preferences was that CaCl2 preferences were significantly higher in 8- week-old female 129 mice than 8-week-old male 129 mice (Table 10).
Variability of preferences in Parts 1–3
To address several hypotheses concerning the variability of preferences, we used the standard error of the mean (SEM) of the preferences for each group in Parts 1–3 (i.e., for each strain, sex, and age tested) as data in subsequent ANOVAs, with strain, sex, diet, and age (where appropriate) as between-subject factors and test solution as a within-subject factor. 1) To test whether the taste preferences of older mice were more variable than those of younger mice, we split the data from male mice into a younger group (≤25 weeks) and an older group (≥30 weeks). The older group had more variable preferences than did the younger group (mean ± SEM of the SEMs; young = 2.75 ± 0.28, old = 3.25 ± 0.17%). 2) To test whether taste preferences of females were more variable than those of males, we compared the SEMs of each of the 18 groups of female mice with the corresponding groups of males. There was no effect of sex on phenotype variability, either overall (mean ± SEM of the SEMs; males = 3.39 ± 0.19, females = 3.68 ± 0.19) or for any of the individual taste solutions. 3) To test whether taste preferences of mice fed chow were more variable than those fed semisynthetic diet, we compared the SEMs of each of the 14 groups of mice fed AIN-93G diet with the corresponding groups fed Teklad 8604 chow. There was no effect of diet on phenotype variability, either overall (mean ± SEM of the SEMs; AIN-93G = 3.77 ± 0.23, chow = 3.31 ± 0.23) or for any of the individual taste solutions.
In all 3 of these analyses, the taste preferences of 129 mice were significantly more variable than those of B6 mice. The variability in preferences for NaCl and ethanol was significantly higher than the variability in preferences for saccharin and IMP, with the variability in preferences for citric acid falling between these extremes. Depending on the analysis, the variability of preferences for QHCl and CaCl2 was either significantly higher than or did not differ from the variability in preferences for saccharin and IMP.
Part 4: concentration-response functions for 8- and 125-week-old mice
In this part of the experiment, we examined the response of young and old mice to different concentrations of taste solutions. All omnibus analyses revealed the expected, highly significant main effect of Concentration on preferences. To simplify, we do not describe these. None of the analyses produced significant interactions involving Concentration × Sex, so we present the results for both sexes combined in Figure 3.
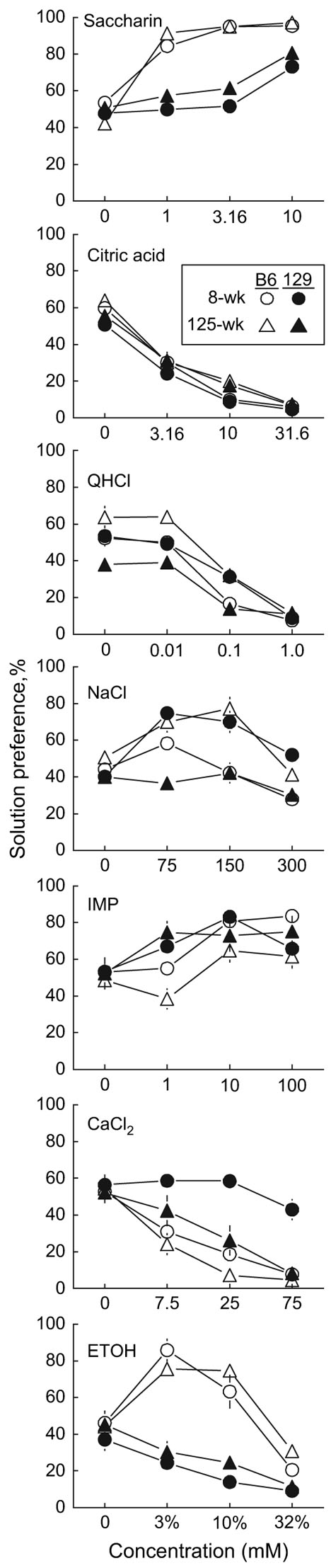
Figure 3.
Preferences for various concentrations of 7 taste solutions by C57BL/6J (B6) and 129X1/SvJ (129) mice aged 8 or 125 weeks. Data from both sexes are combined. All concentrations are given in millimoles except for ethanol (ETOH), which is given in percent (v/v). Symbols represent means; vertical lines are standard errors (usually smaller than the symbols).
There were Strain × Age interactions influencing preferences for QHCl, IMP, and CaCl2. For QHCl, the interpretation of this is unclear because there were differences between groups in the choice test involving 2 bottles of water (Figure 3). For IMP (particularly for 1 mM IMP), 129 mice aged 125 weeks had lower preferences than did 129 mice aged 8 weeks or B6 mice of either age. For CaCl2, 129 mice aged 8 weeks had much higher preferences than did 129 mice aged 125 weeks or B6 mice of either age (Figure 3). There was also an interaction of Strain × Concentration influencing CaCl2 preferences: Whereas the B6 and 129 mice did not differ in the test with water versus water, the 129 mice had consistently higher CaCl2 preference scores than did the B6 mice.
Preferences for NaCl involved a complex Strain × Age × Concentration interaction: B6 mice aged 125 weeks had higher 150mM NaCl preferences than B6 mice aged 8 weeks, and at 75 and 300 mM, the old and young B6 mice did not differ. The 129 mice aged 125 weeks had lower preferences than did the 129 mice aged 8 weeks at all 3 concentrations of NaCl. (Figure 3).
Age had no effects on saccharin, citric acid, or ethanol preferences. Irrespective of age, B6 mice had stronger preferences for saccharin than did 129 mice, particularly at the 1 and 3.16 mM concentrations. Over both ages and all concentrations combined, female 129 mice had higher preferences than did male 129 mice for saccharin (64 ± 3% vs. 56 ± 2%) and ethanol (29 ± 3% vs. 21 ± 2%). There was not a corresponding sex difference for B6 mice in preferences for saccharin (82 ± 2%vs. 80 ± 2%), and male B6 mice had higher preferences for ethanol than did female B6 mice (61 ± 3% vs. 50 ± 3%).
Discussion
We have split the Discussion into 2 parts: First, we discuss the results obtained for water intake and each taste solution. Under each subheading, we describe previous experiments with rodents by other investigators, summarize our results, and attempt to integrate them into this literature. Second, we discuss the implications of the results for phenotyping mice used for genetic analyses.
Discussion of specific taste preference tests
Water
Tests involving a choice between 2 drinking tubes of water provided a measure of habitual fluid intake. Previous studies of daily water intakes of old rodents have produced conflicting results. There are reports of age-related polydipsia (Goodrick 1969; Beck and Yu 1982), eudipsia (Davies et al. 1985), and hypodipsia (Silver et al. 1991, 1993). In the present work, we found hypodipsia in old male B6 mice fed chow but not in old male 129 mice fed chow, old female mice of either strain fed chow, or old male mice of either strain fed AIN-93G diet. Taken with the earlier literature, it seems clear that the effects of age on water intake are complex and dependent on strain, diet, and sex. This conclusion isbased on raw daily water intakes without adjustment for body size (i.e., ml/d/mouse). Old mice were 2.5–4 times heavier than the youngest mice, so adjustment for body weight would render all strain, sex, and diet groups polydipsic when young and hypodipsic when old relative to middle-aged groups.
Saccharin
Previous investigations of age-related changes in sweet taste preferences in rodents have produced mixed results (Colavita 1968; Goodrick 1969; Wurtman JJ and Wurtman RJ 1979; Bertino and Wehmer 1981; Perez and Sclafani 1990). Here, we found no age-related changes in saccharin preference in either the B6 or 129 mouse strain. The largest influence on saccharin preference was genetic. B6 mice had much stronger preferences for 2 mM saccharin than did 129 mice, irrespective of their sex or diet. This marked strain difference is consistent with the presence of a sweet “taster” Tas1r3 allele in the B6 strain and a “nontaster” Tas1r3 allele in the 129 strain (e.g., Bachmanov, Li, et al. 2001; Reed et al. 2004). The present results suggest that the sensitivity of the TAS1R3 receptor is not modified to any appreciable extent by age.
Citric acid
We are unaware of any previous work to examine the influence of age on sour taste solution preference in rodents. Here, there were only 2 effects of age: Citric acid preferences were lower in 4-week-old male B6 mice fed Teklad 8604 and higher in 50-week-old B6 mice fed AIN-93G than in some of the other groups. Irrespective of age, the effect of strain on citric acid preferences was sex and diet dependent. Female 129 mice had higher citric acid preferences than did female B6 mice or male 129 mice, and B6 mice fed AIN-93G diet had higher preferences than did 129 mice fed the same diet, but preferences did not differ between B6 and 129 males fed chow (confirming Tordoff et al. 2002).
QHCl
There are at least 4 previous studies that have examined the response of elderly rodents to bitter compounds: 1) There were no differences in lick rates or bar presses for QHCl between rats aged 6, 21, or 43 weeks (Bloomquist and Candland 1965). 2) Thresholds for quinine detection in 2-bottle preference tests were higher in weanlings than in 14- or 43-weekold rats (Cicala and McMichael 1964). 3) Rats aged 124 weeks were more sensitive to quinine adulteration of their diet than were rats aged 34 weeks (Jakubczak 1977). 4) Quinine preference was higher in rats aged 106 weeks than those aged 4, 20, and 60 weeks (Goodrick 1969). The results of our study appear similar to the latter finding. The only effect of age was that for both strains combined, the 100- and 125-week-old mice (and 75-week-old groups fed AIN-93G) had significantly higher QHCl preferences than did several of the younger groups (Figure 2). Preferences for QHCl were unaffected by strain, sex, diet, or any of their interactions.
NaCl
Previous studies of NaCl preferences in aging rodents have produced contradictory results (McConnell and Henkin 1973; Midkiff and Bernstein 1983; Ferrell and Gray 1985) and the results found here complicate things even further. First, female B6 mice aged 4 weeks (Part 1) or 8 weeks (Part 3) had dramatically lower preferences for NaCl than did older B6 mice. This increase is consistent with the onset of pubertal steroid hormones contributing to NaCl preference (see Introduction); however, arguing against this explanation, a similar change was not observed in female 129 or male mice. Second, 100-week-old B6 males fed AIN-93G diet had lower preferences than did younger B6 mice fed this diet. This is consistent with general trends for NaCl preference to decrease with age in both male and female B6 mice fed chow. Third, there was a complex interaction in Part 4 of the experiment: Preferences for 75, 150, and 300 mM NaCl were lower in 125- than 8-week-old 129 mice but preferences of 125- and 8-week-old B6 mice either did not differ (75 and 300 mM NaCl) or were higher in the 125- than 8-week-old groups (150mM NaCl; Figure 3). We note that there are persistent effects of past experience with NaCl solutions on subsequent NaCl preferences (Beauchamp and Fisher 1993; Bachmanov, Schlager, et al. 1998; Bachmanov, Tordoff, and Beauchamp 1998) and that in this experiment, the 8- week-old mice were experimentally naive but the 125-week-old mice had consumed NaCl solution previously. It is an open question to what extent this difference in experience influenced the results.
IMP
The only previous work we know on the response of old rodents to umami-tasting compounds is a report of no changes in monosodium glutamate preference of hairless rats tested at 10, 15, and 20 weeks old (Smriga et al. 2000). Here, we found that 4-week-old male mice of both strains and 4-week-old female 129 mice had higher preferences for IMP than did some of the older groups. The general pattern of results was for B6 mice to maintain virtually 100% preference for IMP at all ages, whereas 129 mice had IMP preferences that declined gradually with age from levels similar to or slightly lower than B6 mice at 4 weeks to between 40–60% at 125 weeks. There were no sex differences in IMP preference. Both strains had stronger preferences for IMP if fed chow than AIN-93G diet. The results of the concentration-response experiment were consistent with the results of earlier tests with 10 mM IMP, although the effects of age on IMP preference were clearer at 1 mM than 10 mM IMP.
CaCl2
We are unaware of any previous studies of calcium preference in elderly animals, although rats with continuous access to CaCl2 or calcium lactate solutions decrease intakes as they pass from adolescence into adulthood (Tordoff 1992). In the present study, preferences of 129 mice for 50mM CaCl2 were stable between age 4–50 weeks, but plummeted between 54 and 125 weeks. This precipitous drop in preference occurred in both male and female 129 mice, for those fed either chow or AIN-93G, and was expressed in 125-week-old mice at all 3 concentrations of CaCl2 tested. It was arguably the largest and most consistent effect of age observed in this study. The reduction in CaCl2 preferences of elderly mice was confined to the 129 strain, but a similar response in the B6 strain may have been constrained by a floor effect because the CaCl2 preferences of B6 mice were very low at all ages.
It has been suggested that the demand for calcium is a function of growth (see Tordoff 2001), perhaps, of bone. Because growth slows with age, it is possible that the reduction in calcium preference observed in 129 mice at 75 weeks or older is due to lower requirements for this mineral. Indeed, bone loss has been observed in elderly C57BL/6 mice (Massie et al. 1989), although the relationship between age and bone calcium is complex (Massie et al. 1990). Bone reabsorption cannot be the only factor involved in reducing calcium preference because relatively young mice had similar preferences to middle-aged mice, even though growth and calcium requirements are higher in the young. Moreover, the strain difference in calcium preference cannot easily be ascribed to differences in growth because the 129 strain was, if anything, slightly smaller than the B6 strain. The 129 strain does have slightly higher bone mineral density and content than the B6 strain (Tordoff and Bachmanov 2002b; Tordoff, Bachmanov, and Reed forthcoming) that may point to strain differences in calcium metabolism. We note that BIOBR and C57BL/6 mice accumulate calcium in the kidneys at ~40 weeks old (Massie et al. 1990; Morita et al. 1994), which is about the time we observed decreased calcium preference. The cause of this renal calcium accumulation is unclear (see van Abel et al. 2006 for other age-related changes in calcium metabolism of mice).
In all but the oldest mice, the 129 strain had higher CaCl2 preferences than did the B6 strain. This is in contrast to the results with the other 6 taste solutions where, if anything, B6 mice had higher preferences than did 129 mice. Females of both strains had slightly but significantly higher CaCl2 preferences than did males, which, because females are smaller than males, also argues against a simple relationship between growth and calcium intake but is consistent with studies in rats (e.g., Schulkin 1991) and a recent survey showing that 11 of 40 mouse strains had sex differences in this direction (Tordoff, Bachmanov, and Reed forthcoming).
Ethanol
Previous work suggests that ethanol preference decreases in elderly rats and mice (Goodrick 1975; Wood 1976; Yoshimoto et al. 2003). In contrast, we found no concerted changes in ethanol preferences with age in mice over 4 weeks. However, 4-week-old male 129 mice fed Teklad chow had lower preferences and 4-week-old B6 mice fed AIN-93G diet had higher preferences than older groups fed the same diet. It is possible that the marked reduction in ethanol preference of B6 mice fed AIN-93G diet between 4 and 12 weeks reflects maturation of mechanisms underlying orosensory perception, although we think this is unlikely because they were confined to one sex and one diet. Instead, we suspect that a metabolic effect of the diet was responsible; mice tested when 4 weeks old had been fed this diet for only 17 days, whereas older mice had been fed it for at least 7 weeks. The lower ethanol preferences of adult B6 mice fed AIN-93G diet than those fed Teklad chow is consistent with work using 10-week-old 129 and B6 mice (Tordoff et al. 2002).
Consistent with earlier work, there was a large strain difference in alcohol preference, with B6 mice showing much stronger preferences for ethanol than did 129 mice. This has been attributed to the interaction of ethanol with the sweet taste receptor, TAS1R3 (Bachmanov et al. 1996a, 1996b, 2002b).
Implications for taste phenotyping mice
The main conclusion to be drawn from the results of this experiment is that, with the possible exception of very young (4 weeks) and very old (100 or 125 weeks) mice, age has little effect on taste preferences. There were virtually no changes in preference for any of the 7 taste solutions tested between 6 and 75 weeks of age. The only concerted change during this period was a progressive age-related reduction in preferences of 129 mice for IMP, and even here, the effects were small (from 76 ± 3% to 66 ± 4%) and strain specific. It is doubtful that such a small difference would be distinguishable without the considerable power afforded this experiment by the large number of comparison groups, each with a relatively large number of mice.
The primary purpose for conducting this study was to determine whether investigators should be concerned about the age of mice when examining taste phenotypes in genetic studies. Because many inbred strains and transgenic mice do not breed easily and are in short supply, it is often difficult to generate groups that are matched perfectly for age. The results indicate that age is a minor source of variability relative to strain, sex, diet, and even “sporadic” variations with no obvious cause. Of course, this conclusion is based on the results of only the 2 strains tested here, but these are the background strains for most knockout mice, so it is likely that they will have wide applicability.
Mice reach sexual maturity between 3 and 9 weeks (depending on strain and the measure of maturity used, e.g., Nelson et al. 1990). We anticipated that the pubertal hormonal cascade might influence taste preferences. However, the only marked change in preference during this period was for NaCl preference of B6 female mice and ethanol intake of B6 male mice fed AIN-93G diet. These findings must be treated with caution or at least considered in the context of the more general findings of no changes in taste preferences of the 129 strain and no changes in preferences for other compounds tested. Moreover, maturity and dietary experience are confounded, so the cause of the changes in preference is unclear. It will require replication of the pertinent results and additional controls to determine whether the changes in NaCl and ethanol preference between 4 and 12 weeks are a consequence of sexual maturation.
Obtaining groups of mice of the same sex doubles the amount of breeding required and thus is an obstacle to efficient phenotyping studies. Testing females is often avoided because of concerns that fluctuations in reproductive hormones over the 4-day estrus cycle add variability to taste preferences (e.g., Nance et al. 1976; Kanaka et al. 1979). However, we found no evidence that variability was greater in female than male mice at any age. There were, however, indications that sex influenced taste preferences for some solutions. Compared with males, females of both strains had higher saccharin, CaCl2, and ethanol preferences (in the concentration-response experiment only), and 129 females had higher preferences for NaCl and IMP as well. Consistent with their larger size and greater growth, males of both strains ate more food than did females. However, there was an intriguing dissociation of food and water intake; 129 males drank more water than did 129 females but B6 males drank less water than did B6 females. Based on the results of this study and strain surveys of taste preferences in male and female mice (Tordoff, Bachmanov, and Reed forthcoming; Tordoff, Reed, and Bachmanov forthcoming), it appears that sex differences in taste preferences are not as robust as those observed with rats but are still present for several taste solutions and thus cannot be ignored.
Diet and dietary restriction can have profound effects on measures of aging (e.g., Duffy et al. 2001, 2002). Due to historical precedent and cost, most studies are conducted with mice fed cereal-based chow diets. These are a poor choice because there are batch-to-batch differences that may add to variability (see Tordoff et al. 2002; Wang et al. 2005). In the current experiment, we did not find any evidence that mice fed chow had more variable taste preferences than did mice fed semisynthetic diet. We note, however, that it is unlikely that batch-to-batch differences in chow would influence within-group variability because mice in the same group were unlikely to be fed from different batches of chow. A more serious problem is that because the ingredients of chow are unknown, experiments using this food are impossible to replicate.
Although diet did not influence phenotypic variability, it had marked effects on mean preferences for all the solutions tested, often in a strain-specific manner. Relative to their counterparts fed chow, mice of both strains fed AIN-93G had lower QHCl and IMP preferences, the 129 mice had lower citric acid, NaCl, and CaCl2 preferences, and the B6 mice had lower saccharin and ethanol preferences. The B6 mice fed AIN-93G also had lower water intakes, higher energy intakes, and were heavier than those fed Teklad 8604. These results confirm and extend an earlier report involving comparison of several chows and semisynthetic diets (Tordoff et al. 2002).
We were surprised to find that mice fed the AIN-93G diet died younger than those fed chow. We did not discern an obvious cause of early death in the AIN-93G-fed mice. A design challenge was whether to use this diet, which is intended for growing rodents, or to switch in the middle of the experiment from AIN-93G to AIN-93M, which is formulated for maintenance of adult rodents (Reeves et al. 1993). We elected to avoid the diet switch in order to simplify interpretation but wonder if the relatively high casein and soybean oil content of the AIN-93G diet may have contributed to an early death. The mice fed AIN-93G diet were considerably heavier and, at least by visual inspection, fatter. It is thus possible that they suffered from atherosclerosis and other diseases associated with obesity.
A thorny issue for this and many other taste preference experiments concerns carry-over effects; that is, the modification of taste preferences by previous taste experience. In this experiment, there were 2 potential sources of carry-over effects: 1) within each test series and 2) between test series in those animals that were tested more than once. Within each series, mice always received tests in the same order (i.e., saccharin, citric acid, QHCl, NaCl, IMP, CaCl2, and ethanol), so it is possible, for example, that experience drinking saccharin might have influenced preferences for citric acid or the other solutions tested subsequently. However, in unpublished work, we found that groups of 8-week-old B6 or 129 mice given a series of four 2-bottle taste tests (2 mM saccharin, 5 mM citric acid, 30 μM QHCl, and 75 mM NaCl) had indistinguishable preferences from separate groups of mice given each of the latter 3 tests alone. Thus, it is unlikely that within-series carry-over effects influenced the results found here, although we cannot rule out their possible contribution to IMP, CaCl2, or ethanol preferences. Between-series carry-over effects were not an issue for interpretation of results from mice in the 4- to 50-week-old groups because these were all naive. However, the groups aged 54, 75, 100, and 125 weeks had all received taste preference tests previously. To assess the possibility of carry-over effects influencing the results of these mice, we compared the 54- week-old groups (that had been tested previously when 4 weeks old) to groups that were 50 weeks old and never been tested previously (in Part 1). In nearly every case, the preferences of corresponding groups were similar (the only exception was a difference in QHCl preferences of both strains fed AIN-93G). This is reassuring evidence that there are few if any carry-over effects from tests conducted a year previously, although it does not rule out the possibility that carry-over effects influenced the response of some of the older groups.
This study is part of a series to dissect the environmental causes of variation in taste phenotype. Earlier studies suggest that test duration and the position and number of drinking spouts can influence both group mean values and variability (Tordoff 2002; Tordoff and Bachmanov 2002a, 2003a, 2003b, 2003c). Consistent with an earlier study (Tordoff et al. 2002), we found that the type of diet the mice were fed influenced group mean preferences but not the variability of the response. Similarly, here we found that males and females differed in mean preferences for some taste solutions but not in the variability of response. With the exception of the very youngest and oldest mice, in most cases, age had no effect on either taste preference means or variability. We note that variability between groups was much greater than variability within groups. This cannot easily be ascribed to differences in test procedures because, at least for the 4–50 week ages, all the mice were tested simultaneously and none had previous experience with taste solutions. Instead, the greatest source of variation appears to be due to factors differentially affecting individual age groups. The most likely possibilities include differences among the groups in prenatal environment, litter composition, or the shipping experience during transportation from the breeder to our laboratory (see Tordoff et al. 2005). Discovering and then controlling for these sources of variation will require additional experimentation.
Taste preferences are complex phenotypes that involve integration of gustatory, olfactory, and trigeminal information and modulation by hormonal and metabolic state. Although gustatory transduction mechanisms remain largely unaffected by age, olfactory and trigeminal ones decline, and there are many hormonal and metabolic changes associated with puberty and old age. It is an intriguing question why, in the face of these nongustatory effects of age, most taste preferences remain relatively stable.
Acknowledgements
Thanks to Diane Pilchak for superlative technical assistance and to Drs Danielle R. Reed and Beverly Cowart for comments on an earlier draft. This study was supported by grant AA-12715 from the National Institutes of Health (NIH). In fulfillment of NIH requirements, data from the project are available on line at http://www.monell.org/MMTPP.
References
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Body weight, food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Schlager G, Tordoff MG, Beauchamp GK. Consumption of electrolytes and quinine by mouse strains with different blood pressures. Physiol Behav. 1998;64:323–330. doi: 10.1016/s0031-9384(98)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet. 1998;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Fisher AS. Strain differences in consumption of saline solutions by mice. Physiol Behav. 1993;54:179–184. doi: 10.1016/0031-9384(93)90063-l. [DOI] [PubMed] [Google Scholar]
- Beck N, Yu BP. Effect of aging on urinary concentrating mechanism and vasopressin-dependent cAMP in rats. Am J Physiol. 1982;243:F121–F125. doi: 10.1152/ajprenal.1982.243.2.F121. [DOI] [PubMed] [Google Scholar]
- Belknap JK. Effect of within-strain sample size on QTL detection and mapping using recombinant inbred mouse strains. Behav Genet. 1998;28:29–38. doi: 10.1023/a:1021404714631. [DOI] [PubMed] [Google Scholar]
- Bertino M, Wehmer F. Dietary influences on the development of sucrose acceptability in rats. Dev Psychobiol. 1981;14:19–28. doi: 10.1002/dev.420140104. [DOI] [PubMed] [Google Scholar]
- Bloomquist DW, Candland DK. Taste preferences measured by tongue licks and bar presses as a function of age in the rat. Psychon Sci. 1965;3:393–394. [Google Scholar]
- Bushong ME, Mann MA. Gender and intrauterine position influence saccharin preference in mice. Horm Behav. 1994;28:207–218. doi: 10.1006/hbeh.1994.1018. [DOI] [PubMed] [Google Scholar]
- Capaldi ED, Hunter MJ, Privitera GJ. Odor of taste stimuli in conditioned “taste” aversion learning. Behav Neurosci. 2004;118:1400–1408. doi: 10.1037/0735-7044.118.6.1400. [DOI] [PubMed] [Google Scholar]
- Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav Neurosci. 1992;106:172–180. doi: 10.1037//0735-7044.106.1.172. [DOI] [PubMed] [Google Scholar]
- Cicala G, McMichael J. Quinine aversion thresholds in rats as a function of age and psychophysical procedure. Can J Psychol. 1964;18:28–35. doi: 10.1037/h0083293. [DOI] [PubMed] [Google Scholar]
- Colavita FB. Saccharine preference in rats as a function of age and early experience. Psychon Sci. 1968;12:311–313. [Google Scholar]
- Davies I, Goddard C, Fotheringham AP, Moser B, Faragher EB. The effect of age on the control of water conservation in the laboratory mouse—metabolic studies. Exp Gerontol. 1985;20:53–66. doi: 10.1016/0531-5565(85)90009-9. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Lewis SM, Mayhugh MA, McCracken A, Thorn BT, Reeves PG, Blakely SA, Casciano DA, Feuers RJ. Effect of the AIN-93M purified diet and dietary restriction on survival in Sprague-Dawley rats: implications for chronic studies. J Nutr. 2002;132:101–107. doi: 10.1093/jn/132.1.101. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Seng JE, Lewis SM, Mayhugh MA, Aidoo A, Hattan DG, Casciano DA, Feuers RJ. The effects of different levels of dietary restriction on aging and survival in the Sprague-Dawley rat: implications for chronic studies. Aging. 2001;13:263–272. doi: 10.1007/BF03353422. [DOI] [PubMed] [Google Scholar]
- Ferrell F, Gray SD. Longitudinal study of salt preferences in normotensive and hypertensive rats. Hypertension. 1985;7:326–332. [PubMed] [Google Scholar]
- Frasnelli J, Hummel T. Age-related decline of intranasal trigeminal sensitivity: is it a peripheral event? Brain Res. 2003;987:201–206. doi: 10.1016/s0006-8993(03)03336-5. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Taste discrimination and fluid ingestion of male albino rats as a function of age. J Genet Psychol. 1969;115:121–131. doi: 10.1080/00221325.1969.10533879. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Behavioral differences in young and aged mice: strain differences for activity measures, operant learning, sensory discrimination, and alcohol preference. Exp Aging Res. 1975;1:191–207. doi: 10.1080/03610737508257960. [DOI] [PubMed] [Google Scholar]
- Jakubczak LF. Age differences in the effects of palatability of diet on regulation of calorie intake and body weight of rats. J Gerontol. 1977;32:49–57. doi: 10.1093/geronj/32.1.49. [DOI] [PubMed] [Google Scholar]
- Kanaka R, Dua-Sharma S, Sharma KN. Gustatory preferences during estrus cycle in rats. Indian J Physiol Pharmacol. 1979;23:277–284. [PubMed] [Google Scholar]
- Krecek J. Sex differences in salt taste: the effect of testosterone. Physiol Behav. 1973;10:683–688. doi: 10.1016/0031-9384(73)90144-3. [DOI] [PubMed] [Google Scholar]
- Laska M. Perception of trigeminal chemosensory qualities in the elderly. Chem Senses. 2001;26:681–689. doi: 10.1093/chemse/26.6.681. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, DeWolfe LK. Calcium and calmodulin changes with ageing in C57BL/6J mice. Gerontology. 1989;35:100–105. doi: 10.1159/000213006. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Shumway ME, Armstrong T. Calcium, iron, copper, boron, collagen, and density changes in bone with aging in C57BL/6J male mice. Exp Gerontol. 1990;25:469–481. doi: 10.1016/0531-5565(90)90035-z. [DOI] [PubMed] [Google Scholar]
- McConnell SD, Henkin RI. NaCl preference in spontaneously hypertensive rats: age and blood pressure effects. Am J Physiol. 1973;225:624–627. doi: 10.1152/ajplegacy.1973.225.3.624. [DOI] [PubMed] [Google Scholar]
- Midkiff EE, Bernstein IL. The influence of age and experience on salt preference of the rat. Dev Psychobiol. 1983;16:385–394. doi: 10.1002/dev.420160504. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. Aging effects on anatomy and neurophysiology of taste and smell. Gerodontol. 1984;3:131–136. doi: 10.1111/j.1741-2358.1984.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. Anatomy and neurophysiology of the taste system in aged animals. Ann N Y Acad Sci. 1989;561:277–290. doi: 10.1111/j.1749-6632.1989.tb20989.x. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Baum BJ. Quantitative study of taste buds in fungiform and circumvallate papillae of young and aged rats. J Anat. 1984;138:323–332. [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM, Oakley IA. Quantitative anatomical study of taste buds in fungiform papillae of young and old Fischer rats. J Gerontol. 1986;41:315–318. doi: 10.1093/geronj/41.3.315. [DOI] [PubMed] [Google Scholar]
- Morita A, Kimura M, Itokawa Y. The effect of aging on the mineral status of female mice. Biol Trace Elem Res. 1994;42:165–177. doi: 10.1007/BF02785387. [DOI] [PubMed] [Google Scholar]
- Nakayasu C, Kanemura F, Hirano Y, Shimizu Y, Tonosaki K. Sensitivity of the olfactory sense declines with the aging in senescence-accelerated mouse, SAM-P1. Physiol Behav. 2000;70:135–139. doi: 10.1016/s0031-9384(00)00234-1. [DOI] [PubMed] [Google Scholar]
- Nance DM, Gorski RA, Panksepp J. Neural and hormonal determinants of sex differences in food intake and body weight. In: Novin D, Wyrwicka W, Bray G, editors. Hunger: basic mechanisms and clinical implications. New York: Raven Press; 1976. pp. 257–271. [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod. 1990;42:649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- Osada K, Komai M, Bryant BP, Suzuki H, Tsunoda K, Furukawa Y. Age related decreases in neural sensitivity to NaCl in SHR-SP. J Vet Med Sci. 2003;65:313–317. doi: 10.1292/jvms.65.313. [DOI] [PubMed] [Google Scholar]
- Perez C, Sclafani A. Developmental changes in sugar and starch taste preferences in young rats. Physiol Behav. 1990;48:7–12. doi: 10.1016/0031-9384(90)90252-y. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, et al. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses. 1994;19:425–431. doi: 10.1093/chemse/19.5.425. [DOI] [PubMed] [Google Scholar]
- Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lect Ser. 1942–1943;38:63–103. [Google Scholar]
- Schimenti J, Bucan M. Functional genomics in the mouse: phenotype-based mutagenesis screens. Genome Res. 1998;8:698–710. doi: 10.1101/gr.8.7.698. [DOI] [PubMed] [Google Scholar]
- Schulkin J. The ingestion of calcium in female and male rats. Psychobiology. 1991;19:262–264. [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Silver AJ, Flood JF, Morley JE. Effect of aging on fluid ingestion in mice. J Gerontol. 1991;46:B117–B121. doi: 10.1093/geronj/46.3.b117. [DOI] [PubMed] [Google Scholar]
- Silver AJ, Morley JE, Ishimaru-Tseng TV, Morley PM. Angiotensin II and fluid ingestion in old rats. Neurobiol Aging. 1993;14:519–522. doi: 10.1016/0197-4580(93)90033-8. [DOI] [PubMed] [Google Scholar]
- Smriga M, Murakami H, Mori M, Torii K. Use of thermal photography to explore the age-dependent effect of monosodium glutamate, NaCl and glucose on brown adipose tissue thermogenesis. Physiol Behav. 2000;71:403–407. doi: 10.1016/s0031-9384(00)00350-4. [DOI] [PubMed] [Google Scholar]
- Thaw AK. Changes in taste threshold over the life span of the Sprague Dawley rat. Chem Senses. 1996;21:189–193. doi: 10.1093/chemse/21.2.189. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Crouse DA, Mann SL, Saxena SK, Sharp JG. Intestinal glucose uptake is increased in aged mice. Mech Ageing Dev. 1988;46:135–143. doi: 10.1016/0047-6374(88)90121-2. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Influence of dietary calcium on sodium and calcium intake of spontaneously hypertensive rats. Am J Physiol. 1992;262:R370–R381. doi: 10.1152/ajpregu.1992.262.3.R370. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Calcium: taste, intake and appetite. Physiol Rev. 2001;81:1567–1597. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Obesity by choice: The powerful effect of nutrient availability on nutrient intake. Am J Physiol. 2002;282:R1536–R1539. doi: 10.1152/ajpregu.00739.2001. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Byerly EA, Doman SA. Mice acquire flavor preferences during shipping. Physiol Behav. 2005;86:480–486. doi: 10.1016/j.physbeh.2005.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Food intakes, water intakes, and spout side preferences. 2001a doi: 10.1023/a:1020884312053. Mouse Phenome Database Web Site. Available from: http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&id=63. Accessed 26 May 2007. [DOI] [PMC free article] [PubMed]
- Tordoff MG, Bachmanov AA. Monell mouse taste phenotyping project. Philadelphia (PA): Monell Chemical Senses Center; 2001b. Available from: http://www.monell.org/MMTPP. Accessed 26 May 2007. [Google Scholar]
- Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses. 2002a;27:759–768. doi: 10.1093/chemse/27.9.759. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Survey of calcium and sodium intake and metabolism with bone and body composition data (MPD:103) 2002b Mouse Phenome Project. Available from: http://phenome.jax.org/pubcgi/phenome/mpdcgi?rtn=projects/details&id=103. Accessed 26 May 2007.
- Tordoff MG, Bachmanov AA. Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol Clin Exp Res. 2003a;27:600–606. doi: 10.1097/01.ALC.0000060529.30157.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Mouse taste preference tests: Influence of drinking spout position. 2003b doi: 10.1093/chemse/28.4.315. Available from: http://www.monell.org/MMTPP/Verification%20-%20Spout%20position.htm. Accessed 2007 June 4. [DOI] [PMC free article] [PubMed]
- Tordoff MG, Bachmanov AA. Mouse taste preference tests: Why only two bottles? Chem Senses. 2003c;28:315–324. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol Behav. doi: 10.1016/j.physbeh.2007.03.027. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Reed DR, Bachmanov AA. Forty mouse strain survey of water and sodium intake. Physiol Behav. doi: 10.1016/j.physbeh.2007.03.025. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Abel M, Huybers S, Hoenderop JGJ, van der Kemp AWCM, van Leeuwen JPTM, Bindels RJM. Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6. Am J Physiol Renal Physiol. 2006;291:F1177–F1183. doi: 10.1152/ajprenal.00038.2006. [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102:9960–9965. doi: 10.1073/pnas.0501632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WG. Ethanol preference in C57BL/6 and BALB/c mice at three ages and eight ethanol concentrations. Exp Aging Res. 1976;2:425–434. doi: 10.1080/03610737608258000. [DOI] [PubMed] [Google Scholar]
- Wurtman JJ, Wurtman RJ. Sucrose consumption early in life fails to modify the appetite of adult rats for sweet foods. Science. 1979;205:321–322. doi: 10.1126/science.451607. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Cowart BJ, Radil T. Nasal trigeminal chemosensitivity across the adult life span. Percept Psychophys. 2003;65:115–122. doi: 10.3758/bf03194788. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Komura S, Yasuhara M. The age of drinking onset and housing condition influences rat alcohol drinking behavior. Jap J Alcohol Stud Drug Depend. 2003;38:335–340. [PubMed] [Google Scholar]