Abstract
Objective
To investigate whether timing of intensive insulin therapy (IIT) after intensive care unit (ICU) admission influences outcome.
Design and setting
Single-center prospective cohort study in the 14-bed medical ICU of a 1,171-bed tertiary teaching hospital.
Patients
The study included 127 patients started on ITT within 48 h of ICU admission (early group) and 51 started on ITT thereafter (late group); the groups did not differ in age, gender, race, BMI, APACHE III, ICU steroid use, admission diagnosis, or underlying comorbidities.
Measurements and results
The early group had more ventilator-free days in the first 28 days after ICU admission (median 12 days, IQR 0–24, vs. 1 day, 0–11), shorter ICU stay (6 days, IQR 3–11, vs. 11 days, vs. 7–17), shorter hospital stay (15 days, IQR 9–30, vs. 25 days, 13–43), lower ICU mortality (OR 0.48), and lower hospital mortality (OR 0.27). On multivariate analysis, early therapy was still associated with decreased hospital mortality (ORadj 0.29). The strength and direction of association favoring early IIT was consistent after propensity score modeling regardless of method used for analysis.
Conclusions
Early IIT was associated with better outcomes. Our results raise questions about the assumption that delayed administration of IIT has the same benefit as early therapy. A randomized study is needed to determine the optimal timing of therapy.
Keywords: Hyperglycemia, Critical illness, Insulin, Mortality
Introduction
The association between hyperglycemia and critical illness was originally described in a canine model of hemorrhagic shock [1]. Stress hyperglycemia, with or without underlying diabetes, is a frequent problem in the critically ill patient [2]. In recent decades hyperglycemia has been reexamined as a marker of severity of illness, and questions have been raised as to whether this is a distinct condition requiring specific treatment [3–8]. Its association with poor outcomes is now well described in many different clinical scenarios [9–11]. In 2001 a protocol-driven intravenous insulin therapy demonstrated significant reductions in morbidity and mortality in surgical ICU patients [12]. Unanswered questions and controversy abound [13], however, regarding intensive insulin therapy (IIT), in part fueled by recent trials such as the German sepsis trial VISEP [14] and the multicenter European trial GLUCONTROL [15]. Both studies were stopped early because of safety concerns. The follow-up Leuven medical intensive care unit (MICU) trial showed no mortality benefit in intention-to-treat analysis, although benefit was again demonstrated in the target population who required an ICU stay of 3 days or more [16]. On subgroup analysis potential harm was seen in short-term stayers. In both Leuven studies improvements in mortality occurred as a delayed event several weeks after ICU admission.
In light of the potential harm in short-term stayers, delayed clinical benefit and limitations in accurately predicting length of stay (LOS) at the time of ICU admission, some have suggested delaying IIT for hyperglycemia until 2–3 days after admission [17, 18]. Others have advocated aggressive and early therapy by incorporating this as part of an early goal-directed sepsis protocol in the emergency department [19]. Data supporting either of these approaches is lacking. We investigated whether timing of IIT after ICU admission influences outcomes [20].
Methods
Study design
All 670 adult patients admitted to the closed MICU at Mount Sinai Medical Center, New York, N.Y., between 22 February and 21 December 2006 who were started on the nurse-driven IIT protocol (available online as Electronic Supplementary Material) were eligible for this prospective, single-center cohort study. Critically ill patients in the MICU were eligible for IIT when more than one blood glucose exceeded 110 mg/dl and the anticipated LOS in the MICU was greater than 24 h (n = 499). Patients younger than 18 years and those receiving treatment of diabetic ketoacidosis or hyperosmolar hyperglycemic state were excluded. Decisions regarding preinfusion subcutaneous therapy and initiation of IIT were left to the discretion of the treating clinicians in the ICU. Ultimately 178 patients were started on IIT during the ICU stay, and these formed the study group. Patients were screened daily to assess for eligibility. A waiver of consent was obtained from the institutional review board for chart review.
Patients were classified into two groups based on timing of ITT initiation. Those in the “early group” were started on IIT within 48 h of ICU admission (n = 127) while those in the “late group” were started on IIT thereafter (n = 51). The cutoff point of 48 h was chosen based upon previous suggestions to conservatively delay intensive insulin therapy until 2 or 3 days after ICU admission [17, 18].
Data collection
Baseline demographic information and clinical characteristics necessary to calculate an APACHE III score were collected for each patient. All blood glucose, caloric, and insulin doses were recorded. Oral and intravenous corticosteroid use (excluding topicals) was monitored. The hyperglycemic index (HGI) was calculated from the area under the curve for blood glucose of greater than 110 mg/dl when plotting blood glucose against time [21]. The primary outcome measure was all-cause hospital mortality. Secondary, predefined outcome measures included: 28-day ventilator-free days (VFD) as previously described [22], all-cause ICU mortality, ICU LOS, and hospital LOS.
Statistical analysis
Univariate analyses for baseline characteristics, APACHE III, glucose levels, insulin and steroid use, and caloric intake were performed using Fisher’s exact test, Student’s t-test, or Wilcoxon rank sum on SAS version 9.1.3, as appropriate. Multivariate analyses with logistic regression were performed for ICU and hospital mortality, correcting for predictors of death on univariate analysis (p < 0.05) and other clinically relevant factors. The C-statistic value for the final model was 0.81. There was no significant interaction between timing of insulin and any covariate (p > 0.2).
To better account for potential confounders that related to the decision of when to start insulin therapy, propensity score analysis was used. This has been used successfully to compare different treatment options in observational studies to minimize the effects of confounding by indication [23–25]. Propensity score for early vs. late administration of insulin was calculated for all patients. Variables for inclusion into the propensity model were selected as recommended by Brookhart et al. [26] in a stepwise selection algorithm (≤ 0.2). The variables thus selected in the propensity score model included: comorbidities (such as AIDS, cancer, cirrhosis, diabetes), race, gender, age, BMI, APACHE III score, peak glucose in the first 24 h of ICU admission, HGI, hypoglycemia (blood glucose < 70 mg/dl) before initiation of insulin protocol, and steroid use in first 24 h of ICU admission.
Patients in the early and late groups were matched according to their propensity score in two ways: (a) after stratifying the propensity score into quintiles and (b) after applying an optimal matching algorithm that paired two individuals in the early group to one individual in the late group with the closest propensity score so as to minimize the difference in propensity scores between the late and early patients (http://mayoresearch.mayo.edu/mayo/research/biostat/sasmacros.cfm, accessed 2 April 2007). After two-to-one matching the mean difference in propensity scores between the late and early patients was 0.19 ± 0.23. Variables in the propensity score were balanced for all variables except for age in quintile 4 (p < 0.001) and peak glucose in quintile 1 (p = 0.04) and quintile 3 (p = 0.02).
The crude odds ratio (OR) for ICU and hospital mortality was computed by Mantel–Haenszel estimator after stratifying by quintiles of propensity score or by matched trios (2:1 matching as above) [27]. Multivariate adjusted OR was calculated with logistic regression after including the propensity score as an indicator variable. Multivariate propensity scores were analyzed by conditional logistic regression after matching by quintiles of propensity score and after matching two early group patients to one late group patient based on their propensity score. All analyses were performed with SAS version 9.1.3.
Results
Baseline characteristics
Of the 499 patients meeting criteria for our IIT protocol, 178 patients were ultimately started on IIT during their ICU stay. 127 patients were started on IIT early, while 51 were started late. The two groups did not differ in age, gender, race, body mass index (BMI), Acute Physiology and Chronic Health Evaluation (APACHE) III, admission diagnosis, ICU steroid use, or comorbid conditions (Table 1).
Table 1.
Baseline characteristics of patients receiving intensive intravenous insulin treatment initiated within 48 h (early group) or after 48 h (late group) of ICU admission (APACHE, Acute Physiology and Chronic Health Evaluation; AIDS, acquired immunodeficiency syndrome)
| All (n = 178) | Early group (n = 127, 71%) | Late group (n = 51, 29%) | p | |
|---|---|---|---|---|
| Age (years) | 63 ± 17 | 63 ± 17 | 64 ± 18 | 0.7 |
| Females | 89 (50%) | 63 (50%) | 26 (51%) | >0.9 |
| Caucasian | 83 (47%) | 55 (43%) | 28 (55%) | 0.2 |
| Body mass index | 0.2 | |||
| Median | 26.1 | 26.5 | 24.2 | |
| Interquartile range | (22.3–30.8) | (22.8–32.3) | (22–30.3) | |
| APACHE III | 84 ± 29 | 83 ± 30 | 88 ± 25 | 0.2 |
| Admission diagnosis | ||||
| Sepsis | 70 (39%) | 50 (39%) | 20 (39%) | >0.9 |
| Acute respiratory failure | 68 (38%) | 47 (37%) | 21 (41%) | 0.6 |
| Other | 40 (23%) | 30 (24%) | 10 (20%) | 0.7 |
| Comorbidities | ||||
| Diabetes mellitus | 94 (53%) | 72 (57%) | 22 (43%) | 0.1 |
| Cirrhosis | 29 (16%) | 20 (16%) | 9 (18%) | 0.8 |
| AIDS | 7 (4%) | 5 (4%) | 2 (4%) | >0.9 |
| Leukemia or lymphoma | 19 (11%) | 13 (10%) | 6 (12%) | 0.8 |
| Solid tumor with metastasis | 12 (7%) | 7 (6%) | 5 (10%) | 0.3 |
| All malignancy: metastasis, leukemia, lymphoma | 31 (17%) | 20 (16%) | 11 (22%) | 0.5 |
| Steroids in 1st 24 h ICU | 46.3% | 44.9% | 50% | 0.6 |
Data on hyperglycemic patients not started on IIT were analyzed for the 1-month period in December 2006 (n = 30). Compared to those started on IIT (n = 19) the former had similar hospital mortality (43% vs. 47%) but shorter ICU LOS [median 4 days, interquartile range (IQR) 2–6, vs. 8.5 days, IQR, 3–13). The difference in LOS was expected, as the IIT protocol in our MICU was targeted to hyperglycemic patients with anticipated ICU stays greater than 24 h.
In the first 24 h after admission the early group had significantly higher peak glucose (median 240 mg/dl, IQR 199–334, vs. 156 mg/dl, 133–208; p < 0.001) and HGI (45.1, IQR 24.1–78.9, vs. 23.2, 3.9–39.7; p < 0.001; Table 2). Caloric intake in the early group was also higher than the late group (272 kcal, IQR 112–478, vs. 45 kcal, 0–190; p < 0.001) as glucose infusion was required as part of the protocol for insulin infusion. In both groups the majority of calories (early group 77.8% vs. late group 80.7%) in the first 24 h after ICU admission were given as carbohydrates from intravenous dextrose or enteral feeding. Only three patients were started on total parenteral nutrition in the first 24 h. Although the late group had lower peak glucose values, all but two patients were hyperglycemic in the first 24 h after ICU admission. By 48 h after ICU admission all late group patients had evidence of hyperglycemia. There was no difference in the proportion of patients treated with subcutaneous insulin in the first 24 h, but by 48 h significantly more patients in the late group had been administered subcutaneous therapy rather than intravenous insulin (76.5% vs. 40.2%, p < 0.001). The late group, on the other hand, had a higher proportion of blood glucose values below 70 mg/dl before initiation of IIT (19.6% vs. 4.7%, p = 0.003) as well as a lower absolute nadir value before IIT (median 92 mg/dl, IQR 76–114, vs. 141 mg/dl, 108–194; p < 0.001).
Table 2.
Insulin treatment and glycemic parameters of patients receiving intensive intravenous insulin treatment initiated within 48 h (early group) or after 48 h (late group) of ICU admission
| All | Early group | Late group | p | |
|---|---|---|---|---|
| Treated with subcutaneous insulin in 1st 24 h | 60 (34%) | 40 (32%) | 20 (39%) | 0.4 |
| Treated with SQ insulin in 1st 48 h | 90 (51%) | 51 (40%) | 39 (77%) | <0.001 |
| Hours to insulin start | <0.001 | |||
| Median | 15.4 | 8 | 71.5 | |
| Interquartile range | 4.5–51.3 | 3–20 | 55.5–104.8 | |
| Patients with blood glucose < 70 mg/dl before insulin | 16 (9%) | 6 (5%) | 10 (20%) | 0.003 |
| Lowest glucose in 1st 48 h (mg/dl) | <0.001 | |||
| Median | 121 | 141 | 92 | |
| Interquartile range | 91–164 | 108–194 | 76–114 | |
| Peak glucose in 1st 24 h (mg/dl) | <0.001 | |||
| Median | 217 | 240 | 156 | |
| Interquartile range | 171–293 | 199–334 | 133–208 | |
| Hyperglycemic index in 1st 24 h | <0.001 | |||
| Median | 38.4 | 45.1 | 23.2 | |
| Interquartile range | 19.1–72.2 | 24.1–78.9 | 3.9–39.7 | |
| Glucose received/day of ICU (g) | 122.5 ± 70.1 | 114.6 ± 70.4 | 141.7 ± 66.2 | 0.02 |
| Total calories in 1st 24 h (kcal) | <0.001 | |||
| Median | 204 | 272 | 45 | |
| Interquartile range | 20–408 | 112–478 | 0–190 |
Once started on IIT, the percentage of measurements that were within the target range of 80–110 mg/dl was the same in both groups (44%), and there was no significant difference in median glucose levels after initiation of IIT between the early group (median 114 mg/dl, IQR 94–142) and the late group (113 mg/dl, IQR 95–141; p > 0.6). Additionally, there was no difference in rates of hypoglycemia between the two groups (0.07% of all blood glucose measurements) or time to reach goal glucose of 80–110 mg/dl after initiation of IIT (12 ± 8 h vs. 16 ± 15 h, p > 0.1).
Outcomes
The early group had more VFD in the first 28 days after ICU admission (median 12 days, IQR 0–24, vs. 1 day, 0–11; p = 0.001), shorter ICU LOS (6 days, IQR 3–11, vs. 11 days, 7–17; p < 0.001), shorter hospital LOS (15 days, IQR 9–30, vs. 25 days, 13–43; p = 0.007), lower ICU mortality (OR 0.48, 95% CI 0.24–0.96), and lower hospital mortality (OR 0.27, 95% CI 0.13–0.55; Fig. 1). On multivariate analysis after adjusting for potential confounders such as age, gender, race, APACHE III, comorbid conditions, sepsis, lowest glucose prior to insulin therapy, peak glucose, HGI, dextrose given per day of ICU, total calories and steroid use in the first 24 h, whether IIT was given within 48 h of ICU admission was still associated with decreased hospital mortality (ORadj 0.29, 95% CI 0.11–0.77). Neither the attending critical care physician on service (p > 0.7) nor the day of admission (weekend vs. weekday; p > 0.3) was associated with the timing of insulin therapy or mortality on univariate analyses. Forcing these parameters into the multivariate model did not change the significance of the association between timing of insulin therapy and mortality.
Fig. 1.
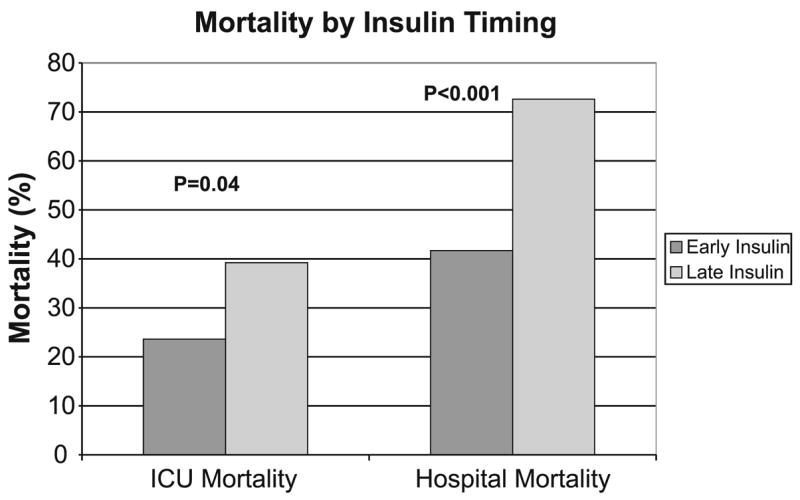
The effect of intensive insulin therapy on ICU and hospital mortality according to timing of insulin
After stratifying by quintiles of propensity score, the early group had a consistently lower mortality than the late group (Fig. 2). In addition, the early administration of insulin was significantly associated with decreased mortality on crude and multivariate analysis regardless of statistical method used for matching and for analysis of propensity scores (Table 3).
Fig. 2.
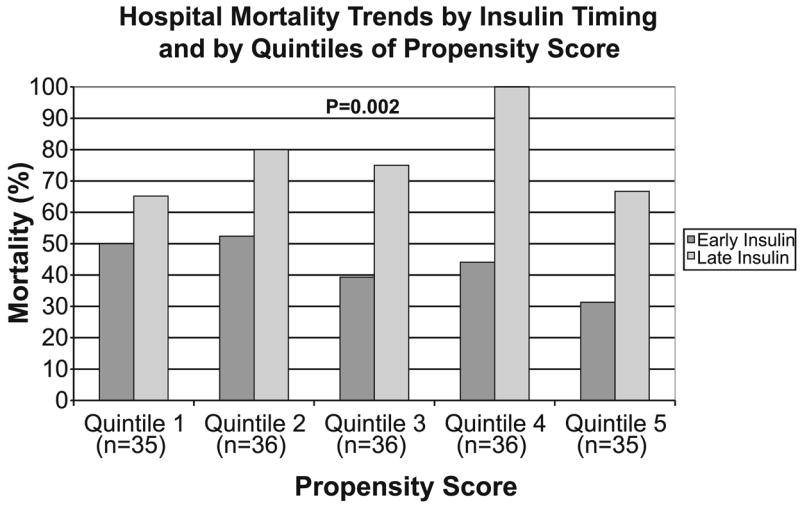
Hospital mortality trends according to timing of insulin and after stratification by quintiles of propensity score. The crude odds ratio and corresponding p-value for hospital mortality was computed using Mantel–Haenszel estimator after stratifying by quintiles of propensity score
Table 3.
Odds ratio (OR) for hospital mortality for patients receiving early intensive insulin therapy compared to late therapy (CI, confidence interval)
| OR | 95% CI | p | |
|---|---|---|---|
| Crude | 0.27 | 0.13–0.55 | <0.001 |
| Multivariate analysisa | 0.29 | 0.11–0.77 | 0.01 |
| Propensity score analysis | |||
| Crude stratified by quintiles | 0.29 | 0.13–0.66 | 0.002 |
| Multivariate analysis adjusted for propensitya | 0.24 | 0.09–0.67 | 0.006 |
| Matched by quintiles and adjusted for other covariatesa | 0.25 | 0.09–0.69 | 0.007 |
| Crude 2:1 matched | 0.31 | 0.15–0.65 | 0.001 |
| 2:1 conditional logistic regressiona | 0.16 | 0.038–0.65 | 0.01 |
Variables included: age, gender, race (white), Acute Physiology and Chronic Health Evaluation, diabetes mellitus, cirrhosis, leukemia, cancer, acquired immunodeficiency syndrome, sepsis, body mass index, peak glucose in first 24 h, lowest glucose in first 48 h, hyperglycemic index in first 24 h, total calories in first 24 h, total dextrose received per day in ICU, steroid use in first 24 h
An a priori decision was made to define the early group as starting insulin within 48 h of ICU admission [17, 18]. We explored whether other time points may be important. After stratifying by the length of time elapsed after ICU admission before initiation of insulin, there was a significant trend toward increasing mortality with increasing delay in initiation of therapy (IIT started < 24 h 41.1%; 24–48 h 45.5%; 48–72 h 84%; > 72 h 62.5%, p = 0.001).
We further explored whether early hypoglycemic events in the late group may explain the association between timing of insulin and mortality. Reanalysis after excluding patients who had episodes of hypoglycemia (blood glucose < 70 mg/dl prior to initiation of IIT; n = 6 in early group, n = 10 in late group) and those patients who were normoglycemic in the first 24 h of ICU admission (n = 2 in late group) demonstrated an odds ratio for hospital mortality still favoring early insulin administration at 0.28 with a 95% CI of 0.095–0.79.
Discussion
In this single-center prospective cohort study the early administration of IIT within 48 h of admission was associated with lower all-cause hospital mortality (ORadj 0.29, 95% CI 0.11–0.77). Patients who received early IIT had improved outcomes in all secondary endpoints such as VFD, ICU and hospital LOS, and all-cause ICU mortality.
Over the past decade a growing body of literature has linked adverse outcomes and hyperglycemia in the critically ill patient [28, 29]. Recent large randomized trials have demonstrated a beneficial effect of IIT in terms of clinically important morbidity and mortality endpoints [12, 16]. However, many unanswered questions remain. The optimal timing of therapy and the appropriate target population are two topics that need clarification from a practical perspective. Results from the recent MICU trial have heightened concern because of a subgroup analysis which suggested a possible harmful effect of IIT on short-term stayers. While we await further studies, some have proposed a conservative approach of delaying IIT for hyperglycemia in ICU patients until 2–3 days after admission [17, 18]. Such recommendations assume that IIT is time insensitive, i.e., that IIT initiated early in the critical illness has the same effect as that started later. Our results raise questions about such assumptions.
Although our study suggests that early therapy is associated with better outcomes, the best time point for initiation of therapy is unknown. It is possible that the maximal benefit is reaped in the first ICU day or perhaps earlier, such as in the emergency department. The initial insult in patients presenting with sepsis and acute respiratory distress syndrome, for example, frequently occurs at a time point much earlier than time zero of ICU admission. In such patients maladaptive cellular pathways have often already firmly established itself, and very early insulin therapy (at time zero in the ICU, or in the emergency department) may theoretically help to improve mortality. For example, insulin, through its pleiotropic effects, has been shown to reverse the state of dyslipidemia and catabolism in critical illness and has anti-inflammatory and anti-apoptotic properties [30]. Thus there are plausible biological mechanisms to support the hypothesis that early administration of insulin would be more beneficial. Indeed, the concept of a crucial window of opportunity is not a new one in the field of critical care medicine. Goal-directed hemodynamic therapy in sepsis was recently found to be beneficial only after early implementation prior to ICU admission [31]. Some centers already implement early glycemic control with intravenous insulin for sepsis while still in the emergency department [19]. However, the benefit and risks of such an approach has not been demonstrated.
The limitations of this study should be noted. Although baseline characteristics such as admission diagnosis, age, and severity of illness were comparable, the late group had lower glucose values than the early group. It is important to note that all late group patients were hyperglycemic and experienced persistently elevated glucose levels within 48 h of ICU admission requiring increasing use of subcutaneous insulin. Furthermore, reanalysis after excluding normoglycemic and hypoglycemic patients in the first 24 h after admission still favored early insulin administration with an OR for hospital mortality at 0.28 (95% CI 0.095–0.79). Thus the relative contribution of hypoglycemia to poor outcomes appeared limited.
Another limitation is that this is an uncontrolled single-center study with a small sample size. The decision to initiate IIT was left to the discretion of the treating ICU team. Complete data on hyperglycemic patients who were not treated with IIT during the study period are not available to allow multivariate analyses between hyperglycemic patients started on IIT and those who were not. However, such analyses would be less informative than the randomized control trials that have been previously completed or that is ongoing [12, 14–16]. Nevertheless, limited available data on 30 consecutive nonintensively treated hyperglycemic patients in this series revealed that hospital mortality was similar (43% vs. 47%, p > 0.9) to that in hyperglycemic patients on IIT.
Lastly, a large effect size was observed. It is possible that the treatment effect was exaggerated in the early group because the severity of hyperglycemia was greater. However, differences in severity of hyperglycemia only occurred early, as glucose control after initiation of IIT was identical, supporting the hypothesis that timing of insulin contributed to the difference in outcomes. We are reassured to see that (a) the admission APACHE score was similar in both groups and that (b) neither the time of admission (weekday vs. weekend, p > 0.3) nor (c) attending critical care physician on service influenced the timing of therapy (p > 0.7). In our closed MICU with a core group of critical care physicians and established practice protocols on a variety of procedures such as ICU prophylaxis, use of activated protein C, corticosteroid use for adrenal insufficiency, and sedation management the wide variability in processes of care that may affect mortality is minimized.
To account for other subtle patient characteristics which may have swayed clinician decision against IIT we adjusted for potential confounding by employing both multivariate and propensity score analyses. Propensity score analysis has been used successfully to compare different treatment options in a nonrandomized design [23]. The strength and direction of association favoring early IIT was consistent after propensity score modeling regardless of method used for analysis (OR 0.16–0.31). Finally, as shown in Fig. 2, at every quintile of propensity score the early group had lower hospital mortality than the late group. Despite the consistency of these results, we cannot exclude the possibility that there remains unmeasured confounding that influenced the late administration of insulin and prognosis. The results should be considered hypothesis generating, in need of further confirmatory studies. Assuming a baseline hospital mortality of 42%, a conservative effect size of 4% (as in the Leuven study [12]), an α value of 0.05 and 80% power, such a trial would require 2,354 patients per group.
Conclusions
In this study the early administration of IIT within 48 h of admission was associated with better outcomes. Despite the limitations of an uncontrolled design the findings from this study raises questions about the assumption that delayed administration of IIT has the same benefit as early initiation of insulin. A randomized study is needed to determine the optimal timing for IIT.
Supplementary Material
The online version of this article (doi:10.1007/s00134-007-0978-3) contains supplementary material, which is available to authorized users.
Acknowledgments
M.N.G. was supported by K23 HL60710, RO1 HL084060, and RO1 HL60710.
The insulin protocol would not have been possible without the dedication and commitment of Maxine Shepard RN, Sandra Buckle RN, and all of the MICU nurses. We thank Jeffrey Mechanick MD and Elise Brett MD for their advice and Thomas Kalb MD for his support.
Contributor Information
Shyoko Honiden, Yale University School of Medicine, Section of Pulmonary and Critical Care Medicine, Department of Medicine, New Haven Conn., USA.
Atara Schultz, Mount Sinai School of Medicine, Department of Medicine, New York N.Y., USA.
Shelly A. Im, Mount Sinai School of Medicine, Department of Medicine, New York N.Y., USA
David M. Nierman, Mount Sinai School of Medicine, Critical Care and Sleep Medicine, Division of Pulmonary, Department of Medicine, 1 Gustave L. Levy Place, Box 1232, New York 10029, NY, USA
Michelle N. Gong, Mount Sinai School of Medicine, Critical Care and Sleep Medicine, Division of Pulmonary, Department of Medicine, 1 Gustave L. Levy Place, Box 1232, New York 10029, NY, USA, e-mail: Michelle.Gong@msnyuhealth.org
References
- 1.Bernard C. Leçons sur le Diabète. Paris: 1877. [Google Scholar]
- 2.Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest. 2004;126:879–887. doi: 10.1378/chest.126.3.879. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 4.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–1062. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 5.Bjerke HS, Shabot MM. Glucose intolerance in critically ill surgical patients: relationship to total parenteral nutrition and severity of illness. Am Surg. 1992;58:728–731. [PubMed] [Google Scholar]
- 6.Margulies DR, Hiatt JR, Vinson D, Shabot MM. Relationship of hyperglycemia and severity of illness to neurologic outcome in head injury patients. Am Surg. 1994;60:387–390. [PubMed] [Google Scholar]
- 7.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 8.Corstjens AM, van der Horst IC, Zijlstra JG, Groeneveld AB, Zijlstra F, Tulleken JE, Ligtenberg JJ. Hyperglycaemia in critically ill patients: marker or mediator of mortality? Crit Care. 2006;10:216. doi: 10.1186/cc4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 10.Woo J, Lam CW, Kay R, Wong AH, Teoh R, Nicholls MG. The influence of hyperglycemia and diabetes mellitus on immediate and 3-month morbidity and mortality after acute stroke. Arch Neurol. 1990;47:1174–1177. doi: 10.1001/archneur.1990.00530110028011. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen C, Toft P, Jorgensen HS, Andersen SK, Tonnesen E. Hyperglycaemia and mortality in critically ill patients. A prospective study. Intensive Care Med. 2004;30:1685–1688. doi: 10.1007/s00134-004-2325-2. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 13.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132:268–278. doi: 10.1378/chest.06-3121. [DOI] [PubMed] [Google Scholar]
- 14.Brunkhorst FM, Kuhnt E, Engel C, Meier-Hellmann A, Ragaller M, Quintel M, Weiler N, Gründling M, Oppert M, Deufel T, Löffler M, Reinhart K the German Competence Network Sepsis (SepNet) Intensive insulin therapy in patient with severe sepsis and septic shock is associated with an increased rate of hypoglycemia—results from a randomized multicenter study (VISEP) Infection. 2005;33:19. [Google Scholar]
- 15.Devos P, Preiser JC. Current controversies around tight glucose control in critically ill patients. Curr Opin Clin Nutr Metab Care. 2007;10:206–209. doi: 10.1097/MCO.0b013e3280147d2d. [DOI] [PubMed] [Google Scholar]
- 16.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra A. Intensive insulin in intensive care. N Engl J Med. 2006;354:516–518. doi: 10.1056/NEJMe058304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brindley PG, Paton-Gay D. Treatment for hyperglycemia in the intensive care unit: a “bittersweet” message. Can J Anaesth. 2006;53:947–949. doi: 10.1007/BF03022838. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro NI, Howell M, Talmor D. A blueprint for a sepsis protocol. Acad Emerg Med. 2005;12:352–359. doi: 10.1197/j.aem.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Honiden S, Schultz A, Im SA, Nierman DM, Gong MN. Early versus late intravenous insulin administration in critically ill patients. American Thoracic Society International Conference. 2007;2007:A595. doi: 10.1007/s00134-007-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care. 2004;8:R122–R127. doi: 10.1186/cc2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenfeld DA, Bernard GR ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials treatment for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Quinn MJ, Aronow HD, Califf RM, Bhatt DL, Sapp S, Kleiman NS, Harrington RA, Kong DF, Kandzari DE, Topol EJ. Aspirin dose and six-month outcome after an acute coronary syndrome. J Am Coll Cardiol. 2004;43:972–978. doi: 10.1016/j.jacc.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 24.Winkelmayer WC, Kurth T. Propensity scores: help or hype? Nephrol Dial Transplant. 2004;19:1671–1673. doi: 10.1093/ndt/gfh104. [DOI] [PubMed] [Google Scholar]
- 25.Cavuto S, Bravi F, Grassi MC, Apolone G. Propensity score for the analysis of observational data: an introduction and illustrative example. Drug Dev Res. 2006;67:208–216. [Google Scholar]
- 26.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2007;26:3078–3094. doi: 10.1002/sim.2781. [DOI] [PubMed] [Google Scholar]
- 28.Langley J, Adams G. Insulin-based regimens decrease mortality rates in critically ill patients: a systematic review. Diabetes Metab Res Rev. 2007;23:184–192. doi: 10.1002/dmrr.696. [DOI] [PubMed] [Google Scholar]
- 29.Pittas AG, Siegel RD, Lau J. Insulin therapy and in-hospital mortality in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2006;30:164–172. doi: 10.1177/0148607106030002164. [DOI] [PubMed] [Google Scholar]
- 30.Vanhorebeek I, Langouche L, Van den Berghe G. Glycemic and nonglycemic effects of insulin: how do they contribute to a better outcome of critical illness? Curr Opin Crit Care. 2005;11:304–311. doi: 10.1097/01.ccx.0000170506.61281.94. [DOI] [PubMed] [Google Scholar]
- 31.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal Directed Therapy Collaboration Group. Early-goal directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this article (doi:10.1007/s00134-007-0978-3) contains supplementary material, which is available to authorized users.


