Abstract
We examined the extent to which maintenance diet influences the taste preferences of mice. C57BL/6J (B6) and 129X1/SvJ (129) mice were fed one of three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, Purina 5001), a cereal-based diet formulated for breeding (Purina 5015), or two purified diets (AIN-76A or AIN-93G). The mice were given 48-h two-bottle choice tests between water and the following seven taste solutions: 2 mmol/L saccharin, 5 mmol/L citric acid, 50 mmol/L citric acid, 30 μmol/L quinine hydrochloride (QHCl), 300 μmol/L QHCl, 75 mmol/L NaCl, and 10% ethanol. There were very few differences in taste solution preference scores among mice of the same strain fed the three different versions of standard cereal-based diet. There were also very few differences in taste solution preference scores between mice of the same strain fed the two purified diets. However, the mice fed standard cereal-based diets generally drank more water and total fluid than did mice fed purified diets. There were larger differences between the B6 and 129 strains in saccharin and ethanol preference scores with mice fed standard cereal-based diets than purified diets. Conversely, there were larger differences between the B6 and 129 strains in citric acid and NaCl preference scores with mice fed purified diets than standard cereal-based diets. These results show that maintenance diet composition can have straindependent effects on taste solution preference. They illustrate that attention must be paid to the effects of diet on phenotype in screens of mutagenized mice and other genetic studies.
Keywords: saccharin, citric acid, quinine, NaCl, ethanol, taste preference, mice
The two-bottle choice test has been used for many years to assess the voluntary intake of nutrients and other solutions. The standard procedure involves giving animals, usually rats, a choice between a solution and water. The ratio of solution intake relative to the total intake of solution and water is considered a measure of preference. Tests are generally 48 h or longer; thus, intakes can be affected by oral, postingestive and experiential factors. However, for many compounds, taste seems to be the dominant determinant of the response to the solutions, and for convenience the method is usually referred to as a “taste preference” test with the tested solutions referred to as “taste solutions.”
The recent impetus to understand the genetic basis of taste perception has provided several challenges for the measurement of taste preferences. First, most genetic studies involve mice, which drink less than do rats. This is a challenge because it is difficult to measure small volumes accurately, the range of intakes is smaller and the errors because of spillage and evaporation are relatively greater. Second, many more animals must be tested than for previous research. For example, several hundred mice are required to identify most quantitative trait loci [e.g., (1,2)], and it is likely that several thousand mice will be required to identify taste-related mutations [e.g., (3)]. Third, genetic studies require highly reliable results. Although interpretation of most previous research has been based on differences between groups of animals, the results of a single mouse in genetic studies can be crucial. Inaccurate phenotyping can lead to incorrect localization of quantitative trait loci or a great deal of wasted effort trying to breed, genotype and phenotype the offspring of mice that do not have a genetic anomaly.
The issue of the robustness of behavioral phenotypes was recently emphasized by work showing that genetically identical mice tested using the same protocols in several different laboratories in North America produced inconsistent results, even though the investigators took heroic measures to maintain identical husbandry and experimental protocols (4). One of the most consistent behaviors across laboratories was ethanol intake in a 6-d, two-bottle choice test. Nevertheless, the investigators could not account for 52% of the variance in ethanol intake.
We suggested that much of this variance in ethanol intake might be attributable to the use of a cereal-based maintenance diet [often referred to as “chow”; (5)]. The composition of most cereal-based diets changes with the sources and quality of the natural ingredients, and diet composition influences taste solution acceptance of rats. For example, feeding rats a high fat diet increases their preference for fat (6,7) and decreases their preference for sucrose (8). Feeding rats diets low in calcium and several other minerals increases their preference for sodium [e.g., (9–11)] and reduces their preference for sweet compounds (12). However, the dietary manipulations used in these experiments are relatively large. There are no published data addressing whether minor variations in diet influence taste solution preference.
As part of a project to improve the efficiency and reliability of the two-bottle choice test for use in genetic studies (13), we examined the extent to which the mouse's maintenance diet influences its acceptance of taste solutions. We determined whether there were differences in taste solution acceptance between mice fed three of the most ubiquitous cereal-based diets used in mouse colonies (Teklad 8604, Zeigler NIH-07, and Purina 5001), a high fat, high-energy “breeder” diet (Purina 5015) and two purified diets (AIN-76A and AIN-93G). The ultimate goal of this work is to provide efficient methods that can be used to identify mutant mice with outlying taste phenotypes relative to population means from the same genetic background (13). Under these circumstances, the identification of such outliers depends upon both the difference of the individual from the population mean and the variance of the population. Tests that provide the greatest distinction between an outlier and its comparison group mean are considered to be the most sensitive. To model this, we compared the results of two strains of mice given various taste solutions to consume. We used the C57BL/6J (B6)3 and 129X1/SvJ (129) strains because these are frequently used in genetic studies, they will be the first strains to have their genomes sequenced, they are likely candidates for mutagenesis experiments and their taste preferences have been well characterized [e.g., (1,14,15)]. The taste solutions were representative sweet (2 mmol/L saccharin), sour (5 and 50 mmol/L citric acid), bitter (30 and 300 μmol/L quinine hydrochloride; QHCl), and salty (75 mmol/L NaCl) compounds, and 10% v/v ethanol. They were chosen because they covered a wide range of acceptance, from avidly preferred (saccharin) to strongly disliked (QHCl). Moreover, according to previous research, there were very large differences between the B6 and 129 strains in saccharin and ethanol preference, moderate differences incitric acid and NaCl preference, and little if any difference in QHCl preference [e.g., (14,15)].
MATERIALS AND METHODS
Male B6 and 129 mice (n = 96 each) were purchased from The Jackson Laboratory (Bar Harbor, Maine). The mice were 5 wk old when they arrived in our facility. They were individually housed in plastic “tub” cages (26.5 cm × 17 cm × 12 cm) with a stainless steel grid lid, and wood shavings scattered on the floor. The vivarium was maintained at 23°C on a 12-h light:dark cycle with lights off at 1900 h. The mice had deionized water to drink and were fed one of six diets (Table 1). All diets were provided as oval pellets (~25 × 16 × 10 mm), which were freely available from a hopper built into the cage lid.
TABLE 1.
Names, manufactures and major ingredients of diets tested
| Diet components |
|||||||
|---|---|---|---|---|---|---|---|
| Diet name(s) | Manufacturer and website | Type | Protein | Carbohydrate | Fat | Fiber | Energy content |
| g/kg (% of total energy) | kJ/g1 | ||||||
| Teklad 8604 a.k.a. Wayne Lab Blox |
Harlan, Madison, WI www.harlan.com/teklad |
Cereal-based diet2 | 245 (30%) | 466 (58%) | 44 (12%) | 37 | 13.0 |
| Zeigler NIH-07 Open Formula |
Zeigler Bros., Gardners, PA www.zeiglerfeed.com |
Cereal-based diet | 225 (27%) | 500 (60%) | 50 (13%) | 45 | 13.4 |
| Purina 5001 | PMI Nutrition International, Purina Mills, Richmond, IN www.labdiet.com |
Cereal-based diet | 234 (28%) | 499 (60%) | 45 (12%) | 53 | 12.6 |
| Purina 5015 | PMI Nutrition International, Purina Mills, Richmond, IN www.labdiet.com |
Cereal-based diet with high fat content, designed for breeding |
175 (18%) | 540 (56%) | 110 (26%) | 25 | 15.5 |
| AIN-76A | Dyets, Bethlehem, PA www.dyets.com |
Purified diet3 | 200 (21%) | 650 (68%) | 50 (12%) | 50 | 15.9 |
| AIN-93G | Dyets, Bethlehem, PA www.dyets.com |
Purified diet designed for growing rodents |
200 (20%) | 629 (64%) | 70 (16%) | 50 | 15.9 |
kJ/g = metabolizable energy per gram of diet.
The four cereal-based diets were supplied by North Penn Feeds (Lansdale, PA). Ingredients for the cereal-based diets are taken from materials provided by the manufacturers, which generally list minimum amounts of protein and fat, and maximum amount of fiber. Nitrogen-free extract is considered an approximation of carbohydrate content. The diets also differ in micronutrient and vitamin content, texture, and color. More detailed ingredient compositions can be found on the manufacturers' web sites.
The formulas for the purified diets were suggested by the American Institute of Nutrition (AIN), now named the American Society for Nutritional Sciences. The Dyets catalog numbers were 100000 for AIN-76A and 110700 for AIN-93G.
We tested 16 mice of each strain with most diets. However, because of deaths shortly after arrival, there were only 15 mice in the group of B6 mice fed Teklad 8604 and in the group of 129 mice fed AIN-93G. Before testing began, deionized water was available from an inverted 300-mL glass water bottle with a stainless steel spout. During tests, graduated drinking tubes were placed to the (mouse's) right of the food hopper. Their tips were 15 mm apart and extended into the cage 25 mm. Each spout had a 3.175-mm diameter hole from which the mice could lick fluids. Specifics of cage sizes, general maintenance conditions, construction of the drinking tubes and other general test procedures are available elsewhere (13). Experimental protocols were approved by the Animal Care and Use Committee of the Monell Chemical Senses Center, and complied with the NIH guidelines.
The mice were given 21 d to adapt to their new diets and laboratory conditions. They then received a series of eight 48-h two-bottle choice tests. The mice first had a choice between two identical drinking tubes of deionized water. They then had a choice between deionized water and each of the following compounds, one at a time, in the order listed: 2 mmol/L sodium saccharin, 5 mmol/L citric acid, 50 mmol/L citric acid, 30 μmol/L QHCl, 300 μmol/L QHCl, 75 mmol/L NaCl, and 10% ethanol. All compounds were obtained from Sigma Chemical (St. Louis, MO) except for the ethanol, which was obtained from Pharmco Products (Brookfield, CT). They were dissolved in deionized water and stored in glass bottles until required.
The taste solution was initially presented on the mouse's left but the positions of the two drinking tubes were switched after 24 h. Fluid intakes were measured to the nearest 0.1 mL every day in the middle of the light period. Body weights were measured at the beginning and end of the 16-d test series. Starting 3 d after the last two-bottle choice test, food intakes were measured over a 4-d period. The mice had one bottle of deionized water to drink during this test. The wire lid of each mouse cage, which contained the food, was weighed (± 0.1 g) and then reweighed 4 d later. Any large chunks of spilled food were collected and accounted for. As a control for evaporation, the lids of two empty cages containing each type of diet (10 cages total) were weighed at the same time as were the cages containing mice. However, the weight change of these empty cage lids was so small (<0.13 g/d) that it was ignored in subsequent analyses.
Data analyses
Fluid intakes from the two drinking tubes available each day for each mouse were collated according to the taste solution and maintenance diet (i.e., 8 taste solutions, including water × 6 diets = 48 tests). A total of 51 of the 10,144 measurements made were lost because of spillage or other technical errors. When this occurred, intakes from the other day of the 2-d test were used as an estimate of the missing value. This allowed us to avoid problems caused by within-subject unequal group sizes.
For each mouse on each day, solution preference ratios were calculated on the basis of the following formula, preference score (%) = taste solution intake/(taste solution intake + water intake) × 100. For conditions in which water was presented in both drinking tubes, the tube presented on the mouse's left was considered as a taste solution. A summary body weight for each mouse was determined by averaging the body weight measurement collected at the beginning and end of the 16-d two-bottle choice test series. Daily food intake was calculated by dividing the weight of food consumed during the 4-d test by 4.
Subsequent analyses were conducted using taste solution intakes, water intakes, total intakes, taste solution preference scores, body weights, and food intakes as dependent variables. We did not conduct analyses in which fluid intakes were adjusted for body weight because, although there were significant differences among the groups in body weight, these were small (Table 2) and thus had little influence on outcomes [see (16) for discussion of the problems related to adjustment for body weight in mice].
TABLE 2.
Body weights, food intakes, and water intakes of B6 and 129 mice fed various maintenance diets1
| Strain |
|||
|---|---|---|---|
| Diet | B6 | 129 | |
| Body weight,2 g | |||
| Teklad 8604a | 23.8 ± 0.4 | 22.2 ± 0.4 | |
| Zeigler NIH-07a | 23.4 ± 0.3 | 23.2 ± 0.5 | |
| Purina 5001a | 22.8 ± 0.4 | 22.1 ± 0.4 | |
| Purina 5015b | 25.1 ± 0.5 | 23.5 ± 0.4 | |
| AIN-76Ab | 25.7 ± 0.5 | 25.1 ± 0.4 | |
| AIN-93Ga | 23.8 ± 0.3 | 22.3 ± 0.5 | |
| Food intake,3 g/d | |||
| Teklad 8604a | 3.91 ± 0.06 | 4.13 ± 0.11 | |
| Zeigler NIH-07ab | 3.43 ± 0.07 | 4.06 ± 0.15 | |
| Purina 5001bc | 3.58 ± 0.08 | 3.85 ± 0.08 | |
| Purina 5015d | 3.20 ± 0.11 | 3.24 ± 0.05 | |
| AIN-76Ad | 3.28 ± 0.09 | 3.47 ± 0.07 | |
| AIN-93Gd | 2.85 ± 0.06 | 3.11 ± 0.06 | |
| Water intake,4 g/d | |||
| Teklad 8604a | 5.73 ± 0.24 | 5.70 ± 0.19 | |
| Zeigler NIH-07a | 5.83 ± 0.77 | 5.01 ± 0.29 | |
| Purina 5001a | 5.66 ± 0.16 | 5.34 ± 0.17 | |
| Purina 5015b | 4.77 ± 0.45 | 4.89 ± 0.53 | |
| AIN-76Ab | 4.41 ± 0.27 | 4.02 ± 0.15 | |
| AIN-93Gb | 4.30 ± 0.39 | 4.83 ± 0.50 | |
Values are means ± SEM, n = 15 or 16 per strain. Diet influenced body weights and intakes of both strains combined; diet names with different letter superscripts indicate that they differed significantly from each other (P < 0.01). Over all diets combined, the B6 mice weighed slightly but significantly more than did the 129 mice [F(1, 178) = 25.5, P < 0.00001]. The 129 mice ate significantly more food than did the B6 mice [(g/d): F(1, 178) = 29.4, P < 0.00001; (kJ/d): F(1, 177) = 28.7, P < 0.00001].
Body weight = mean of body weight measurements collected at beginning and end of 16-d two-bottle test series.
Food intake = average of 4-d test with only water to drink.
Water intake = intake from both bottles in two-bottle test of water vs. water.
The initial approach to hypothesis testing was to conduct omnibus ANOVA with factors of strain and diet. It was clear from these analyses that the results from mice fed the three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, and Purina 5001) generally concurred, as did the results from mice fed the two purified diets (AIN-76A and AIN-93G). We therefore conducted additional ANOVA as planned comparisons to examine differences in response related to the type of diet (standard cereal-based, breeder cereal-based and purified).
Tukey's post-hoc tests were conducted to distinguish between individual pairs of means. The analyses involved a great number of comparisons, leading to the strong possibility of Type II errors. A criterion of P < 0.01 was used for statistical significance as a compromise between the typical P < 0.05 significance level and a Bonferroni correction, which would be excessively restrictive. Readers who wish to apply other criteria for significance can derive exact probabilities from the F-test values presented in the text and tables, and in supplementary material (13).
RESULTS
The results are presented in the following manner: for each dependent variable, we first describe overall differences between the B6 and 129 strains. These main effects of strain are derived from the omnibus analyses and refer to the difference between B6 and 129 mice irrespective of their diet. We then describe the effects of diet on all mice (the B6 and 129 strains combined). There are three separate analyses involved, i.e., the comparison of mice fed the three standard cereal-based diets with each other, the comparison of mice fed the two purified diets with each other, and the comparison of the combined responses of mice fed standard cereal-based diets with the combined responses of mice fed purified diets and those fed Purina 5015. Finally, we describe interactions between strain and diet. If there were no differences in the response of mice fed each standard cereal-based diet or each purified diet (i.e., within diet type), then the interactions are based on analyses of diet types [i.e., strain (B6 and 129) × diet type (standard cereal-based diets, purified diet, Purina 5015)]. However, if differences within these groups were present, then a more complete description of the interaction based on the omnibus analyses is reported.
We tested two concentrations of citric acid and two of QHCl but the results from tests with similar compounds were not informative, except to demonstrate that the higher concentrations of these compounds were less preferred. Consequently, to save space, the results of the tests with 50 mmol/L citric acid and 300 μmol/L QHCl are not presented. A complete set of the raw data and results of all analyses can be found elsewhere (13).
Body weight
Strain differences
The B6 mice were heavier than the 129 mice when they arrived from the vendor at age 5 wk [B6, 18.2 ± 0.2 g; 129, 14.9 ± 0.2; F(1,178) = 188.6, P < 0.00001]. After 3 wk of maintenance consuming the six diets, the B6 mice still weighed slightly more than the 129 mice [Table 2, F(1,178) = 25.5, P < 0.00001].
Diet differences
Mice were randomly assigned to groups; thus it is not surprising that the 6 diet groups initially had similar body weights. However, at the start of taste tests, after 21 d of consuming the various diets, differences in body weight related to diet were present [F(5,178) = 11.5, P < 0.00001]. Mice fed standard cereal-based diets had similar body weights, mice fed AIN-76A were heavier than those fed AIN-93G [F(1,59) = 23.5, P < 0.00001] and mice fed standard cerealbased diets were lighter than were mice fed purified diets or Purina 5015 [F(2,84) = 13.4, P < 0.00001].
Strain × diet interactions
There was no evidence for an interaction between strain and diet on body weight, either when the mice arrived or when they began tests 3 wk later (Table 2).
Food intake
Strain differences
During the 4-d food intake test, the 129 mice ate more food than did the B6 mice [(g/d): F(1,178) = 29.4, P < 0.00001; (kJ/d): F(1,177) = 28.7, P < 0.00001; Table 2].
Diet differences
Mice fed Teklad 8604 ate more food than did mice fed Purina 5001 [F(2,89) = 5.91, P < 0.004]. Intakes of Zeigler NIH-07 fell between, and were not different from the other two standard cereal-based diets. There was no difference in intakes of AIN-76A and AIN-93G. Mice fed standard cerealbased diets ate more food by weight than did mice fed purified diets [(g): cereal-based diets, 3.8 ± 0.1; AIN, 3.2 ± 0.1; Purina 5015, 3.2±0.1; F(2,184)=82.8, P<0.00001; Table 2]; this was apparently attributable to the different energy densities of the diets because diet type had no influence on energy intake.
Strain × diet interactions.
There were no significant interactions between strain and diet that influenced food intake.
Fluid intake (water only available)
Strain differences
There were no differences between B6 and 129 mice in water intake when two bottles of water and no taste solutions were available (Table 2). Consistent with earlier results, 129 mice drank approximately two-thirds of their daily water intake from the left tube (67 ± 2% preference for the left tube), whereas B6 mice were indifferent (55 ± 1% preference for the left tube). These differences were eliminated by the switching of tube positions after 24 h of the 48-h test [2-d averages, preference for tube starting on the left (%): 129, 54 ± 2; B6, 51 ± 2].
Diet differences
Mice fed the three standard cereal-based diets had similar water intakes, as did mice fed the two purified diets. The mice fed standard cereal-based diets drank significantly more water than did those fed the purified diets. Water intakes of the mice fed Purina 5015 were intermediate between, and did not differ reliably from those fed standard cereal-based diets and purified diets [(mL): cereal-based diets, 5.5 ± 0.2; AIN, 4.4 ± 0.2; Purina 5015, 4.8 ± 0.3; F(2,184) = 11.9, P < 0.00002; Table 2].
Strain ×diet interactions
There were no significant interactions between strain and diet that influenced water intake.
Intake of 2 mmol/L saccharin
Strain differences
Irrespective of diet, B6 mice had significantly higher preferences for saccharin solution than did 129 mice (Fig. 1; Table 3). This was because the B6 mice had both significantly higher saccharin intakes and significantly lower water intakes than did 129 mice. The lower water intakes were insufficient to completely counteract the higher saccharin intakes; thus total fluid intakes were also significantly higher in B6 than 129 mice.
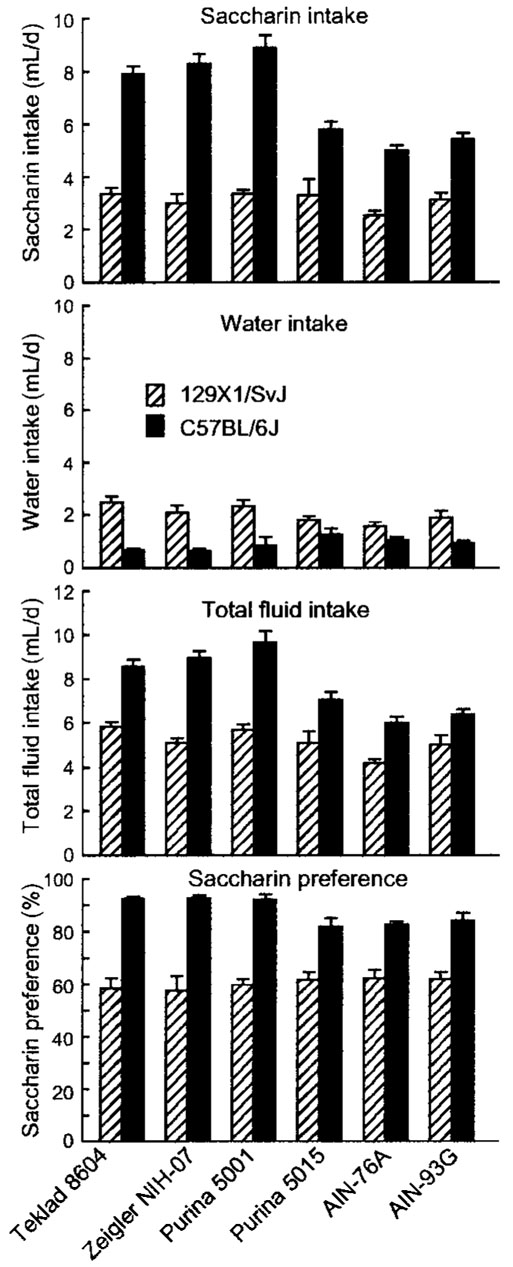
FIGURE 1.
Intake of 2 mmol/L saccharin solution, water, and total fluid, and saccharin preference in B6 and 129 mice fed one of three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, and Purina 5001), a “breeder” diet (Purina 5015), or two purified diets (AIN-76A and AIN-93G). Values are means ± SEM, n = 15 or 16 per strain for each diet. The following differences were significant (P < 0.01; see also Table 4): Saccharin intake: B6 > 129; standard cereal-based diets > Purina 5015 or purified diets; B6 fed standard cereal-based diets > B6 fed Purina 5015 or B6 fed purified diets: Water intake: B6 < 129; Total intake: B6 > 129; standard cereal-based diets > Purina 5015 or purified diets; B6 fed standard cereal-based diets > B6 fed Purina 5015 or B6 fed purified diets; Saccharin preference: B6 > 129; B6 fed standard cereal-based diets > B6 fed Purina 5015 or B6 fed purified diets.
TABLE 3.
Magnitude of the effects of strain and maintenance diet and their interaction on fluid intake and preference scores of B6 and 129 mice1
| Dependent variable |
|||||||
|---|---|---|---|---|---|---|---|
| Compound tested | Water intake | Solution intake | Total intake | Preference score | |||
| Main effect of strain (df = 1 and 178) | |||||||
| 2 mmol/L saccharin | 99.9***** | 396.5***** | 176.7***** | 267.9***** | |||
| 5 mmol/L citric acid | 4.1* | 3.3 | 0.0 | 6.2* | |||
| 30 μmol/L QHCl | 3.5 | 3.0 | 0.8 | 0.0 | |||
| 75 mmol/L NaCl | 26.4***** | 74.9***** | 14.1*** | 80.0***** | |||
| 10% ETOH | 264.8***** | 331.6***** | 3.1 | 568.4***** | |||
| Main effect of diet (df = 5 and 178) | |||||||
| 2 mmol/L saccharin | 0.7 | 16.4***** | 16.9***** | 0.7 | |||
| 5 mmol/L citric acid | 17.4***** | 1.7 | 13.9***** | 1.3 | |||
| 30 μmol/L QHCl | 6.3**** | 3.9** | 6.2**** | 5.3*** | |||
| 75 mmol/L NaCl | 5.1*** | 0.8 | 4.1** | 2.8* | |||
| 10% ETOH | 2.1 | 8.9***** | 4.5*** | 6.7**** | |||
| Strain × diet interaction (df = 5 and 178) | |||||||
| 2 mmol/L saccharin | 3.8** | 10.9***** | 5.0*** | 3.1* | |||
| 5 mmol/L citric acid | 4.5*** | 0.5 | 2.7* | 2.5* | |||
| 30 μmol/L QHCl | 1.9 | 0.8 | 1.0 | 2.0 | |||
| 75 mmol/L NaCl | 2.6* | 1.4 | 1.3 | 3.4** | |||
| 10% ETOH | 5.5*** | 6.4**** | 1.4 | 5.5** | |||
Numbers in the body of the table are F-values obtained from omnibus ANOVA:
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001;
P < 0.00001;
Diet differences
There were no differences in saccharin intake, water intake, total intake or saccharin preference scores among mice fed the three standard cereal-based diets. There was also no difference between mice fed the two purified diets. However, relative to the other two diet types, mice fed standard cereal-based diets had greater saccharin intakes [(mL/d): cereal-based diets, 5.8 ± 0.3; AIN, 4.0 ± 0.2; Purina 5015, 4.5 ± 0.4; F(2,184) = 38.2, P < 0.00001] and total intakes [(mL/d): cereal-based diets, 7.3 ± 0.2; AIN, 5.4 ± 0.2; Purina 5015, 6.1 ± 0.4; F(2,184) = 38.6, P < 0.00001], but the diet types did not significantly influence water intakes or saccharin preference scores.
Strain × diet interactions
There were no strain × diet interactions involving mice fed the same type of diet. However, B6 mice fed standard cereal-based diets drank significantly more saccharin, more total fluid and had higher saccharin preferences than did B6 mice fed either purified diets or Purina 5015. In contrast, diet type had no effect on the response of 129 mice to saccharin [Fig. 1; interactions, saccharin intake, F(2,184) = 25.7, P < 0.00001; total intake, F(2,184) = 10.1, P < 0.00007, saccharin preference score, F(2, 184) = 7.50, P < 0.0008].
Intake of 5 mmol/L citric acid
Strain differences
B6 mice had slightly but significantly higher preference scores for 5 mmol/L citric acid than did 129 mice [26 ± 2% vs. 21 ± 2%; Fig. 2; Table 3]. This was attributable to nonsignificant tendencies for the B6 mice to have higher citric acid intakes [1.3 ± 0.1 vs. 1.1 ± 0.1 mL/d] and lower water intakes [3.7 ± 0.1 vs. 4.0 ± 0.1 mL/d]. Total intakes also did not differ between the two strains.
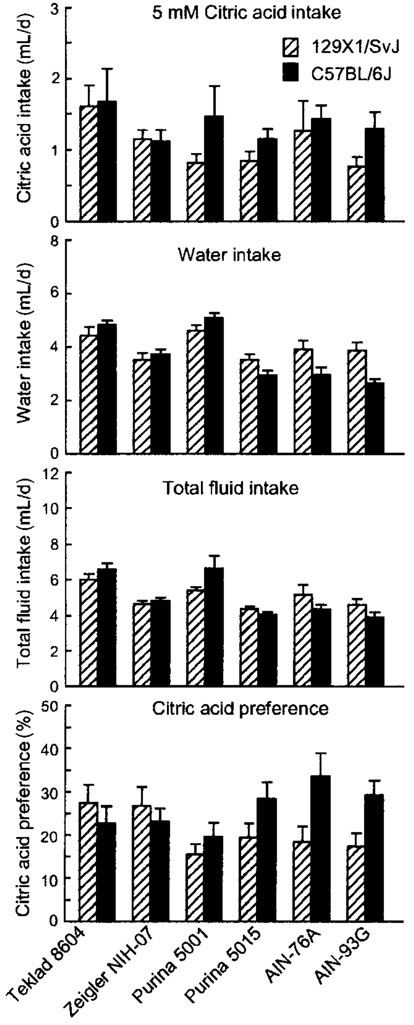
FIGURE 2.
Intake of 5 mmol/L citric acid solution, water, and total fluid, and citric acid preference in B6 and 129 mice fed one of three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, and Purina 5001), a “breeder” diet (Purina 5015), or two purified diets (AIN-76A and AIN-93G). Values are means ± SEM, n = 15 or 16 per strain for each diet. The following differences were significant (P < 0.01; see also Table 4): Citric acid intake: no differences; Water intake: standard cerealbased diets > Purina 5015 or purified diets; B6 fed standard cerealbased diets > B6 fed Purina 5015 or B6 fed purified diets; Total intake: standard cereal-based diets > Purina 5015 or purified diets; Citric acid preference, B6 > 129; B6 fed standard cereal-based diets < B6 fed Purina 5015 or B6 fed purified diets.
Diet differences
Relative to mice fed purified diet or Purina 5015, mice fed standard cereal-based diets had markedly higher water intakes [(mL/d): cereal-based diets, 4.4 ± 0.1; AIN, 3.3 ± 0.2; Purina 5015, 3.2 ± 0.2; F(2,184) = 25.2, P < 0.00001] and total fluid intakes [(mL/d): cereal-based diets, 5.7 ± 0.2; AIN, 4.5 ± 0.2; Purina 5015, 4.2 ± 0.1; F(2,184) = 20.3, P < 0.00001]. However, there were no effects of diet type on citric acid intake or preference scores.
Strain × diet interaction
There was a significant interaction involving water intake when 5 mmol/L citric acid was available. B6 mice fed standard cereal-based diets drank significantly more water than those fed purified diet or Purina 5015, whereas water intake of 129 mice was unaffected by diet [F(2,184) = 9.53, P < 0.0002]. This led to an interaction involving citric acid preference scores (P < 0.05; Table 3), with significantly larger differences between B6 and 129 mice fed purified diets than standard cereal-based diets.
Intake of 30 μmol/L QHCl
Strain differences
There were no differences between B6 and 129 mice in any response when 30 μmol/L QHCl was available (Fig. 3).
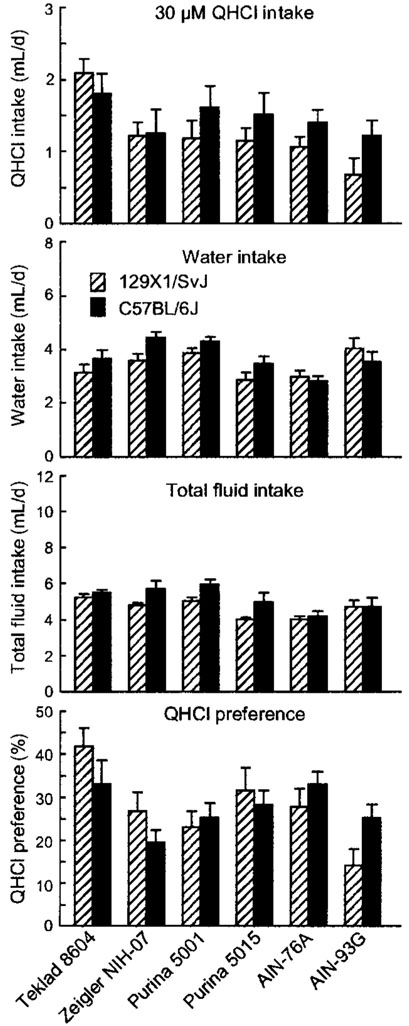
FIGURE 3.
Intake of 30 μmol/L quinine hydrochloride (QHCl) solution, water, and total fluid, and QHCl preference in B6 and 129 mice fed one of three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, and Purina 5001), a “breeder” diet (Purina 5015), or two purified diets (AIN-76A and AIN-93G). Values are means ± SEM, n = 15 or 16 per strain for each diet. The following differences were significant (P < 0.01; see also Table 4): QHCl intake: Teklad 8604 > Zeigler NIH-07 or Purina 5001; Water intake: AIN-76A < AIN-93G; standard cereal-based diets > Purina 5015 or purified diets; Total intake: standard cereal-based diets > purified diets; QHCl preference: Teklad 8604 > Zeigler NIH-07 or Purina 5001; AIN-76A > AIN-93G; 129 fed AIN-76A > 129 fed AIN-93G.
Diet differences
Mice fed Teklad 8604 had significantly higher preferences for 30 μmol/L QHCl than did mice fed Zeigler NIH-07 or Purina 5001 [F(2,89) = 7.89, P < 0.0004]. This was due largely to a tendency for higher solution intakes of the groups fed Teklad 8604 [F(1,89) = 4.83, P < 0.03]. Preference scores of the groups fed AIN-76A were higher than those fed AIN-93G [F(1,56) = 8.63, P < 0.005], which was attributable to lower water intakes in the AIN-76A–fed groups than AIN-93G–fed groups [F(1,56) = 7.34, P < 0.01]. There were differences in water intake and total fluid intake among mice fed the three diet types, but because there were differences between mice fed the same diet type (both for standard cereal-based diets and purified diets), these were not meaningful.
Strain × diet interaction
There were no interactions between diet and strain in any of the analyses.
Intake of 75 mmol/L NaCl
Strain differences
B6 mice drank significantly more NaCl, less water, more total fluid and had significantly higher NaCl preference scores than did 129 mice (Fig. 4, Table 3).
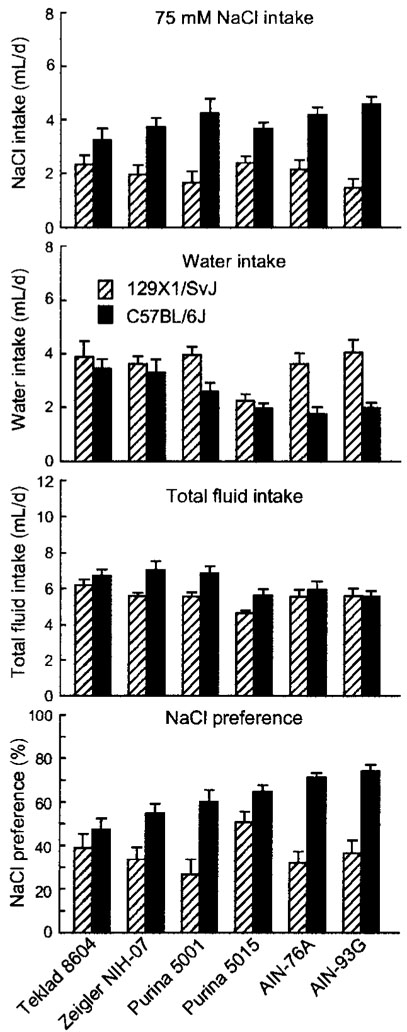
FIGURE 4.
Intake of 75 mmol/L NaCl solution, water, and total fluid, and NaCl preference in B6 and 129 mice fed one of three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, and Purina 5001), a “breeder” diet (Purina 5015), or two purified diets (AIN-76A and AIN-93G). Values are means ± SEM, n = 15 or 16 per strain for each diet. The following differences were significant (P < 0.01; see also Table 4): NaCl intake: B6 > 129; Water intake: B6 < 129; Purina 5015 < standard cereal-based diets or purified diets; B6 fed standard cereal-based diets > B6 fed Purina 5015 or B6 fed purified diets; Total intake: B6 > 129; Purina 5015 < standard cereal-based diets or purified diets; NaCl preference: B6 > 129; Purina 5015 > standard cereal-based diets or purified diets; B6 fed Teklad 8604 or B6 fed Zeigler NIH-07 < B6 fed AIN-76A or B6 fed AIN-93G; 129 fed Purina 5015 > 129 fed Purina 5001 or 129 fed AIN-76A.
Diet differences
There were no differences among mice fed the three standard cereal-based diets in any response, nor were there differences between the mice fed purified diets. The mice fed Purina 5015 had significantly greater preference scores for NaCl than did the mice fed standard cereal-based diets or purified diets [(%): cereal-based diets, 44 ± 3; AIN, 49 ± 3; Purina 5015, 58 ± 3; F(2,184) = 6.15, P < 0.003]. This was attributable to significant differences in water intake [(mL/d): cereal-based diets, 3.5 ± 0.2; AIN, 2.8 ± 0.2; Purina 5015, 2.1 ± 0.1; F(2,184) = 11.9, P < 0.00002] and total intake [(mL/d): cereal-based diets, 6.3 ± 0.1; AIN, 5.7 ± 0.2; Purina 5015, 5.2 ± 0.2; F(2,184) = 10.2, P < 0.0007], but not NaCl intake [(mL/d): cereal-based diets, 2.9 ± 0.2; AIN, 2.9 ± 0.2; Purina 5015, 3.0 ± 0.2].
Strain × diet interactions
There was a significant interaction involving water intake when NaCl was available [F(2,184) = 5.22, P < 0.007]. B6 mice fed cereal-based diet drank significantly more water than did B6 mice fed purified diet or Purina 5015. However, 129 mice fed cereal-based diet or purified diet drank significantly more water than did 129 mice fed Purina 5015. This led to an interaction involving NaCl preference scores (Table 3), with significantly larger differences between B6 and 129 mice fed AIN-76A and AIN-93G than Teklad 8604 or Purina 5015. The Zeigler NIH-07 and Purina 5001 diets produced strain differences intermediate in magnitude between these two extremes (Fig. 4).
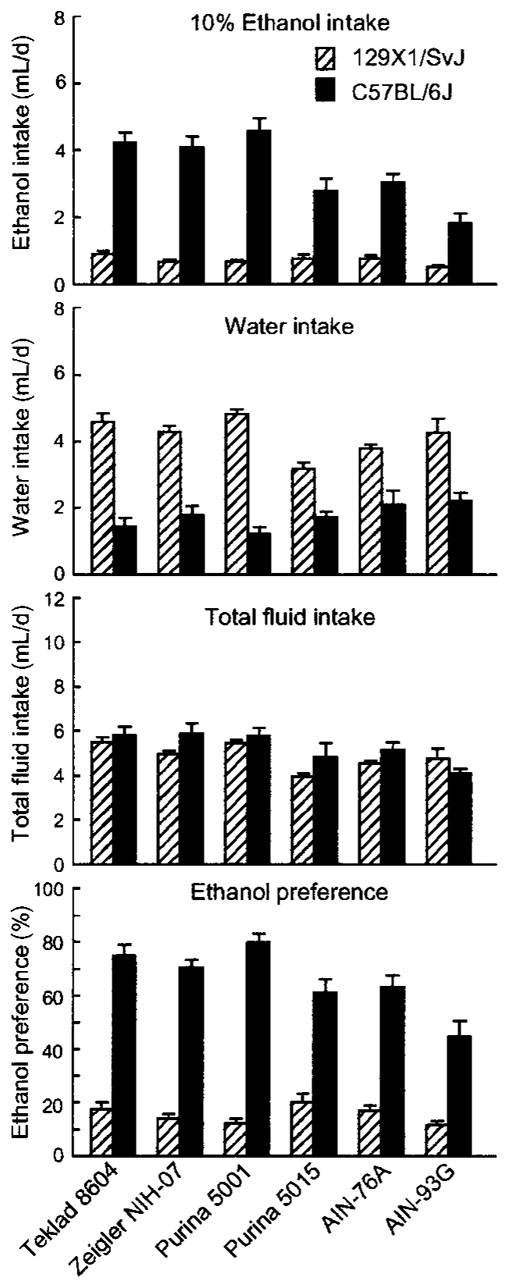
Intake of 10% ethanol
Strain differences
B6 mice drank significantly more ethanol and had significantly higher ethanol preference scores than did 129 mice (Fig. 5., Table 3). They drank significantly less water, in a manner reciprocal to ethanol intake, so that there was no difference between the strains in total fluid intake.
FIGURE 5.
Intake of 10% ethanol solution, water, and total fluid, and ethanol preference in B6 and 129 mice fed one of three standard cereal-based diets (Teklad 8604, Zeigler NIH-07, and Purina 5001), a “breeder” diet (Purina 5015), or two purified diets (AIN-76A and AIN-93G). Values are means ± SEM, n = 15 or 16 per strain for each diet. The following differences were significant (P < 0.01; see also Table 4): Ethanol intake: B6 > 129; AIN-76A > AIN-93G; standard cereal-based diets > Purina 5015 or purified diets; B6 mice fed standard cerealbased diets > B6 mice fed Purina 5015 or B6 mice fed purified diets; Water intake: B6 < 129; 129 mice fed standard cereal-based diets > 129 mice fed Purina 5015; Total intake: standard cereal-based diets > Purina 5015 or purified diets; Ethanol preference: B6 > 129; AIN-76A > AIN-93G; standard cereal-based diets > Purina 5015 or purified diets; B6 mice fed standard cereal-based diets > B6 mice fed Purina 5015 or B6 mice fed purified diets.
Diet differences
There were no differences among mice fed the three standard cereal-based diets in any response. Relative to mice fed AIN-93G, mice fed AIN-76A drank significantly more ethanol [F(1,56) = 13.9, P < 0.0005] and had higher ethanol preference scores [F(1,56) = 12.0, P < 0.001]. Mice fed the three standard cereal-based diets drank significantly more ethanol and had significantly higher ethanol preference scores than did mice fed purified diets or Purina 5015 [ethanol intakes (mL/d): standard cereal-based diets, 2.5 ± 0.2; AIN, 1.5 ± 0.2; Purina 5015, 1.8 ± 0.3; F(2,184) = 10.6, P < 0.00005; preference scores (%): standard cerealbased diets, 44 ± 3; AIN, 34 ± 3; Purina 5015, 31 ± 5; F(2,184) = 7.88, P < 0.0006].
Strain × diet interactions
In the analyses involving diet types, there were significant interactions involving ethanol intake [F(2,184) = 10.6, P < 0.00005; water intake, F(2,184) = 11.0, P < 0.00004; and ethanol preference, F(2,184) = 9.28, P < 0.0002]. For ethanol intake and preference, B6 mice fed standard cereal-based diets had significantly higher values than did B6 mice fed purified diet or Purina 5015. However, there was no effect of diet type on 129 mice. For water intake, the 129 mice fed standard cereal-based diets drank significantly more than did 129 mice fed Purina 5015. The 129 mice fed purified diets were intermediate between, and did not differ significantly from the mice fed cereal-based diets or Purina 5015. There were no effects of diet type on water intake of B6 mice.
Effect of diet on variability of the response to various taste solutions
To determine the effect of diet on the sensitivity of twobottle choice tests (see above), we compared the variability of the response in solution intakes and preference scores produced by each diet. To do this, we normalized all data from each test to the B6 group mean. That is, we calculated Zscores, based on the mean of the B6 group and the average standard deviations of the B6 and 129 groups. The normalized values of the 129 mice were collated for each diet condition and then compared using one-way ANOVA [Table 4; see (15) for additional justification]. To conserve space, only the analyses of taste preferences are presented here [raw Z-scores for intake and preference are available elsewhere (13)].
TABLE 4.
Influence of diet on the variability of preference scores: deviation of B6 and 129 mice from the mean value of the B6 strain1
| Taste solution and strain |
Teklad 8604 | Zeigler NIH-07 | Purina 5001 | Purina 5015 | AIN-76A | AIN-93G | |
|---|---|---|---|---|---|---|---|
| 2 mmol/L saccharin | |||||||
| B6 | 0.00 ± 0.10 | 0.00 ± 0.09 | 0.00 ± 0.26 | 0.00 ± 0.24 | 0.00 ± 0.15 | 0.00 ± 0.19 | |
| 129 | 3.75 ± 0.41b | 2.70 ± 0.41ab | 3.60 ± 0.24b | 1.92 ± 0.26a | 2.47 ± 0.35ab | 2.39 ± 0.32ab | |
| 5 mmol/L citric acid | |||||||
| B6 | 0.00 ± 0.25 | 0.00 ± 0.22 | 0.00 ± 0.28 | 0.00 ± 0.26 | 0.00 ± 0.27 | 0.00 ± 0.29 | |
| 129 | −0.22 ± 0.26a | −0.21 ± 0.28a | 0.45 ± 0.22ab | 0.63 ± 0.24ab | 0.73 ± 0.23b | 1.04 ± 0.21b | |
| 30 μmol/L QHCl | |||||||
| B6 | 0.00 ± 0.29 | 0.00 ± 0.21 | 0.00 ± 0.23 | 0.00 ± 0.21 | 0.00 ± 0.21 | 0.00 ± 0.22 | |
| 129 | −0.43 ± 0.22a | −0.45 ± 0.29a | 0.20 ± 0.27b | 0.07 ± 0.29b | 0.40 ± 0.29b | 0.76 ± 0.29b | |
| 75 mmol/L NaCl | |||||||
| B6 | 0.00 ± 0.22 | 0.00 ± 0.23 | 0.00 ± 0.23 | 0.00 ± 0.19 | 0.00 ± 0.14 | 0.00 ± 0.17 | |
| 129 | 0.30 ± 0.29a | 1.00 ± 0.27ab | 1.38 ± 0.27abc | 0.86 ± 0.31ab | 2.54 ± 0.36c | 2.17 ± 0.35ab | |
| 10% ethanol | |||||||
| B6 | 0.00 ± 0.34 | 0.00 ± 0.36 | 0.00 ± 0.35 | 0.00 ± 0.29 | 0.00 ± 0.34 | 0.00 ± 0.41 | |
| 129 | 4.65 ± 0.17c | 3.94 ± 0.14bc | 6.66 ± 0.15d | 2.64 ± 0.21a | 3.79 ± 0.16b | 2.29 ± 0.10a | |
Values in the body of the text show mean ± SEM of Z-scores, based on the mean of the B6 group and the average standard deviations of the B6 and 129 groups. Thus, for example, for 2 mmol/L saccharin, the mean of the 129 mice fed Teklad 8604 differed from the mean of B6 mice by 3.75 SD. A significant difference in the ANOVA signifies that there were differences among the diets in their ability to distinguish B6 from 129 mice. Values with the same superscript did not differ significantly (P < 0.01, Tukey's test).
Significant effects of diet were found for all taste solutions except 30 μmol/L QHCl (which was significant at the P < 0.05 level; Table 4). When saccharin and ethanol were available, the 129 groups fed the standard cereal-based diets had the largest deviations from the B6 means. For the other solutions, the largest differences were found with the 129 groups fed purified diets. It was clear that the statistical effects were attributable to differences in the separation of strain means; there were no consistent differences in within-strain variability (i.e., the standard errors in Table 4).
DISCUSSION
On the whole, mice fed different versions of diets of the same type (i.e., standard cereal-based diets or purified diets) had similar responses to each taste solution. However, many of the responses of mice fed standard cereal-based diets differed from those of mice fed purified diets. These results demonstrate that the maintenance diet mice are fed influences their taste solution intakes and preference scores. Moreover, the effects of diet are specific to the taste solution and genotype being investigated.
Influence of diet on the variability of response to taste solutions
Unlike purified diets, which are rigorously formulated and made from relatively pure ingredients, cereal-based diets are made from natural ingredients. The nutrient composition of natural ingredients varies; thus, the nutrient content of cereal-based diets varies accordingly. Manufacturers of these diets provide chemical composition sheets (Table 1), but these are simply averages of many assays. The composition of one frequently used cereal-based diet (Purina 5001) is analyzed by the manufacturer only approximately three times per year (17). Based on these considerations, we hypothesized that the poorer control of ingredients in cereal-based diets would lead to higher variability in response relative to mice fed purified diets. However, the results did not support this hypothesis, i.e., in all tests, measures of within-strain variability were very similar for mice fed cereal-based diet and those fed purified diets.
Our experiment underrepresented the variability inherent in the use of laboratory cereal-based diet under most circumstances because the diets we used most likely came from the same batches; that is, each mouse in the same group ate diet that was formulated, mixed, packaged and shipped at the same time. Thus, it is probable that all the mice fed a particular brand of diet ate identical ingredients, even though the exact diet constitution was unknown and uncontrolled. This would not necessarily be the case if different investigators received cereal-based diet from different lots, or if an investigator attempted a replication at a later time. Nevertheless, the finding that mice fed different brands of cereal-based diet had similar taste preference responses argues that minor differences in diet formulation do not necessarily cause differences in taste preferences.
The mice in these experiments were fed purified diets for only 3 wk before taste tests began. All mice (and their mothers) were fed cereal-based diet while at The Jackson Laboratory, before they arrived in our facility. It is possible that differences in ingredients the mice ate when they were young could influence behavior throughout life, even when they were fed purified diets.
Given our predictions concerning increased variability with cereal-based diets, we were surprised to find that strain differences were attributable to different effects of the diets on strain means, not on strain variances. This was also seen in a recent study comparing two-bottle choice tests of various durations. Contrary to expectations, the variance associated with short tests (1 or 2 d) was no greater than that associated with long tests [4 or 6 d; (15)]. The finding that the difference in response is mediated by differences in strain means argues that the effects are attributable to an interaction of diet with genes rather than differences in the variability produced by the various diets.
Differences in taste solution preference of mice fed diets of different types
Differences in saccharin and ethanol preference scores between B6 and 129 mice were larger if the mice were fed standard cereal-based diets rather than purified diets. In contrast, differences in citric acid, NaCl and perhaps QHCl preference scores between B6 and 129 mice were larger if the mice were fed purified diets rather than standard cereal-based diets (interpretation of the results with QHCl depend on the statistical test used; see Fig. 3). The six diets tested differed in many properties, including nutrient, mineral, vitamin and fiber composition, texture and orosensory characteristics, any one or combination of which could be responsible for this complex finding. An attractive possibility involves an interaction between the sweetness of the diet with sweet solution consumption. Saccharin and ethanol have sweetness in common because ethanol has a sweet component to mice (14,18–20). Because of their high sugar content, the purified diets are sweet, unlike the cereal-based diets. It may be that this sweetness inhibits intake of other sweet compounds by a mechanism akin to sensory specific satiety (21,22). The differential response would be observed in B6 but not 129 mice because unlike the B6 strain, the 129 strain has a “low preferring” allele of the Tas1r3 (sweet receptor) gene that causes it to be relatively insensitive to sweet compounds (23). However, this explanation does not account for the results of mice fed Purina 5015 “breeder” diet, which is a cereal-based diet but which produced a pattern of results similar to those of mice fed purified diets.
An alternative possibility is that the intake of saccharin and ethanol is related to diet energy density. The three standard cereal-based diets contained less energy (and more indigestible content) per gram than did the breeder diet or purified diets. It may be that the B6 mice, which are more prone to dietary obesity than are 129 mice (24), compensate for the lower energy density of the standard cereal-based diets by consuming more saccharin and ethanol. In this scheme, the mice drink ethanol because it is a source of energy. They drink saccharin because, being sweet, it is a signal for energy (25). A related possibility is that the intake of taste solutions relies on a balance between the palatability of the taste solution and the palatability of the maintenance diet. Differences in response to the maintenance diet are more obviously reflected in saccharin and ethanol intakes because these are the most palatable of the taste solutions we tested, at least for the B6 mice. It will require additional work to discriminate among these (and other) potential explanations.
Appropriate diet for genetic studies using the two-bottle choice test
A major impetus for this study was to determine the most suitable diet to feed mice while measuring phenotypes in mutagenesis screens and other genetic studies (see introduction). Currently, nearly all investigators conducting behavioral phenotyping (of any behavior, not just taste-related behavior) feed their subjects a standard cereal-based diet. Different facilities use different brands. In the direct comparisons conducted here, we found a few differences in response between mice fed different standard cereal-based diets but these were inconsistent and thus probably due to chance. We conclude from this that the brand of cereal-based diet has little influence on taste solution intakes or preference scores; therefore, at least from the perspective of conducting standardized two-bottle choice tests, there seems little need for all laboratories to use a particular brand of cereal-based diet.
There were consistent differences in response related to whether the mice were fed standard cereal-based diets or purified diets. The cereal-based diets produced larger differences between B6 and 129 mice in saccharin and ethanol preference scores than did the purified diets; in contrast, the purified diets produced larger differences between B6 and 129 mice in preference scores for citric acid, NaCl and perhaps QHCl. Thus, the “best” type of diet to use depended on which taste compounds were being tested. The ideal approach would be to match diet to the taste solution being tested but this is usually impractical because it is too time-consuming to change diets during a battery of phenotyping tests containing several taste solutions.
Given the different effects of diet on consumption of different taste solutions, a case can be made for using either cereal-based or purified diets for taste phenotyping. However, there are other advantages and disadvantages of each diet type that investigators planning phenotyping studies should consider. The chief advantages of using cereal-based diet are the low cost and the wealth of existing data available. The chief advantages of purified diets are that they have defined constituents, they can be closely replicated and they can be easily manipulated to allow examination of the interaction between genes, diet and behavior. We suspect that the rigorous controls afforded by purified diets outweigh the advantages of cerealbased diets, and this will become increasingly apparent as other gains toward reducing environmental variance occur (e.g., the elimination of viral infections, better controls for perinatal effects on behavior). Nevertheless, the results found here suggest that choosing the best diet for analyzing a particular phenotype is not straightforward. Our results provide guidelines for choosing the most appropriate diet for testing specific taste solutions but a general recommendation covering all taste solutions can not be made.
ACKNOWLEDGMENTS
The expert technical assistance of Lindsey Curtis and Sydrick Rabusa is gratefully acknowledged.
Footnotes
Supported by grant AA-12715 from the National Institutes of Health.
Abbreviations used: 129, 129X1/SvJ mice; AIN, AIN-76A and AIN-93G diets; B6, C57BL/6J mice; QHCl, quinine hydrochloride.
LITERATURE CITED
- 1.Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm. Genome. 1997;8:545–548. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal chromosome 4. Mamm. Genome. 2001;12:13–16. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schimenti J, Bucan M. Functional genomics in the mouse: phenotype-based mutagenesis screens. Genome Res. 1998;8:698–710. doi: 10.1101/gr.8.7.698. [DOI] [PubMed] [Google Scholar]
- 4.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science (Wash., DC) 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 5.Tordoff MG, Bachmanov AA, Friedman MI, Beauchamp GK. Testing the genetics of behavior in mice [Letter] Science (Wash., DC) 1999;285:2069. [PubMed] [Google Scholar]
- 6.Reed DR, Friedman MI. Diet composition alters the preference for fat by rats. Appetite. 1990;14:219–230. doi: 10.1016/0195-6663(90)90089-q. [DOI] [PubMed] [Google Scholar]
- 7.Reed DR, Tordoff MG, Friedman MI. Enhanced acceptance and metabolism of fats by rats fed a high-fat diet. Am. J. Physiol. 1991;261:R1084–R1088. doi: 10.1152/ajpregu.1991.261.5.R1084. [DOI] [PubMed] [Google Scholar]
- 8.Bertino M, Wehmer F. Dietary influences on the development of sucrose acceptability in rats. Dev. Psychobiol. 1981;14:19–28. doi: 10.1002/dev.420140104. [DOI] [PubMed] [Google Scholar]
- 9.Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. Am. J. Physiol. 1996;271:R1–R10. doi: 10.1152/ajpregu.1996.271.1.R1. [DOI] [PubMed] [Google Scholar]
- 10.Tordoff MG. Salt intake of rats fed diets deficient in calcium, iron, magnesium, phosphorus, potassium, or all minerals. Appetite. 1992;18:29–41. doi: 10.1016/0195-6663(92)90208-n. [DOI] [PubMed] [Google Scholar]
- 11.Tordoff MG. Calcium: taste, intake and appetite. Physiol. Rev. 2001;81:1567–1597. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- 12.Tordoff MG, Rabusa SH. Calcium-deprived rats avoid sweet compounds. J. Nutr. 1998;128:1232–1238. doi: 10.1093/jn/128.7.1232. [DOI] [PubMed] [Google Scholar]
- 13.Tordoff MG, Bachmanov AA. Monell mouse taste phenotyping project. Monell Chemical Senses Center. www.monell.org/MMTPP, accessed Apr. 29, 2002. [Google Scholar]
- 14.Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin. Exp. Res. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle choice test. Chem. Senses. 2002 doi: 10.1093/chemse/27.9.759. in press. [DOI] [PubMed] [Google Scholar]
- 16.Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav. Genet. 1998;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strobel V. What Do We Mean by Quality Assurance and Control? Purina Mills LabDiet Animal Nutrition Symposia; Philadelphia, PA: 2000. [Google Scholar]
- 18.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav. Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- 20.Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem. Senses. 1993;18:509–522. [Google Scholar]
- 21.Rolls BJ. Sensory specific satiety. Nutr. Rev. 1986;44:93–101. doi: 10.1111/j.1753-4887.1986.tb07593.x. [DOI] [PubMed] [Google Scholar]
- 22.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am. J. Clin. Nutr. 1990;51:963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 23.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem. Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol. Behav. 2001;72:603–613. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tordoff MG. How do nonnutritive sweeteners increase food intake? Appetite. 1998;11:5–11. [PubMed] [Google Scholar]