Abstract
Metallothionein (MT) is an enigmatic protein, and its physiological role remains a matter of intense study and debate fifty years after its discovery. This is particularly true of its function in the central nervous system (CNS), where the challenge remains to link its known biochemical properties of metal binding and free radical scavenging to the intricate workings of brain. In this compilation of four reports, first delivered at the 11th International Neurotoxicology Association (INA-11) meeting, June 2007, the authors present the work of their laboratories, each of which gives an important insight into the actions of MT in the brain. What emerges is that MT has the potential to contribute to a variety of processes, including neuroprotection, regeneration, and even cognitive functions. In this article, the properties and CNS expression of MT are briefly reviewed before Dr Juan Hidalgo describes his pioneering work using transgenic models of MT expression to demonstrate how this protein plays a major role in the defence of the CNS against neurodegenerative disorders and other CNS injuries. His group’s work leads to two further questions, what are the mechanisms at the cellular level by which MT acts, and does this protein influence higher order issues of architecture and cognition. These topics are addressed in the second and third sections of this review by Dr Adrian West, and Drs Edward Levin and Donnie Eddins, respectively. Finally, Dr Michael Aschner examines the ability of MT to protect against a specific toxicant, methymercury, in the CNS.
Introduction
Fifty years ago, an unusual cadmium binding protein was isolated from horse kidney (Margoshes and Vallee, 1957). Due to its high content of metals and cysteine residues, this protein was named metallothionein (MT) (Kagi and Vallee, 1961; Kagi and Vallee, 1960). In these 50 years, an overwhelming flow of information about these proteins has been published. It is clear that they constitute a superfamily of proteins, widely distributed in the animal kingdom as well as other phylogenetic groups (Andrews, 2000; Bremner, 1987a; Coyle et al., 2002; Ghoshal and Jacob, 2001; Hamer, 1986; Vasak and Hasler, 2000). In humans, the MT genes are tightly clustered in the q13 region of chromosome 16 (Karin et al., 1984a; Palmiter et al., 1992; Quaife et al., 1994; West et al., 1990), consisting of 7 functional MT-I genes (MT-1A, -B, -E, -F, -G, -H and –X) and a single gene encoding each of the other MT isoforms, namely MT-II (the MT-2A gene), MT-III and MT-IV. Mice have a much simpler MT gene structure, with only one functional gene for each isoform MT-I to MT-IV, all located on chromosome 8 (Cox and Palmiter, 1983; Palmiter et al., 1992; Quaife et al., 1994; Searle et al., 1984). The genes Mt1 and Mt2 are expressed coordinately in most tissues including the central nervous system (CNS) (Campagne et al., 2000; Searle et al., 1984; Yagle and Palmiter, 1985) et al., 2000), while Mt3 and Mt4 show a much more restricted tissue expression (mainly CNS and stratified squamous epithelia, respectively). The evolutionary retention of MT genes argues that they contribute important physiological properties; nonetheless these remain elusive to some extent.
All CNS MTs are composed of a single polypeptide chain of 61–68 amino acids, 20 of which are highly conserved cysteine residues, and there are no disulfides, aromatic amino acids or histidine. A major feature of their amino acid sequences is the occurrence of Cys-Xaa-Cys, Cys-Xaa-Yaa-Cys and Cys-Cys motifs, where Xaa and Yaa stands for an amino acid residue other than Cys (Kagi and Schaffer, 1988). These proteins usually bind 7 divalent metal ions (eg Zn(II)) and up to 12 monovalent copper ions, partitioned into two metal-thiolate clusters (Bogumil et al., 1998; Faller et al., 1999; Vašák M, 1994). Each cluster is located in a separate protein domain designated α (residues 32–61) and β (residues 1–31). When compared with the classical 61–62 amino acid sequences of MT-I and -II (hereafter referred to generically as MT-I/II, unless otherwise indicated) the sequence of MT-III (68 amino acids) shows two inserts: a single Thr in the N-terminal region and an acidic hexapeptide in the C-terminal region (Uchida et al., 1991a).
It is generally accepted that the expression of MT-I/II proteins is highly inducible in response to a range of stimuli, including metals, hormones, cytokines, oxidative agents, inflammation and stress (Bremner, 1987b; Sato and Bremner, 1993). Mt-1 and Mt-2 genes are co-ordinately regulated in mice by metals and glucocorticoids (Searle et al., 1984) (Yagle and Palmiter, 1985). Metal induced synthesis is mediated through the action of short cis-acting DNA sequences known as metal responsive elements (MREs), which are present in the promotor region of all mammalian MT genes (Karin et al., 1984b); (Radtke et al., 1993), and is mediated mainly by metal response element-binding transcription factor (MTF-1), a zinc sensitive trans-acting factor (Andrews, 2000; Radtke et al., 1993; Westin and Schaffner, 1988). Similarly, glucocorticoid responsive elements (GREs) are responsible for expression of some MT genes in response to glucocorticoids (Karin et al., 1984b) (Kelly et al., 1997). MT gene expression is also regulated by antioxidant response elements (AREs), although some MREs also respond to oxidants, again MTF-1 being involved (Samson and Gedamu, 1998). Increased levels of MT-I/II in response to inflammatory factors is very likely to be influenced by cytokines such as interleukin (IL-6) through signal transducer and activator of transcription (STAT) factors (Lee et al., 1999).. Nevertheless, it is feasible that multiple signals participate in stress, inflammation and oxidative stress induction of the MT genes (Figure 1). The regulation of MT-III and MT-IV gene expression is poorly known.
Figure 1.
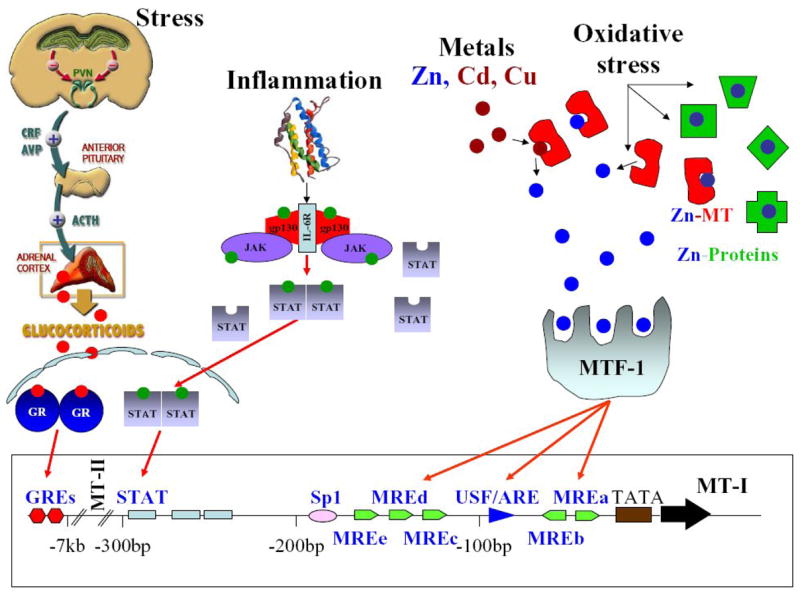
Metallothionein-I&II regulation
It has been demonstrated that MT-I/II occur throughout the brain and spinal cord, and that the main cell expressing these MT isoforms is the astrocyte, especially the reactive astrocyte (Holloway et al., 1997). Nevertheless, MT-I/II expression is also found in ependymal cells, epithelial cells of choroid plexus, meningeal cells of the pia mater and endothelial cells of blood vessels. Neurons appear to express MT-I/II to a much lower extent than astrocytes, while in the normal brain, oligodendrocytes and microglia are essentially devoid of MT-I/II, but the latter cells do upregulate MT-I/II expression in response to injury (Hidalgo et al., 2001). MT-III was discovered as a putative factor important in Alzheimer disease (Uchida et al., 1991b). In contrast to MT-I/II, there is still significant uncertainty regarding the cellular source of MT-III in the CNS, and astrocytes as well as neurons have been suggested to be the main cellular sources (Kobayashi et al., 1993); (Masters et al., 1994b; Uchida, 1994b);(Hidalgo et al., 2001).
It is clear from the literature that MT-I/II and MT-III not only show a distinct pattern of expression in the CNS, but also respond differently to a number of insults. MT-I/II are typically upregulated in response to tissue injury, even to subtle insults. For instance, brain MT-I/II are known to be upregulated by psychogenic stress (Hidalgo et al., 1990) or by the administration of bacterial endotoxin (Palmiter et al., 1992; Searle et al., 1984), glutamate analogues (Acarin et al., 1999b; Dalton et al., 1995), cryogenic injury (Penkowa et al., 1999a; Penkowa et al., 1999c), or by stroke/ischemia (Campagne et al., 1999; Campagne et al., 2000; Neal et al., 1996; Tang et al., 2002; Trendelenburg et al., 2002);. It is thus clear that MT-I/II may play an important role in the overall response of the brain to damage, a response that is thought to be orchestrated by a number of cytokines in a complex manner (Allan, 2001). Results obtained with microarrays and transgenic mice with null mutations have demonstrated that IL-6 (Poulsen et al., 2005) and TNF-α (Quintana et al., 2007) play major roles in the response of the cortex to injury. These studies also demonstrated that both cytokines regulate MT-I/II expression, which is in line with results obtained with transgenic mice overexpressing either IL-6 (Carrasco et al., 1999; Hernandez et al., 1997) or TNF-α (Carrasco et al., 2000a) and with the presence of STAT response elements in the promoters (Lee et al., 1999). In contrast to MT-I/II, MT-III expression shows up- or downregulation depending on the model, time, etc. in animal models of brain injury. MT-III expression has been shown to be increased by stab wounds (Anezaki et al., 1995; Hozumi et al., 1995; Hozumi et al., 1996) and kainic acid administration (Anezaki et al., 1995), but decreased by cortical ablation of the somatosensory cortex (Yuguchi et al., 1995a), facial nerve transection (Yuguchi et al., 1995b), and middle cerebral artery occlusion (Inuzuka et al., 1996). A biphasic response of MT-III to CNS injury, with initial downregulation followed by upregulation, was observed in response to N-methyl-D-aspartate (NMDA) (Acarin et al., 1999a) or to a cryolesion (Penkowa and Hidalgo, 2000b; Penkowa et al., 1999c).
Tissue injury elicits an inflammatory response and oxidative stress. There is increasing experimental evidence that oxidative stress contributes significantly to the neuropathology of several adult neurodegenerative disorders as well as to stroke, trauma, seizures and neuronal degeneration caused, among other reasons, by persistent activation of glutamate-gated ion channels (Coyle and Puttfarcken, 1993). As could be expected, brain MT-I/II levels have been consistently reported to be increased in Alzheimer’s disease (Duguid et al., 1989; Uchida, 1994a) (Adlard et al., 1998; Chuah and Getchell, 1999; Zambenedetti et al., 1998), Pick’s disease (Duguid et al., 1989) short-course Creutzfeld-Jakob disease (Kawashima et al., 2000), amyotrophic lateral sclerosis (Sillevis Smitt et al., 1992) (Sillevis Smitt et al., 1994) (Blaauwgeers et al., 1996), multiple sclerosis (Lock et al., 2002; Penkowa et al., 2003b), and aging (Suzuki et al., 1994).
Taken together, the above studies strongly suggest a significant role of MT-I/II and MT-III during neurodegenerative diseases and in response to brain injury, but demonstration of their involvement in specific processes in the CNS has been problematical, due in large part to the broader lack of a definitive understanding of the physiological role for MT in any tissue. In the sections to follow, four research groups present their investigations into the involvement of MT in physiological and protective CNS processes. It is interesting, and consistent with the long history of MT investigation, that convincing arguments can be made for a multi-faceted role of MT, spanning normal physiological processes and also a major reactive role in the face of injurious events such as toxicity or lesion.
Use of transgenic models to explore the role of MT in CNS disorders (J.H.)
The generation of genetically modified mice which overexpress (Dalton et al., 1996) or do not produce MT-I and MT-II (Masters et al., 1994a; Michalska and Choo, 1993) has been a key strategy for understanding the roles of these proteins in the CNS. Mice overexpressing MT-I were partially protected against mild focal cerebral ischemia and reperfusion, since the volume of affected tissue was smaller and the motor performance (3 weeks after the lesion) better (Campagne et al., 1999). Conversely, mice with null mutations in Mt-1 and Mt-2 (hereafter MT-I/II null mice) developed approximately threefold larger infarcts than wild-type mice and a significantly worse neurological outcome (Trendelenburg et al., 2002). These results highlight an important role of MT-I/II to cope with ischemic damage of the brain. Other studies have clearly demonstrated a similar essential role to cope with damage elicited by kainic acid-induced seizures (Carrasco et al., 2000b), the gliotoxin 6-aminonicotinamide (Penkowa et al., 2002; Penkowa et al., 1999b), 6-hydroxydopamine (Asanuma et al., 2002), mutated Cu,Zn-superoxide dismutase (Nagano et al., 2001; Puttaparthi et al., 2002a), 2002), multiple sclerosis models (Penkowa et al., 2001a; Penkowa et al., 2003a), traumatic brain injury (Giralt et al., 2002b; Penkowa et al., 1999b; Penkowa and Hidalgo, 2000a), and transgenic IL-6-induced neuropathology (Giralt et al., 2002a; Molinero et al., 2003; Penkowa et al., 2003a). Overall the results obtained in these studies are compatible with a role of MT-I/II decreasing oxidative stress, inflammation and apoptosis in the CNS, which is in accordance with results in other tissues (Kang YJ, 1997; Kondo et al., 1995; Lazo et al., 1995; Youn et al., 2002). It is worth noting again that besides being potent antioxidant proteins, MT-I/II are induced by oxidative stress (Andrews, 2000; Aschner, 1996a; Sato and Bremner, 1993). Alzheimer disease is the most prevalent neurodegenerative disease, and as stated above MT-I/II levels are consistently elevated; recently MT induction in response to amyloid plaques/production has been demonstrated in three different mouse models of AD (Carrasco et al., 2006), and thus are suitable models for analyzing the putative neuroprotective role of MTs.
The exact mechanisms through which MT-I/II elicit these neuroprotective effects are ill defined, and may include not only the putative roles of the intracellular protein but also those of the extracellular one: we have shown that exogenously applied MT-II protein mimics MT-I transgenic overexpression, causing a significant clinical improvement in the animal model of multiple sclerosis experimental autoimmune encephalomyelitis (Penkowa and Hidalgo, 2000a; Penkowa and Hidalgo, 2003), and protecting against traumatic brain injury (Chung et al., 2003a; Giralt et al., 2002a), which opens exciting perspectives about the use of the MT family as therapeutic agents. A microarray study comparing MT-I/II null mice with wildtype mice clearly shows the complexity of the challenge of the understanding fully the roles of these proteins (Penkowa et al., 2006).
MT-III was discovered unexpectedly while pursuing putative mechanisms underlying the neuropathology of Alzheimer’s disease (Uchida et al., 1991b). It was originally suggested that MT-III is down-regulated during AD, but unfortunately this is not a consistent finding (Amoureux et al., 1997; Carrasco et al., 1999; Erickson et al., 1994; Tsuji et al., 1992; Uchida, 1994b; Uchida et al., 1991b; Yu et al., 2001). Also, in line with the unclear nature of its response following injury, MT-III expression increases or decreases in other human diseases such as Down syndrome (Arai et al., 1997), Creutzfeld-Jakob disease (Kawashima et al., 2000), pontosubicular necrosis (Isumi et al., 2000), Parkinson disease, meningitis, or amyotrophic lateral sclerosis (Uchida, 1994b). As with MT-I/II, the generation of genetically modified mice (Erickson et al., 1997; Erickson et al., 1995) could help significantly to understand the potential biological roles of MT-III. In normal conditions, MT-III null mice do not show an appreciable phenotype (Erickson et al., 1997), but if challenged with the seizures elicited with the glutamate analogue kainic acid, they showed enhanced sensitivity, convulsing longer and having greater mortality than littermate controls, and showing increased neuronal death in the CA3 pyramidal cell layer of hippocampus; transgenic mice overexpressing MT-III showed the opposite trends (Erickson et al., 1997). Consistent with these results, MT-III was found to prevent glutamate neurotoxicity in primary cultures of cerebellar neurons (Montoliu et al., 2000). The increased neuronal death in the CA3 following kainic acid treatment of MT-III null mice has been confirmed recently, but these authors have also shown a decreased neuronal death in the CA1 area (Lee et al., 2003), indicating a protective or detrimental role of MT-III depending on the brain area. In the transgenic G93A SOD1 mice (a mouse model of ALS), crossed with MT-III null mice (Puttaparthi et al., 2002b), the deficiency of MT-III potentiated motor neuron impairment as revealed by stride length and grip strength, likely because of increased motor neuron death. In line with this study, a recent report shows that an adenoviral vector encoding rat MT-III prevents the degeneration of injured motoneurons (Sakamoto et al., 2003). In the cryolesion model of cortical injury, MT-III deficiency did not affect the inflammatory response, oxidative stress and apoptotic death (Carrasco et al., 2003), in sharp contrast to what is observed in MT-I/II deficient mice (see above). However, MT-III deficiency did increase the expression of some neurotrophins and other factors that may significantly affect neuronal survival and/or growth, such as GAP43 (Benowitz and Routtenberg, 1997; Bibel M, 2000), which would support an inhibitory role of MT-III in line with some in vitro bioassays (Chung et al., 2002a; Chung et al., 2002; Chung et al., 2003b; Chung and West, 2004b; Erickson et al., 1994; Sewell et al., 1995; Uchida et al., 1991b). A recent report analyzing the response of MT-III null mice in a peripheral nerve model supports a role of MT-III as an inhibitory factor of neuronal sprouting (Ceballos et al., 2003), since axonal regeneration was faster as substantiated electrophysiologically and histologically. Thus, it might be envisaged that MT-III could serve different functions in the CNS, promoting neuronal survival (perhaps only in specific neuronal populations such as motor neurons or hippocampal CA3 neurons) or death (hippocampal CA1 neurons) while inhibiting neuronal sprouting. The mechanisms underlying these effects remain to be established despite much effort with in vitro studies since again significant discrepancies are observed in the literature (i.e. (Uchida et al., 2002) vs. (Chen et al., 2002; Shi Y, 2003) for instance).
Thus, a considerable body of work has demonstrated that transgenic models in which MT expression is modified develop important phenotypes after injury, excitotoxicity or after the onset of neurological disorders, with a strong theme that MT-I/II have regenerative and protective properties. The interesting properties of MT-III following challenge are less clear. These animals will continue to be a valuable resource for investigating the role of MT specifically, and the pathological basis of neurological damage in general.
Interactions between neurons, astrocytes and metallothionein after CNS injury (A.K.W.)
An unexpected outcome of investigation of MT in the CNS is that it is shedding light on how glial cells in the CNS interact to help or hinder the recovery of neurons from physical and chemical injury. Following neuronal injury, astrocytes respond in a variety of ways that change over time and according to their distance from the lesion site (Myer et al., 2006). A common theme however is that the astrocytic response is complex and contains both inhibitory and stimulatory components towards regenerative neuronal growth after injury, each of which contributes to the overall outcome following injury. Understanding this response is essential to the development of therapeutic strategies for any CNS injury or degenerative condition and is likely to contain multiple points at which a therapeutic strategy might act. As an example of how astrocytes can influence neuronal regeneration, Penkowa and Hidalgo (Giralt et al., 2002a; Penkowa et al., 1999a), as discussed above, showed that mice lacking proteis produced predominantly by astrocytes in the CNS, MT-I/II exhibited significantly worse outcomes following a range of CNS injuries. Likewise, MT-I/II deficient animals had severely worse outcomes following stroke, the onset of EAE (a model for MS) and motor neuron disease (Campagne et al., 1999; Penkowa and Hidalgo, 2001; Puttaparthi et al., 2002b). Hence, perturbation of a purely astrocytic protein has major consequences in the injured CNS, including an increase in apoptotic neurons and deficient neuronal regenerative growth. Indeed, genetically modified animals have been produced that exhibit the full range of possible astrocytic MT-I/II expression, ranging from null to overexpressing strains, and there is an extremely robust correlation between MT-I/II expression and the ability of the animal to recover from CNS insult or degenerative disease (Hidalgo et al., 2001; Penkowa et al., 1999b; Penkowa et al., 2001b; Xie et al., 2004). No definitive mechanism has been demonstrated for these observations, including the key question of how these astrocytic proteins promote the recovery of injured neurons. MT-I/II are not significantly expressed in neurons in either the normal or injured state (Chung et al., 2004a; Dittmann et al., 2005; Holloway et al., 1997; West et al., 2004).
There are a number of ways that MT-I/II might potentially enhance the ability of astrocytes to promote neuronal regeneration, for example by zinc binding, with roles in metal homeostasis, or free radical scavenging (Hidalgo et al., 2001; West et al., 2004) although their function in any tissue remains to be definitively described. Since they demonstrably accumulate in the astrocytic cytoplasm after neuronal injury (Chung et al., 2004b), MTs likely act within the astrocyte itself, and they may, for example, be part of the mechanism by which astrocytes handle toxic intermediates such as reactive oxygen species. Astrocytes produce a number of proteins which are induced following injury and which are known to be strongly protective and as well as MT-I/II (Chung et al., 2004b; Holloway et al., 1997) include proteins such as Cu,Zn, superoxide dismutase (Turner et al., 2005), HSP70 (Robinson et al., 2005), prion protein (Lima et al., 2007) and S100β (Businaro et al., 2006). In this model, MT-I/II and other protective proteins act primarily to allow astrocytes to reduce the noxious environment surrounding a lesion thereby increasing the likelihood of neuronal survival and regeneration (Chung and West, 2004a; Chung et al., 2007). This may be a partial explanation for the phenomenon of reactive astrocytes in which these cells respond to neuronal stress with a program of morphological and gene expression changes.
However, we have shown that the role of MT-I/II is more complex than simply acting within astrocytes and we have demonstrated, for example, that MT-I/II strongly increases post-injury regenerative sprouting when added directly to injured neurons in culture (Chung et al., 2003b). In these experiments there are no glial or immune system cells present, showing that MT-I/II can act directly on injured neurons, outside of the context of the astrocyte cytoplasm. These experiments have been extended by work done elsewhere and we now know “exogenous” MT-I/II strongly promote regenerative neurite growth of cortical (Chung et al., 2003b), cerebellar (Ambjorn et al., 2007; Kohler et al., 2003) and retinal ganglionic cells (Fitzgerald et al., 2007) suggesting that there is a robust and generic neuronal response to extracellular MT-I/II. The physiological significance of these findings therefore hinges on whether MT-I/II are actually released by astrocytes, something which would initially appear to be unlikely since they do not have a secretory leader sequence. However, we have now unequivocally demonstrated that i) cultured astrocytes can release significant amounts of MT-I/II into medium after stimulation by zinc and Il-1, in an active, non-lytic manner and ii) substantial amounts of MT-I/II can be observed in the extracellular milieu of lesions by western blotting and immunohistochemistry (Chung et al, under review). These observations are consistent with the hypothesis that a major component of MT-I/II action in the injured CNS follows secretion by astrocytes and thus direct action on injured neurons to enhance regenerative growth.
How does MT-I/II act on neurons to promote regenerative sprouting and growth cone turning? We have shown that MT-I/II tethered to the substrate does not promote neurite extension in vitro (Fung, Chung and West, unpublished data). When MT-I/II was directly bound to glass coverslips, neuronal extension was not observed, yet this could be overcome by adding soluble MT-I/II to the system. One interpretation of this experiment is that MT-I/II must be internalised by neurons to promote neurite extension. To investigate this, we have labelled MT-I/II (Alexofluor594) and successfully observed uptake into cultured cortical neurons and retinal ganglionic cells both in vitro and in vivo. We have also shown that other labelled proteins, including albumin, are not similarly taken up.
We have further shown that a significant proportion of MT-I/II is transported into neurons by the cell surface protein megalin, a scavenger receptor which has been implicated in the uptake of cadmium-MT in the kidney (Klassen et al., 2004; Wolff et al., 2006). Our evidence is that i) neurons in culture and in vivo express megalin receptor, ii) the competitive inhibitor of megalin, RAP significantly blocks MT-I/II uptake in cultured cortical neurons and iii) anti-megalin antibodies block MT uptake (Chung and West, unpublished data). Furthermore, anti-megalin antibodies block the ability of MT-I/II to promote neurite outgrowth in mixed retinal cultures (Fitzgerald et al., 2007). The concept that MT-I/II promotes regenerative neurite growth in part by a receptor-mediated mechanism is novel.
Therefore, we now have strong evidence that MT-I/II is produced in astrocytes following neuronal damage, that it can be released by astrocytes in vitro and in vivo, and that it can be internalised by neurons to produce regenerative growth following injury. Taken together, our recent findings have two key implications. Firstly, the ability of MT-I/II to act directly on neurons to promote regenerative sprouting has major therapeutic potential. Indeed, we have demonstrated that exogenous MT promotes exuberant neuronal extension when applied to cortical lesions (Chung et al., 2003a) and to the cell bodies of lesioned retinal ganglionic cells (Chung et al, under review). The second major implication is that the properties of MT-I/II offer insight into the unanswered question of how astrocytes are able to support neuronal regeneration after injury: we propose they are able to produce and release proteins normally considered to be cytoplasmic which then act on neurons to promote axonal recovery, in this case by a mechanism dependent in part on neuronal uptake. We have proposed a theoretical model on the basis of our experimental findings (Chung and West, 2004a; Chung et al., 2007) which may have general applicability to protective astrocytic proteins other than MT-I/II. In particular, it is now becoming clear that proteins such as Cu,Zn superoxide dismutase (Turner et al., 2005), HSP70 (Robinson et al., 2005), prion protein (Lima et al., 2007) and S100β (Businaro et al., 2006) can be found outside the cell, and that these might confer protective effects on injured neurons.
Both of these implications are important. Current therapeutic agents to assist in CNS injury and disease are few and are generally unsatisfactory, whilst our understanding of the interaction between glia and injured neurons is a poorly understood field which limits our attempts to develop treatments for injury and neurodegenerative illness. In ongoing investigations, we are examining the potential of MT-I/II as a therapeutic agent in CNS disorders and have obtained promising outcomes in animal models of cortical injury (Chung et al., 2003a) and optic nerve lesion..
A number of important questions remain unanswered at this time. For example, little is known about how the interaction between megalin and MT-I/II promotes regenerative growth, and which intracellular pathways mediate this effect. It remains possible that the action of MT-I/II depends on its zinc binding property and that the role of MT-I/II uptake by injured neurons is to ferry zinc to a physiologically important location within the cell. Likewise, the free radical scavenging properties of MT-I/II might also be similarly targeted to a sensitive intracellular site after neuronal uptake. Alternatively, a megalin MT-I/II complex might activate signalling pathways within the cell that promote regenerative neuronal growth (Fig 3). The conundrum that the related isoform, MT-III appears to inhibit neuronal growth under some conditions is also interesting; it has not yet been established whether this isoform interacts with megalin.
Figure 3.
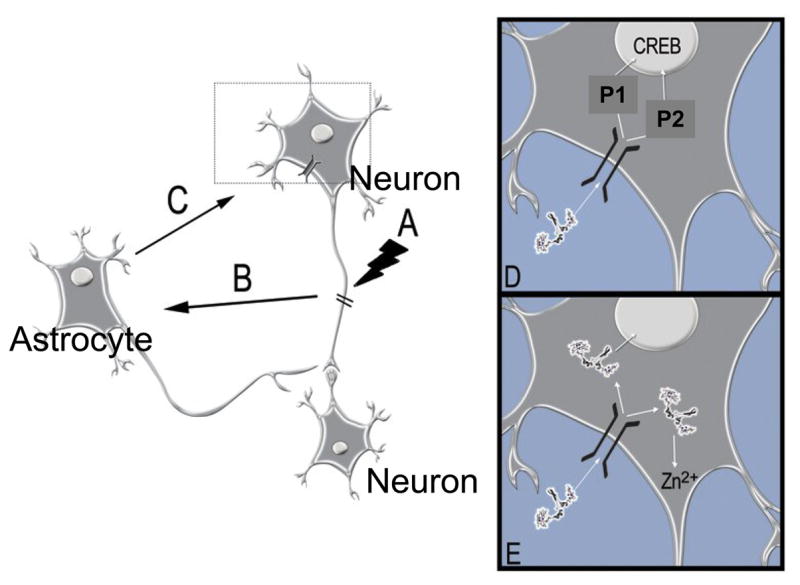
A model of astrocyte-neuron interaction after injury. Injury to neurons (A) causes signaling (B) to adjacent astrocytes leading them to increase synthesis of intracellular MT-I/II. Following non-lytic release (C), MT-I/II interacts with neurons and promotes regenerative neurite growth via an interaction with the cell surface receptor megalin, either by interaction of the megalin-MT-I/II complex with potential intracellular pathways or (D) by the targeted release of zinc ions into the neuron interior (D).
In summary, the observation that extracellular MT-I/II have a previously unsuspected capacity to influence neuronal regeneration after injury has implications for our understanding of the interaction of astrocyte and neurons, and also suggests that MT-based molecules might have therapeutic potential in a range of injury and neurodegenerative disorders. It also raises the question of whether MT-I/II might have roles outside regenerative growth of neurons, for example, in development of the CNS or in cognitive processes.
Cognitive Effects of Metallothionein 1 and 2 Knockout in Mice (E.D.L. and D.E.)
MTs are known to act in key ways in the physiology of transition metals, but their roles in neurobehavioral function have not been well characterized. There are reasons to believe that alterations in MT function could alter neural function in ways that would impair behavioral function. For example, the hippocampus, which plays a central role in cognitive functions such as memory depends on correct physiological actions of zinc, which is heavily concentrated in the hippocampal mossy fiber system (Daumas et al., 2004). MT-I/II protect against the adverse effects of both zinc under and overload (Kelly et al., 1996). In the striatum, alterations of copper functions can lead to cognitive and behavioral impairments seen in Wilson’s disease (Portala et al., 2001). Other transition metals such as mercury have no identified normal physiological role, but are environmental contaminants, which have known neurotoxic actions, which result in behavioral abnormalities including cognitive impairment (Goulet et al., 2003; Peixoto et al., 2007; Yoshida et al., 2005). Abnormalities in MT function could alter neurobehavioral function through dysregulation of all of these transition metals.
To begin determining the potential roles MTs may play in neurobehavioral function we have tested the effects of knocking out Mt1 and Mt2 genes (MT-I/II null mice) on cognitive function. The impact of MT-1/II knockout on choice accuracy and locomotor behavior in the 8-arm radial-arm maze was determined during acquisition and maintenance of response, with drug treatment and after toxicant challenge.
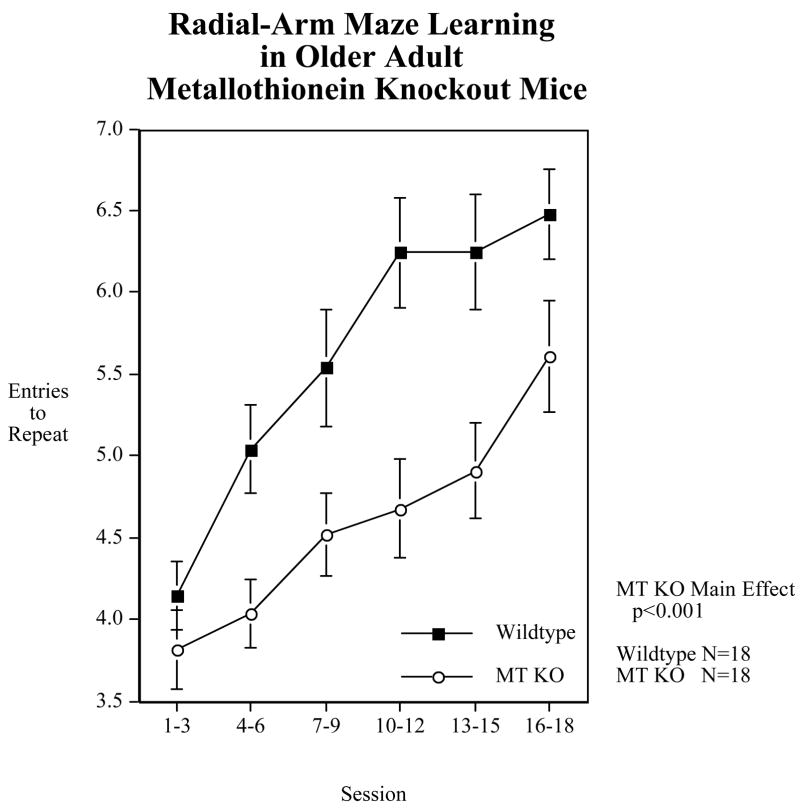
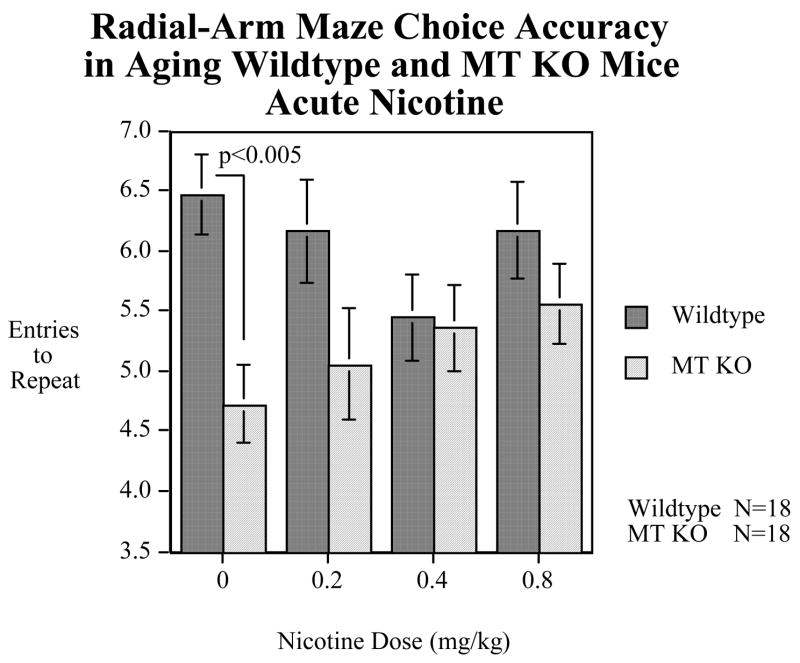
In our initial set of studies (Figure 1) we found that MT-I/II null mice (Jackson Labs) showed a significant (p<0.001) impairment in choice accuracy on the 8-arm radial maze test of spatial learning and memory (Levin et al., 2006). This was seen both in younger adult and older adult mice (Levin et al., 2006). Interestingly, as shown in Figure 2, the impairment of the MT-I/II null mice vs. their wildtype controls (129 strain) was significantly (p<0.05) ameliorated by acute nicotine treatment (Levin et al., 2006). This indicated that the MT-I/II knockout-induced impairment was not due to learning per se because the effect of nicotine was immediate and did not depend on further training in the maze. More likely it was due to attentional or memory aspects of the task which could be immediately changed from session to session as the nicotine treatments changed.
Figure 2.
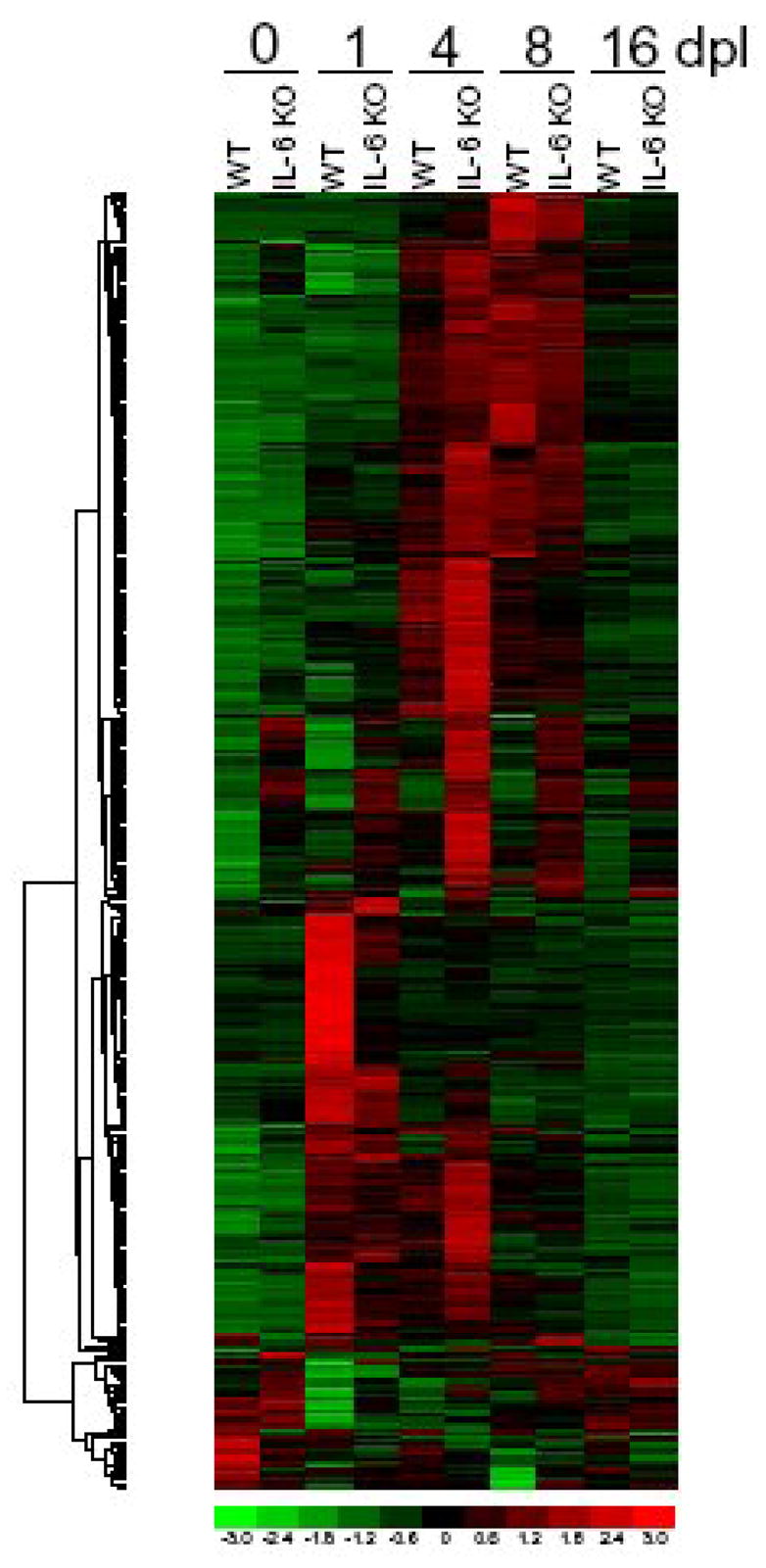
Microarray study with WT and IL-6 KO mice, with 1, 4, 8 and 16 days post lesion as well as unlesioned mice (Poulsen et al., 2005).
Developmental heavy metal exposure has long been known to impair neurobehavioral function. Physiologic mechanisms controlling metal distribution and metabolism such as MTs can greatly influence the actions of toxic as well as essential metals. However, only now is their role in neurobehavioral functioning being determined. In a recent study we have shown that MT-I/II null mice show an enhanced effect of neonatal mercury (HgCl2) exposure (Eddins, Levin et al. under review). In that study, a significant impairment in choice accuracy on the radial-arm maze was seen in MT-I/II null mice treated with a history of neonatal mercury exposure during the initial phase of training. During this initial phase neither mercury alone nor the MT-1/2 null mutation alone caused an impairment. To further characterize the role of metallothionein in learning and memory, mice with deletions of genes for MT-1/II were trained on a win-shift task in an 8-arm radial maze. MT-I/II null mice had similar choice accuracy levels as wildtype controls in the early stage of training, but had poorer accuracy during the later stages as the controls continued to improve. Acute nicotine treatment, which can improve working memory, eliminated the impairment suggesting that the MT-I/II null mice had learned the task across sessions, but that they were deficient in the attentional or memory requirements for accurate performance within each session. Then we replicated this effect and demonstrated that MT-I/II null mice were more vulnerable to the persisting adverse cognitive effects of developmental mercury exposure. Mercury, as mercuric chloride, was injected (0, 2 and 5 mg/kg) on days 7, 14 and 21 after birth. During the early stage of training the MT-I/II null mice exposed to 5 mg/kg mercuric chloride had significantly lower accuracy scores than either wildtype mice exposed to the same mercury dose or MT-I/II null mice not exposed to mercury. Frontal cortical dopamine (DA) showed a differential effect of developmental mercury exposure in MT-I/II null and wildtype mice. The 5 mg/kg mercury dose caused a significant increase in frontal DA levels in the wildtype mice. No such effect was seen in the MT-I/II null mice. MT knockout significantly increased frontal cortical levels of serotonin and its metabolite 5-HIAA regardless of mercury condition. The mercury-induced increased frontal cortical DA level seen in wildtype but not MT-I/II null mice may be relevant to the protection against memory impairment since the MT-I/II null mice showed a significant initial learning deficit with this dose of mercury while the wildtype controls did not. MT-I/II null mice were more sensitive to the persisting adverse cognitive effects of developmental mercury exposure. Alterations in frontal cortical DA response may be related to the functional impairment.
Thus, the absence of MT correlates with quantifiable cognitive changes, and furthermore, this work demonstrates that cognitive changes produced by neonatal exposure to mercury are sensitive to the presence or absence of MT. These pivotal observations are exciting because they provide a direct connection between higher order processes such as cognition and our understanding of the molecular properties of MT in metal binding and cell specific expression. In particular, the linkage with mercury toxicity allows reference to the large body of work in this area, and thereby suggests several specific mechanisms by which MT might interact with neurobehavioural pathways.
The Role of Metallothioneins in Methylmercury (MeHg)-Induced Neurotoxicity (M.A.)
Mercury is a global pollutant, which knows no environmental boundaries. Even the most stringent control of man-made sources of mercury pollution will not eliminate human exposure to potentially toxic quantities, given its ubiquitous presence in the environment. Environmental exposure to mercury occurs primarily via the food chain due to accumulation of methylmercury (MeHg) in fish. Human poisoning outbreaks as a result of food-borne MeHg consumption are evidenced by the tragic epidemics of MeHg poisoning in Japan and Iraq. Because methylation of inorganic mercury species to MeHg by microorganisms is known to take place in waterways, resulting in its accumulation in the food chain, any source of environmental mercury represents a potential source for MeHg poisoning. Anthropogenic sources culminating in the acidification of freshwater streams and lakes in North America, and the impoundment of water for large hydroelectric schemes have led to increases in MeHg concentrations in fish, posing increasingly greater risks to human populations. Latest statistics in the US indicate that 46 states have fish consumption advisories covering 40% of the nation’s rivers, lakes and streams. In addition, mercury is a common pollutant in hazardous waste sites in the nation (EPA, 2001). It is estimated that 3–4 million children live within one mile of at least one of the 1,300+ active hazardous waste sites in the USA (EPA, 2001).
Astrocytes make up ~50% of human brain volume (Chen and Swanson, 2003) and perform several functions that are essential for normal neuronal activity, including glutamate uptake (80% of synaptic glutamate), glutamate release, K+ and H+ buffering, and water transport. The “foot” processes of these cells are known to be closely associated with synapses, axonal tracts, nodes of Ranvier and capillaries. Astrocytes also induce the high electrical resistance (tightness) of the blood-brain barrier (BBB) that limits the transport of noxious substances entering the brain, and modulates optimal transport of nutrients and metabolites (reviewed by (Aschner et al., 1999). During neurodevelopment, cues for axon guidance of migrating neurons are regulated by interactions between axons and astrocytes (radial glia). The understanding of molecular signals that regulate neuron-glia interactions has increased greatly with the advent of molecular and cellular biological techniques, as well as genetically modified mice. Studies in which cell ablations are genetically targeted with ectopic gene expression, and gene knockout with single cell specificity have established distinct roles played by astrocytes during development (reviewed by (Aschner et al., 2002)).
We do not maintain that astrocytes are the only cell type affected by methylmercury (MeHg). Nevertheless, there is abundant evidence in support of their pivotal role in mediating neurotoxicity, establishing astrocytes as a unique and relevant experimental model for the assessment of mechanisms underlying MeHg-induced cytotoxicity.
Chronic exposure to MeHg in primates is associated with preferential accumulation of MeHg in astrocytes (and to some extent in microglia) (Charleston et al., 1996; Charleston et al., 1994).
MeHg inhibits astrocytic glutamate uptake and stimulates its efflux (Allen et al., 2001a; Aschner, 1996b; Aschner et al., 1993; Brookes and Kristt, 1989), increasing in vivo glutamate concentrations in the extracellular fluid (ECF) and sensitizing neurons to excitotoxic injury (Juarez et al., 2002). Damage associated with MeHg correlates with brain areas with dense glutamatergic innervation (Miyamoto et al., 2001). The ionotropic glutamate receptor N-methyl-D-aspartate (NMDA) antagonist, dizocilpine (MK801) protects against neuronal damage induced by in vivo MeHg (Miyamoto K, 1999; Miyamoto et al., 2001) (Lafon-Cazal et al., 1993).
MeHg selectively inhibits astrocytic (but not neuronal) uptake systems for cystine and cysteine transport (Allen et al., 2001b; Shanker et al., 2001a; Shanker et al., 2001b), compromising glutathione (GSH) synthesis and the CNS redox potential. Compared with astrocytes, neurons have lower levels of GSH as well as of a second putative antioxidant, MT (Maret, 1994; Philbert et al., 1991) making them more susceptible to the effects of increased ROS. Since GSH synthesis is dependent upon precursors derived from astrocytes (Dringen et al., 1999; (Wang and Cynader, 2000), MeHg-induced inhibition of cystine transport and astrocytic GSH production would ultimately lead to decreased neuronal GSH levels and increased glutamate toxicity.
MeHg-induced ROS formation in astrocytes can be attenuated by antioxidants (Shanker et al., 2002), reversing its functional effects on glutamate uptake inhibition (Allen et al., 2001a; Aschner et al., 2007; Yin et al., 2007).
Co-application of non-toxic concentrations of mercury with glutamate results in the appearance of typical neuronal lesions associated with excitotoxic stimulation (Matyja and Albrecht, 1993).
In human and non-human primates chronic in vivo exposure to MeHg is associated with swelling of astrocytes (Charleston et al., 1996; Charleston et al., 1994; Oyake et al., 1966; Vahter et al., 1994), a process associated with release of glutamate into the extracellular fluid.
In the absence of glutamate, neurons are unaffected by exposure to mercury, suggesting that neuronal dysfunction is secondary to disturbances in astrocytes (Brookes, 1992).
Additional data invoke astrocytic cPLA2 as a target for MeHg toxicity, supporting the notion that cPLA2-stimulated hydrolysis and release of arachidonic acid (AA) play a role in MeHg-induced neurotoxicity (Shanker et al., 2002).
Thiol groups play fundamental structural and functional roles in protein chemistry, being located mainly within the active catalytic sites of many enzymes. Mercury compounds react specifically with active sulfhydryl (-SH) groups forming complexes of defined stoichiometry, -S-Hg-R. The high affinity of MeHg for the anionic form of -SH groups is responsible for the toxicological behavior of this compound (Hughes, 1957) (Bramanti et al., 1999). The affinity of MeHg for the anionic form of -SH groups (log K, where K is the association constant) is extremely high, on the order of 15–23, whereas its affinity constants for oxygen-, chloride-, or nitrogen-containing ligands such as carboxyl or amino groups are about 10 orders of magnitude lower (Carty AJ, 1979). Indeed, wherever a MeHg compound has been identified in biological media, it has been complexed to -SH-containing ligands. Complexes with cysteine and GSH have been identified in blood (Naganuma and Imura, 1979; Rabenstein and Fairhurst, 1975), and complexes with GSH have been identified in brain (Thomas DJ, 1979), liver (Omata et al., 1978), and bile (Refsvik and Norseth, 1975).
An intriguing concept is that MTs not only account for the preferential accumulation of MeHg in astrocytes, but also endow these cells with tolerance to MeHg. Owing to their inducibility, MTs provide astrocytes with a potential mechanism to attenuate, at least temporarily, the toxicity of MeHg. An astrocyte can be envisioned to experience several stages, beginning with non-exposure, a state of grace that evidently no longer exists in nature, and ending with cell death or pervasive cell dysfunction once tolerance mechanisms have been overwhelmed. Between these extremes lies a continuum of metabolic changes consisting of initial injuries that take place at discrete molecular sites, followed by the metabolic amplification of such injuries. The toxic effects of MeHg become more pervasive until the astrocytes exhibit significant biochemical impairments, affecting the function of juxtaposed neurons. Additional MeHg exposure would presumably hasten the process. Because MeHg turnover in the CNS is very slow, the process may continue without additional exposure. During all stages, the cells may develop tolerance for MeHg, brought about by amplification of the MT genes.
While numerous studies support the ability of inorganic mercury to induce MT expression in various in vivo and in vitro models (reviewed by (Aschner et al., 2006), 2006), there are few studies on the interaction between MeHg and MT and the ability of MTs to attenuate MeHg-induced toxicity. MeHg was shown to be ineffective in inducing MTs, both in cultured neurons and astrocytes (Kramer et al., 1996a; Kramer et al., 1996b), consistent with in vivo findings (Yasutake et al., 1998) in which brain MT levels in rats exposed for 10 days to MeHg (40 μmol/kg per day × 5 days, po) remained unchanged even in the presence of neurological symptoms. The effects of dietary MeHg administration on gene expression were examined by (Gonzalez P, 2005) in three organs (liver, skeletal muscle, and brain) of the zebrafish. Adult male fish were fed for 7, 21, and 63 days with one of three different diets: control diet and two diets contaminated by MeHg at 5 and 13.5 μg of Hg/g. Genes (13 in total) known to be involved in antioxidant defenses, metal chelation, active efflux of organic compounds, mitochondrial metabolism, DNA repair, and apoptosis were investigated by quantitative real-time reverse transcription–polymerase chain reaction and normalized according to actin gene expression. Surprisingly, no change in the expression levels of these genes, including MT-II, was observed in brain samples, although this organ accumulated the highest Hg concentration. Thus, all studies conducted refute the inability of MeHg to induce MT. In rats exposed to MeHg by gastric gavage (5 × 10 mg/kg body wt over a 15-day period), or in drinking water (20 mg MeHgCl for 14 or 42 days), Hg was detected in undamaged Purkinje neurons and Bergmann glial cells; no Hg was detected in granule cells although these cells had a high incidence of pyknotic nuclei (Leyshon-Sorland et al., 1994). (1994). MT was detected primarily in Bergmann glial cells, Purkinje cells, astrocytes, and glial cells of white matter and no MT was detected in granule cells. The resistance of Purkinje cells to MeHg-induced damage was hypothesized to reflect their ability to transform organic mercurials to inorganic mercury, which in turn, induces the synthesis of radical-scavenging MT molecules.
Similar to the “housekeeping” functions ascribed to MTs in other mammalian tissues, brain MTs are implicated in metal metabolism, cellular repair processes, growth and differentiation where they are likely to serve as the source of zinc for newly synthesized apoenzymes, as well as regulator molecules in gene expression. Additional functions include control of intracellular redox potential, and metal detoxification. The adult human brain consumes >20% of the oxygen utilized by the body although it comprises only 2% of the body weight, indicating that ROS are generated at high rates during oxidative metabolism of the brain. Arrayed against oxygen-free radical-induced damage is an extensive system of defenses capable of scavenging and transforming oxygen-free radicals into non-toxic species. These systems include a range of specific antioxidants such as catalase, GSH peroxidases, and non specific antioxidants such as reduced glutathione (GSH), ceruloplasmin, and transferrin. MT itself has also been invoked as a thiol donor and free radical scavenger, thus providing a line of protection against oxidative damage (Lazo et al., 1995). Accordingly, studies in our laboratory have specifically addressed the ability of MT to attenuate MeHg-induced neurotoxicity in astrocytes. To explore the possibility that astrocytic MTs afford a protective role in heavy metal-induced neurotoxicity, we investigated the effects of MeHg on the induction of MTs both at the mRNA and protein level, and the ability of cultures which express high MT levels (by pre-treatment with ZnSO4 or CdCl2) to protect against the cytotoxic effects of MeHg (Aschner et al., 1998; Rising et al., 1995; Yao et al., 1999).
In the first series of studies (Rising et al., 1995), the cytotoxic effect of MeHg on cultured astrocyte monolayers was assayed by measuring its effect on the initial rate of uptake of radiolabeled D-aspartic acid, a non-metabolizable analog of L-glutamate. Treatment of astrocytes with 10 μM MeHg had a significant inhibitory effect on the initial rate (30 minutes) of radiolabeled aspartate uptake. This effect could be completely reversed by pre-treatment of astrocytes with CdCl2 (48–96 hours) at a concentration as low as 1 μM. To correlate the temporal sequence between astrocytic MT protein levels and resistance to MeHg additional studies were carried out to determine MT levels in astrocytes upon CdCl2 pre-treatment by means of western blot analysis. MT-I/II protein levels increased in astrocytes pre-treated with CdCl2 in a time-dependent fashion, with maximal inducibility at 72 hours. Thus, associated with the protective effect of CdCl2 on MeHg-induced inhibition of D-aspartate uptake is a marked increase in astrocytic MT levels.
Additional studies (Aschner et al., 1998) revealed that pretreatment of astrocytes for 24 hours with 100 μM ZnSO4 are also associated with a significantly higher MT expression level compared to controls (2.9 fold increase). The relative abundance of MT-I mRNA transcripts in ZnSO4 pretreated cells showed a 5.6 fold increase compared with controls. Exposure of astrocytes to MeHg (10 μM) led to approximately 20% cell shrinkage, followed by marked swelling with approximately 30% increase in cell volume over baseline. Pretreatment of astrocytes for 24 hours with 100 μM ZnSO4 abolished the stimulatory effect of MeHg on cell volume (p<0.01), such that the Zn pretreated group was indistinguishable from controls. Since exposure of astrocytes to MeHg is associated with the activation of the Na+/H+ antiporter, studies were also performed to determine whether the Zn-associated attenuation of MeHg-induced swelling could be correlated with reduced influx of Na+. MeHg exposure led to a significant increase in the initial uptake of 22Na+, which was completely abolished by 24-hour pretreatment of the cells with 100 μM ZnSO4. Since a common mechanism of regulatory volume decrease whereby astrocytes re-establish basal cellular volume involves activation of conductive K+ and Cl− channels, we determined whether the Zn-associated attenuation of MeHg-induced swelling could be correlated with reduced astrocytic efflux of Rb+, a marker of K+ efflux. Swelling induced by MeHg led to a significant increase in the release of Rb+. This effect was significantly attenuated by pretreatment with ZnSO4.
In additional studies (Yao et al., 1999), we used routine transfection techniques to assess the potential of MT to mitigate MeHg-induced neurotoxicity. The pGfa2-cLac plasmid, which contains a 2.2 kb promoter of the human GFAP gene \was placed in front of the Escherichia coli LacZ gene, which in turn was followed by a fragment of the mouse protamine-I gene as a polyadenylation signal. Construction of the recombinant plasmids entailed excision of the LacZ gene by digestion with BamHI and its replacement by blunt end ligation with a 0.55 kb HindIII–XbaI fragment containing the entire MT-I coding region. These recombinant plasmids were identified with enzyme digestions and confirmed by DNA sequence analysis. Transfection with the pGFAP-MT-I plasmid was associated with a significant increase both in MT-I mRNA and MT-I protein levels compared to controls.
To determine the relationship between the cytotoxicity of MeHg and the relative abundance of MT expression, Na251CrO4 efflux measurements were carried out. The fractional release of 51Cr is a good index of membrane integrity, and under non-toxic conditions, the basal release of the radiolabeled compound is exceedingly small. As expected, MeHg induced a concentration-dependent increase in the fractional release of 51Cr as its concentration in the perfusion buffer increased. The release of 51Cr in the MT-I transfected groups was lower in pGFAP-MT-I transfected astrocytes compared to their controls, indicative of the neuroprotective effect of MTs.
The series of studies presented above shows that chemical treatments (CdCl2 or ZnSO4) or transient transfection with the MT-I gene, are associated with increased expression of MT-I mRNA transcription and MT-I protein synthesis. Although the astrocytic increase in MT-I expression is in most cases relatively modest (<10-fold over controls) it is in close agreement to those published by others. For example, Liu et al. (Liu J, 1992) reported a relatively small 4-fold increase in astrocytic MT protein in response to metal exposure compared to a robust 10-fold increase in MT protein in similarly treated hepatocytes. Kramer et al. (Kramer KK, 1995) reported a 3–4-fold increase in MT expression in cultured murine astrocytes upon zinc exposure. (Hidalgo et al., 1994) reported a 2.25-fold increase in astrocytic expression of MTs subsequent to zinc treatment. The same authors suggested, that compared to other cell types, astrocytes might not be capable of mounting a dramatic MT response to metal exposure, because they posses only a limited capacity to overexpress this protein. The reason for this limited ability to overexpress MTs is unknown, but undoubtedly it may reflect the fact that constitutive MT levels are 10 times higher in astrocytes compared to neurons (Hidalgo et al., 1994). Other reports suggested that MT could be increased between 2.5-and 15-fold in a variety of cell types by homologous and heterologous MT gene transfection (Abdel-Mageed and Agrawal, 1997; Morton et al., 1992), but to the best of our knowledge, such studies in primary astrocyte cultures are lacking.
The results of our studies support the hypothesis that intracellular MT levels confer resistance to MeHg toxicity. MT-I can be efficiently expressed both in primary astrocyte cultures by MT-I gene transfection under the control of the astrocyte-specific GFAP promoter or treatments with CdCl2 or ZnSO4, known potent inducers of MTs. As mentioned above, by virtue of their high -SH content, MTs have a very high affinity for MeHg, potentially providing for a concentrative mechanism for MeHg in astrocytes. Furthermore, induction of MTs may endow the astrocytes, at least during early stages of heavy metal accumulation, with tolerance to MeHg. The MeHg-MT complex is well suited for the detoxification of MeHg. The stability of MTs against proteolysis is greatly increased after binding to MeHg and the degradative release of the metal results in its association with other MTs. This cycle can keep MeHg in a relatively non-toxic form within the astrocytes, protecting both the astrocytes themselves and juxtaposed neurons from the cytotoxic effects of MeHg. Thus, we propose that astrocytes can play a “double” role in MeHg intoxication. Whereas, at high levels and chronic exposure MeHg can short circuit astrocytic function, at low concentrations of MeHg, MTs may serve to “buffer” MeHg itself, or indirectly attenuate its toxicity and protect vulnerable astrocytic -SH groups, such as those on the glutamate transporter, from MeHg. However, once MT induction mechanisms are overwhelmed, pervasive astrocytic dysfunction leads to failure to control the extracellular fluid, and neuronal impairment. In vitro MT enrichment confers resistance against the cytotoxicity of MeHg in multiple assays. This interpretation is consistent with the observation that resistance to heavy metal toxicity is closely related to MT expression levels and raises interesting questions regarding the potential involvement of heavy metals in neurodegeneration where MT synthesis may be compromised.
SUMMARY
Using various experimental approaches we have established that [1] in transgenic models of MT expression this protein plays a major role in the defence against neurodegenerative disorders and other injuries, [2] MTs influence tissue architecture and [3] cognition. Finally, [4] MTs protect against mercury neurotoxicity. The review establishes multi-functional and critical roles afforded by MTs describing a diverse range of processes that are mediated by the unique MT protein family. Fifty years after its discovery, a greater appreciation exists for the MT’s metal binding and free radical scavenging properties, yet clearly its role in the intricate workings of brain is much broader and yet to be fully detailed.
Figure 4.
Metallothionein 1 and 2 knockout effects vs. wildtype control (129 strain) performance on choice accuracy during acquisition of choice accuracy (entries to repeat) on the 8-arm radial maze (mean±sem) (Levin et al., 2006)
Figure 5.
Acute nicotine effects on radial-arm maze choice accuracy deficits (entries to repeat) in Metallothionein 1 and 2 knockout mice vs. wildtype control (129 strain) performance (mean±sem).
Acknowledgments
Ministerio de Ciencia y Tecnología and Feder SAF2005-00671 and European Comission FP6 Integrated Project Exgenesis (Ref. LSHM-CT-2004-005272) (J.H.); PHS grants NIEHS 07331 (M.A); Australia Alzheimer’s Research and Australian Research Council (A.K.W); Autism Speaks and the Duke University Superfund Basic Research Center ES010356 (E.D.L. and D.E.). Thanks to Ann Petro, Ninitia Pollard, Charles Perraut and Jonathan H. Freedman (E.L.) and the NeuroRepair Group, Menzies Research Institute and colleagues (A.K.W.) for their valuable work on these projects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Mageed A, Agrawal KC. Antisense down-regulation of metallothionein induces growth arrest and apoptosis in human breast carcinoma cells. Cancer Gene Ther. 1997;4:199–207. [PubMed] [Google Scholar]
- Acarin L, Carrasco J, Gonzalez B, Hidalgo J, Castellano B. Expression of growth inhibitory factor (metallothionein-III) mRNA and protein following excitotoxic immature brain injury. J Neuropathol Exp Neurol. 1999a;58:389–97. doi: 10.1097/00005072-199904000-00009. [DOI] [PubMed] [Google Scholar]
- Acarin L, Gonzalez B, Hidalgo J, Castro AJ, Castellano B. Primary cortical glial reaction versus secondary thalamic glial response in the excitotoxically injured young brain: astroglial response and metallothionein expression. Neuroscience. 1999b;92:827–39. doi: 10.1016/s0306-4522(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Adlard PA, West AK, Vickers JC. Increased density of metallothionein I/II-immunopositive cortical glial cells in the early stages of Alzheimer’s disease. Neurobiol Dis. 1998;5:349–56. doi: 10.1006/nbdi.1998.0203. [DOI] [PubMed] [Google Scholar]
- Allan SMRNJ. Cytokines and acute neurodegeneration. Nature reviews. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001a;902:92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- Allen JW, Shanker G, Aschner M. Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res. 2001b;894:131–40. doi: 10.1016/s0006-8993(01)01988-6. [DOI] [PubMed] [Google Scholar]
- Ambjorn M, Asmussen JW, Lindstam M, Gotfryd K, Jacobsen C, Kiselyov VV, et al. Metallothionein and a peptide modeled after metallothionein, EmtinB, induce neuronal differentiation and survival through binding to receptors of the low-density lipoprotein receptor family. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.05036.x. [DOI] [PubMed] [Google Scholar]
- Amoureux MC, Van Gool D, Herrero MT, Dom R, Colpaert FC, Pauwels PJ. Regulation of metallothionein-III (GIF) mRNA in the brain of patients with Alzheimer disease is not impaired. Mol Chem Neuropathol. 1997;32:101–21. doi: 10.1007/BF02815170. [DOI] [PubMed] [Google Scholar]
- Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- Anezaki T, Ishiguro H, Hozumi I, Inuzuka T, Hiraiwa M, Kobayashi H, et al. Expression of growth inhibitory factor (GIF) in normal and injured rat brains. Neurochem Int. 1995;27:89–94. doi: 10.1016/0197-0186(94)00170-y. [DOI] [PubMed] [Google Scholar]
- Arai Y, Uchida Y, Takashima S. Developmental immunohistochemistry of growth inhibitory factor in normal brains and brains of patients with Down syndrome. Pediatr Neurol. 1997;17:134–8. doi: 10.1016/s0887-8994(97)00085-4. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Higashi Y, Tanaka K, Haque ME, Fujita N, et al. Aggravation of 6-hydroxydopamine-induced dopaminergic lesions in metallothionein-I and -II knock-out mouse brain. Neurosci Lett. 2002;327:61–5. doi: 10.1016/s0304-3940(02)00346-4. [DOI] [PubMed] [Google Scholar]
- Aschner M. The functional significance of brain metallothioneins. Faseb J. 1996a;10:1129–1136. doi: 10.1096/fasebj.10.10.8751715. [DOI] [PubMed] [Google Scholar]
- Aschner M. Methylmercury in astrocytes--what possible significance? Neurotoxicology. 1996b;17:93–106. [PubMed] [Google Scholar]
- Aschner M, Allen JW, Kimelberg HK, LoPachin RM, Streit WJ. Glial cells in neurotoxicity development. Annu Rev Pharmacol Toxicol. 1999;39:151–73. doi: 10.1146/annurev.pharmtox.39.1.151. [DOI] [PubMed] [Google Scholar]
- Aschner M, Conklin DR, Yao CP, Allen JW, Tan KH. Induction of astrocyte metallothioneins (MTs) by zinc confers resistance against the acute cytotoxic effects of methylmercury on cell swelling, Na+ uptake, and K+ release. Brain Res. 1998;813:254–61. doi: 10.1016/s0006-8993(98)00947-0. [DOI] [PubMed] [Google Scholar]
- Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993;602:181–6. doi: 10.1016/0006-8993(93)90680-l. [DOI] [PubMed] [Google Scholar]
- Aschner M, Sonnewald U, Tan KH. Astrocyte modulation of neurotoxic injury. Brain Pathol. 2002;12:475–81. doi: 10.1111/j.1750-3639.2002.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB. Metallothioneins: mercury species-specific induction and their potential role in attenuating neurotoxicity. Exp Biol Med (Maywood) 2006;231:1468–73. doi: 10.1177/153537020623100904. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–91. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Bibel MBY-A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes & 2919–2937. Development. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Blaauwgeers HG, Anwar Chand M, van den Berg FM, Vianney de Jong JM, Troost D. Expression of different metallothionein messenger ribonucleic acids in motor cortex, spinal cord and liver from patients with amyotrophic lateral sclerosis. J Neurol Sci. 1996;142:39–44. doi: 10.1016/0022-510x(96)00013-5. [DOI] [PubMed] [Google Scholar]
- Bogumil R, Faller P, Binz PA, Vasak M, Charnock JM, Garner CD. Structural characterization of Cu(I) and Zn(II) sites in neuronal- growth-inhibitory factor by extended X-ray absorption fine structure (EXAFS) Eur J Biochem. 1998;255:172–7. doi: 10.1046/j.1432-1327.1998.2550172.x. [DOI] [PubMed] [Google Scholar]
- Bramanti E, D’Ulivo A, Lampugnani L, Zamboni R, Raspi G. Application of mercury cold vapor atomic fluorescence spectrometry to the characterization of mercury-accessible -SH groups in native proteins. Anal Biochem. 1999;274:163–73. doi: 10.1006/abio.1999.4257. [DOI] [PubMed] [Google Scholar]
- Bremner I. Interactions between metallothionein and trace elements. Prog Food Nutr Sci. 1987a;11:1–37. [PubMed] [Google Scholar]
- Bremner I. Nutritional and physiological significance of metallothionein. Experientia Suppl. 1987b;52:81–107. doi: 10.1007/978-3-0348-6784-9_5. [DOI] [PubMed] [Google Scholar]
- Brookes N. In vitro evidence for the role of glutamate in the CNS toxicity of mercury. Toxicology. 1992;76:245–56. doi: 10.1016/0300-483x(92)90193-i. [DOI] [PubMed] [Google Scholar]
- Brookes N, Kristt DA. Inhibition of amino acid transport and protein synthesis by HgCl2 and methylmercury in astrocytes: selectivity and reversibility. J Neurochem. 1989;53:1228–37. doi: 10.1111/j.1471-4159.1989.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Businaro R, Leone S, Fabrizi C, Sorci G, Donato R, Lauro GM, et al. S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J Neurosci Res. 2006;83:897–906. doi: 10.1002/jnr.20785. [DOI] [PubMed] [Google Scholar]
- Campagne M, Thibodeaux H, van Bruggen N, Cairns B, Gerlai R, Palmer J, et al. Evidence for a protective role of metallothionein-1 in focal cerebral ischemia. PNAS. 1999;96:12870–5. doi: 10.1073/pnas.96.22.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagne MV, Thibodeaux H, van Bruggen N, Cairns B, Lowe DG. Increased binding activity at an antioxidant-responsive element in the metallothionein-1 promoter and rapid induction of metallothionein-1 and -2 in response to cerebral ischemia and reperfusion. J Neurosci. 2000;20:5200–7. doi: 10.1523/JNEUROSCI.20-14-05200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco J, Giralt M, Molinero A, Penkowa M, Moos T, Hidalgo J. Metallothionein (MT)-III: generation of polyclonal antibodies, comparison with MT-I+II in the freeze lesioned rat brain and in a bioassay with astrocytes, and analysis of Alzheimer’s disease brains. J Neurotrauma. 1999;16:1115–29. doi: 10.1089/neu.1999.16.1115. [DOI] [PubMed] [Google Scholar]
- Carrasco J, Giralt M, Penkowa M, Stalder AK, Campbell IL, Hidalgo J. Metallothioneins are upregulated in symptomatic mice with astrocyte- targeted expression of tumor necrosis factor-alpha. Exp Neurol. 2000a;163:46–54. doi: 10.1006/exnr.1999.7335. [DOI] [PubMed] [Google Scholar]
- Carrasco J, Penkowa M, Giralt M, Camats J, Molinero A, Campbell IL, et al. Role of metallothionein-III following central nervous system damage. Neurobiol Dis. 2003;13:22–36. doi: 10.1016/s0969-9961(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Carrasco J, Penkowa M, Hadberg H, Molinero A, Hidalgo J. Enhanced seizures and hippocampal neruodegeneration following kainic acid-induced seizures in metallothionein-I + II-deficient mice. Eur J Neurosci. 2000b;12:2311–22. doi: 10.1046/j.1460-9568.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- Carty AJMS. The chemistry of mercury in biological systems. In: JO N, editor. The Biogeochemistry of Mercury in the Environment. Amsterdam: Elsevier/North Holland Biomedical Press; 1979. pp. 433–479. [Google Scholar]
- Ceballos D, Lago N, Verdu E, Penkowa M, Carrasco J, Navarro X, et al. Role of metallothioneins in peripheral nerve function and regeneration. Cellular and Molecular Life Sciences. 2003;60:1209–1216. doi: 10.1007/s00018-003-3047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charleston JS, Body RL, Bolender RP, Mottet NK, Vahter ME, Burbacher TM. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long-term subclinical methylmercury exposure. Neurotoxicology. 1996;17:127–38. [PubMed] [Google Scholar]
- Charleston JS, Bolender RP, Mottet NK, Body RL, Vahter ME, Burbacher TM. Increases in the number of reactive glia in the visual cortex of Macaca fascicularis following subclinical long-term methyl mercury exposure. Toxicol Appl Pharmacol. 1994;129:196–206. doi: 10.1006/taap.1994.1244. [DOI] [PubMed] [Google Scholar]
- Chen Y, Irie Y, Keung WM, Maret W. S-nitrosothiols react preferentially with zinc thiolate clusters of metallothionein III through transnitrosation. Biochemistry. 2002;41:8360–7. doi: 10.1021/bi020030+. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003;84:1332–9. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- Chuah MI, Getchell ML. Metallothionein in olfactory mucosa of Alzheimer’s disease patients and apoE-deficient mice. Neuroreport. 1999;10:1919–24. doi: 10.1097/00001756-199906230-00023. [DOI] [PubMed] [Google Scholar]
- Chung R, Adlard P, Dittmann J, Vickers J, Chuah M, West A. Neuron-glia communication: metallothionein expression is specifically up-regulated by astrocytes in response to neuronal injury. J Neurochem. 2004a;88:454–61. doi: 10.1046/j.1471-4159.2003.02193.x. [DOI] [PubMed] [Google Scholar]
- Chung R, Holloway A, Eckhardt B, Harris J, Vickers J, Chuah M, et al. Sheep have an unusual variant of the brain-specific metallothionein, metallothionein-III. Biochem J. 2002a;365:323–8. doi: 10.1042/BJ20011751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung R, Vickers J, Chuah M, West A. Metallothionein-IIA promotes initial neurite elongation and postinjury reactive neurite growth and facilitates healing after focal cortical brain injury. J Neurosci. 2003a;23:3336–42. doi: 10.1523/JNEUROSCI.23-08-03336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung R, West A. A role for extracellular metallothioneins in CNS injury and repair. Neuroscience. 2004a;123:595–9. doi: 10.1016/j.neuroscience.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Chung RS, Adlard PA, Dittmann J, Vickers JC, Chuah MI, West AK. Neuron-glia communication: metallothionein expression is specifically up-regulated by astrocytes in response to neuronal injury. J Neurochem. 2004b;88:454–61. doi: 10.1046/j.1471-4159.2003.02193.x. [DOI] [PubMed] [Google Scholar]
- Chung RS, Hidalgo J, West AK. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- Chung RS, Vickers JC, Chuah MI, Eckhardt BL, West AK. Metallothionein-III inhibits initial neurite formation in developing neurons as well as postinjury, regenerative neurite sprouting. Exp Neurol. 2002;178:1–12. doi: 10.1006/exnr.2002.8017. [DOI] [PubMed] [Google Scholar]
- Chung RS, Vickers JC, Chuah MI, West AK. Metallothionein-IIA Promotes Initial Neurite Elongation and Postinjury Reactive Neurite Growth and Facilitates Healing after Focal Cortical Brain Injury. J Neurosci. 2003b;23:3336–3342. doi: 10.1523/JNEUROSCI.23-08-03336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RS, West AK. A role for extracellular metallothioneins in CNS injury and repair. Neuroscience. 2004b;123:595–9. doi: 10.1016/j.neuroscience.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Cox DR, Palmiter RD. The metallothionein-I gene maps to mouse chromosome 8: implications for human Menkes’ disease. Hum Genet. 1983;64:61–4. doi: 10.1007/BF00289481. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–47. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T, Fu K, Palmiter RD, Andrews GK. Transgenic mice that overexpress metallothionein-I resist dietary zinc deficiency. J Nutr. 1996;126:825–33. doi: 10.1093/jn/126.4.825. [DOI] [PubMed] [Google Scholar]
- Dalton T, Pazdernik TL, Wagner J, Samson F, Andrews GK. Temporalspatial patterns of expression of metallothionein-I and -III and other stress related genes in rat brain after kainic acid-induced seizures. Neurochem Int. 1995;27:59–71. doi: 10.1016/0197-0186(94)00168-t. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Lassalle JM. Disruption of hippocampal CA3 network: effects on episodic-like memory processing in C57BL/6J mice. Eur J Neurosci. 2004;20:597–600. doi: 10.1111/j.1460-9568.2004.03484.x. [DOI] [PubMed] [Google Scholar]
- Dittmann J, Fung SJ, Vickers JC, Chuah MI, Chung RS, West AK. Metallothionein biology in the ageing and neurodegenerative brain. Neurotox Res. 2005;7:87–93. doi: 10.1007/BF03033779. [DOI] [PubMed] [Google Scholar]
- Duguid JR, Bohmont CW, Liu NG, Tourtellotte WW. Changes in brain gene expression shared by scrapie and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7260–4. doi: 10.1073/pnas.86.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency Water Quality Criterion for the Protection of Human Health: Methylmercury, Office of Science and Technology, Office of Water, Environmental Protection Agency Washington, DC 20460, EPA-823-R-01-001, 2001
- Erickson JC, Hollopeter G, Thomas SA, Froelick GJ, Palmiter RD. Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J Neurosci. 1997;17:1271–81. doi: 10.1523/JNEUROSCI.17-04-01271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JC, Masters BA, Kelly EJ, Brinster RL, Palmiter RD. Expression of human metallothionein-III in transgenic mice. Neurochem Int. 1995;27:35–41. doi: 10.1016/0197-0186(94)00166-r. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Sewell AK, Jensen LT, Winge DR, Palmiter RD. Enhanced neurotrophic activity in Alzheimer’s disease cortex is not associated with down-regulation of metallothionein-III (GIF) Brain Res. 1994;649:297–304. doi: 10.1016/0006-8993(94)91076-6. [DOI] [PubMed] [Google Scholar]
- Faller P, Hasler DW, Zerbe O, Klauser S, Winge DR, Vasak M. Evidence for a dynamic structure of human neuronal growth inhibitory factor and for major rearrangements of its metal-thiolate clusters. Biochemistry. 1999;38:10158–67. doi: 10.1021/bi990489c. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Nairn P, Bartlett CA, Chung RS, West AK, Beazley LD. Metallothionein-IIA promotes neurite growth via the megalin receptor. Exp Brain Res. 2007;183:171–80. doi: 10.1007/s00221-007-1032-y. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Jacob ST. Regulation of metallothionein gene expression. Prog Nucleic Acid Res Mol Biol. 2001;66:357–84. doi: 10.1016/s0079-6603(00)66034-8. [DOI] [PubMed] [Google Scholar]
- Giralt M, Penkowa M, Hernandez J, Molinero A, Carrasco J, Lago N, et al. Metallothionein-1+2 deficiency increases brain pathology in transgenic mice with astrocyte-targeted expression of interleukin 6. Neurobiol Dis. 2002a;9:319–38. doi: 10.1006/nbdi.2002.0480. [DOI] [PubMed] [Google Scholar]
- Giralt M, Penkowa M, Lago N, Molinero A, Hidalgo J. Metallothionein-1+2 protect the CNS after a focal brain injury. Exp Neurol. 2002b;173:114–28. doi: 10.1006/exnr.2001.7772. [DOI] [PubMed] [Google Scholar]
- Giralt M, Penkowa M, Lago N, Molinero A, Hidalgo J. Metallothionein-1+2 protect the CNS after a focal brain injury. Exp Neurol. 2002a;173:114–28. doi: 10.1006/exnr.2001.7772. [DOI] [PubMed] [Google Scholar]
- Gonzalez PDY, Massabuau JC, Boudou A, Bourdineaud JP. Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio) Environ Sci Technol. 2005;39:3972–3980. doi: 10.1021/es0483490. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol. 2003;25:335–47. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Hamer D. Metallothionein. Ann Rev Biochem. 1986;55:913–51. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hernandez J, Molinero A, Campbell IL, Hidalgo J. Transgenic expression of interleukin 6 in the central nervous system regulates brain metallothionein-I and -III expression in mice. Brain Res Mol Brain Res. 1997;48:125–31. doi: 10.1016/s0169-328x(97)00087-9. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Aschner M, Zatta P, Vasak M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull. 2001;55:133–45. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Borras M, Garvey JS, Armario A. Liver, brain, and heart metallothionein induction by stress. J Neurochem. 1990;55:651–4. doi: 10.1111/j.1471-4159.1990.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Garcia A, Oliva AM, Giralt M, Gasull T, Gonzalez B, et al. Effect of zinc, copper and glucocorticoids on metallothionein levels of cultured neurons and astrocytes from rat brain. Chem Biol Interact. 1994;93:197–219. doi: 10.1016/0009-2797(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Holloway AF, Stennard FA, Dziegielewska KM, Weller L, West AK. Localisation and expression of metallothionein immunoreactivity in the developing sheep brain. Int J Dev Neurosci. 1997;15:195–203. doi: 10.1016/s0736-5748(96)00091-3. [DOI] [PubMed] [Google Scholar]
- Hozumi I, Inuzuka T, Hiraiwa M, Uchida Y, Anezaki T, Ishiguro H, et al. Changes of growth inhibitory factor after stab wounds in rat brain. Brain Res. 1995;688:143–8. doi: 10.1016/0006-8993(95)00522-r. [DOI] [PubMed] [Google Scholar]
- Hozumi I, Inuzuka T, Ishiguro H, Hiraiwa M, Uchida Y, Tsuji S. Immunoreactivity of growth inhibitory factor in normal rat brain and after stab wounds--an immunocytochemical study using confocal laser scan microscope. Brain Res. 1996;741:197–204. doi: 10.1016/s0006-8993(96)00912-2. [DOI] [PubMed] [Google Scholar]
- Hughes W. A physicochemical rationale for the biological activity of mercury and its compound. Ann N Y Acad Sci. 1957;65:454–460. doi: 10.1111/j.1749-6632.1956.tb36650.x. [DOI] [PubMed] [Google Scholar]
- Inuzuka T, Hozumi I, Tamura A, Hiraiwa M, Tsuji S. Patterns of growth inhibitory factor (GIF) and glial fibrillary acidic protein relative level changes differ following left middle cerebral artery occlusion in rats. Brain Res. 1996;709:151–31. doi: 10.1016/0006-8993(95)01444-6. [DOI] [PubMed] [Google Scholar]
- Isumi H, Uchida Y, Hayashi T, Furukawa S, Takashima S. Neuron death and glial response in pontosubicular necrosis. The role of the growth inhibition factor. Clin Neuropathol. 2000;19:77–84. [PubMed] [Google Scholar]
- Kagi J, Vallee B. Metallothionein: a cadmium and zinc-containing protein from equine renal cortex. J Biol Chem. 1961;236:2435–42. [PubMed] [Google Scholar]
- Kagi JH, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–15. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Kagi JH, Vallee BL. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960;235:3460–3465. [PubMed] [Google Scholar]
- Kang YJCY, Yu A, Voss McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest. 1997;100:1501–1506. doi: 10.1172/JCI119672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Eddy RL, Henry WM, Haley LL, Byers MG, Shows TB. Human metallothionein genes are clustered on chromosome 16. Proc Natl Acad Sci U S A. 1984a;81:5494–8. doi: 10.1073/pnas.81.17.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Haslinger A, Holtgreve H, Richards R, Krauter P, Westphal H, et al. Characterisation of DNA sequences through which cadmium and glucocorticoid hormones influence human metallothionein-IIA gene. Nature. 1984b;308:513–9. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Doh-ura K, Torisu M, Uchida Y, Furuta A, Iwaki T. Differential expression of metallothioneins in human prion diseases. Dement Geriatr Cogn Disord. 2000;11:251–62. doi: 10.1159/000017247. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782–90. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc Natl Acad Sci U S A. 1997;94:10045–50. doi: 10.1073/pnas.94.19.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen RB, Crenshaw K, Kozyraki R, Verroust PJ, Tio L, Atrian S, et al. Megalin mediates renal uptake of heavy metal metallothionein complexes. Am J Physiol Renal Physiol. 2004;287:F393–403. doi: 10.1152/ajprenal.00233.2003. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Uchida Y, Ihara Y, Nakajima K, Kohsaka S, Miyatake T, et al. Molecular cloning of rat growth inhibitory factor cDNA and the expression in the central nervous system. Brain Res Mol Brain Res. 1993;19:188–94. doi: 10.1016/0169-328x(93)90025-k. [DOI] [PubMed] [Google Scholar]
- Kohler LB, Berezin V, Bock E, Penkowa M. The role of metallothionein II in neuronal differentiation and survival. Brain Res. 2003;992:128–36. doi: 10.1016/j.brainres.2003.08.049. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Woo ES, Michalska AE, Choo KH, Lazo JS. Metallothionein null cells have increased sensitivity to anticancer drugs. Cancer Res. 1995;55:2021–3. [PubMed] [Google Scholar]
- Kramer KK, Liu J, Choudhuri S, Klaassen CD. Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol Appl Pharmacol. 1996a;136:94–100. doi: 10.1006/taap.1996.0011. [DOI] [PubMed] [Google Scholar]
- Kramer KKLJ, Choudhuri S, Klaassen CD. Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol Appl Pharmacol 1995. 1995;136:94–100. doi: 10.1006/taap.1996.0011. [DOI] [PubMed] [Google Scholar]
- Kramer KK, Zoelle JT, Klaassen CD. Induction of metallothionein mRNA and protein in primary murine neuron cultures. Toxicol Appl Pharmacol. 1996b;141:1–7. doi: 10.1006/taap.1996.0253. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–7. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KH, Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270:5506–10. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- Lee DK, Carrasco J, Hidalgo J, Andrews GK. Identification of a signal transducer and activator of transcription (STAT) binding site in the mouse metallothionein-I promoter involved in interleukin-6-induced gene expression. Biochem J. 1999;337(Pt 1):59–65. [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–47. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- Levin ED, Perraut C, Pollard N, Freedman JH. Metallothionein expression and neurocognitive function in mice. Physiol Behav. 2006;87:513–8. doi: 10.1016/j.physbeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Leyshon-Sorland K, Jasani B, Morgan AJ. The localization of mercury and metallothionein in the cerebellum of rats experimentally exposed to methylmercury. Histochem J. 1994;26:161–9. doi: 10.1007/BF00157965. [DOI] [PubMed] [Google Scholar]
- Lima FR, Arantes CP, Muras AG, Nomizo R, Brentani RR, Martins VR. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04904.x. [DOI] [PubMed] [Google Scholar]
- Liu JMJ, Liu YP, Klaassen CD. Effects of butyrate homologues on metallothionein induction in rat primary hepatocyte culture. In Vitro Cell Dev Biol. 1992;28:320–326. doi: 10.1007/BF02877055. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci U S A. 1994;91:237–41. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoshes m, Vallee BL. A cadmium protein from equine kidney cortex. J Am Chem Soc. 1957;79:4813–4814. [Google Scholar]
- Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A. 1994a;91:584–8. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, et al. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci. 1994b;14:5844–57. doi: 10.1523/JNEUROSCI.14-10-05844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyja E, Albrecht J. Ultrastructural evidence that mercuric chloride lowers the threshold for glutamate neurotoxicity in an organotypic culture of rat cerebellum. Neurosci Lett. 1993;158:155–8. doi: 10.1016/0304-3940(93)90252-g. [DOI] [PubMed] [Google Scholar]
- Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci U S A. 1993;90:8088–92. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto KMK, Wakamiya J, Eto K, Sakamoto M, Yasutake A, et al. Protective effect of MK-801 in methylmercury-induced neuronal injury. Mercury as a Global Pollutant. 5th International Conference; 1999. p. 376. [Google Scholar]
- Miyamoto K, Nakanishi H, Moriguchi S, Fukuyama N, Eto K, Wakamiya J, et al. Involvement of enhanced sensitivity of N-methyl-D-aspartate receptors in vulnerability of developing cortical neurons to methylmercury neurotoxicity. Brain Res. 2001;901:252–8. doi: 10.1016/s0006-8993(01)02281-8. [DOI] [PubMed] [Google Scholar]
- Molinero A, Penkowa M, Hernandez J, Camats J, Giralt M, Lago N, et al. Metallothionein-I overexpression decreases brain pathology in transgenic mice with astrocyte-targeted expression of interleukin-6. J Neuropath Exp Neurol. 2003;62:315–28. doi: 10.1093/jnen/62.3.315. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Monfort P, Carrasco J, Palacios O, Capdevila M, Hidalgo J, et al. Metallothionein-III prevents glutamate and nitric oxide neurotoxicity in primary cultures of cerebellar neurons. J Neurochem. 2000;75:266–73. doi: 10.1046/j.1471-4159.2000.0750266.x. [DOI] [PubMed] [Google Scholar]
- Morton KA, Jones BJ, Sohn MH, Schaefer AE, Phelps RC, Datz FL, et al. Uptake of cadmium is diminished in transfected mouse NIH/3T3 cells enriched for metallothionein. J Biol Chem. 1992;267:2880–3. [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nagano S, Satoh M, Sumi H, Fujimura H, Tohyama C, Yanagihara T, et al. Reduction of metallothioneins promotes the disease expression of familial amyotrophic lateral sclerosis mice in a dose-dependent manner. Eur J Neurosci. 2001;13:1363–70. doi: 10.1046/j.0953-816x.2001.01512.x. [DOI] [PubMed] [Google Scholar]
- Naganuma A, Imura N. Methylmercury binds to a low molecular weight substance in rabbit and human erythrocytes. Toxicol Appl Pharmacol. 1979;47:613–6. doi: 10.1016/0041-008x(79)90532-5. [DOI] [PubMed] [Google Scholar]
- Neal JW, Singhrao SK, Jasani B, Newman GR. Immunocytochemically detectable metallothionein is expressed by astrocytes in the ischaemic human brain. Neuropathol Appl Neurobiol. 1996;22:243–7. [PubMed] [Google Scholar]
- Omata S, Sakimura K, Ishii T, Sugano H. Chemical nature of a methylmercury complex with a low molecular weight in the liver cytosol of rats exposed to methylmercury chloride. Biochem Pharmacol. 1978;27:1700–2. doi: 10.1016/0006-2952(78)90184-3. [DOI] [PubMed] [Google Scholar]
- Oyake Y, Tanaka M, Kubo H, Chichibu M. [Neuropathological studies on organic mercury poisoning with special reference to the staining and distribution of mercury granules] Shinkei Kenkyu No Shimpo. 1966;10:744–50. [PubMed] [Google Scholar]
- Palmiter RD, Findley SD, Whitmore TE, Durnam DM. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992;89:6333–7. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto NC, Roza T, Morsch VM, Pereira ME. Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Dev Neurosci. 2007;25:39–46. doi: 10.1016/j.ijdevneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Caceres M, Borup R, Nielsen FC, Poulsen CB, Quintana A, et al. Novel roles for metallothionein-I + II (MT-I + II) in defense responses, neurogenesis, and tissue restoration after traumatic brain injury: insights from global gene expression profiling in wild-type and MT-I + II knockout mice. J Neurosci Res. 2006;84:1452–74. doi: 10.1002/jnr.21043. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Camats J, Giralt M, Molinero A, Hernandez J, Carrasco J, et al. Metallothionein-I overexpression alters brain inflammation and stimulates brain repair in transgenic mice with astrocyte-targeted interleukin-6 expression. Glia. 2003a;42:287–306. doi: 10.1002/glia.10208. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J. CNS wound healing is severely depressed in metallothionein I- and II-deficient mice. J Neurosci. 1999a;19:2535–45. doi: 10.1523/JNEUROSCI.19-07-02535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J. CNS wound healing is severely depressed in metallothionein-I and II- deficient mice. J Neurosci. 1999b;19:2535–45. doi: 10.1523/JNEUROSCI.19-07-02535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Espejo C, Martinez-Caceres EM, Poulsen CB, Montalban X, Hidalgo J. Altered inflammatory response and increased neurodegeneration in metallothionein I+II deficient mice during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001a;119:248–60. doi: 10.1016/s0165-5728(01)00357-5. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Espejo C, Martinez-Carceres E, Montalban X, Hidalgo J. Increased demyelination and axonal damage in metallothionein-I + II-deficient mice during experimental autoimmune encephalomyelitis. Cell Mol Life Sci. 2003a:60. doi: 10.1007/s000180300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Espejo C, Martinez-Carceres E, Poulsen C, Montalban X, Hidalgo J. Altered inflammatory response and increased neurodegeneration in metallothionein I + II deficient mice during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001b;119:248–60. doi: 10.1016/s0165-5728(01)00357-5. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Espejo C, Ortega-Aznar A, Hidalgo J, Montalban X, Martinez Caceres EM. Metallothionein expression in the central nervous system of multiple sclerosis patients. Cell Mol Life Sci. 2003b;60:1258–66. doi: 10.1007/s00018-003-3021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Giralt M, Camats J, Hidalgo J. Metallothionein 1+2 protect the CNS during neuroglial degeneration induced by 6-aminonicotinamide. J Comp Neurol. 2002;444:174–89. doi: 10.1002/cne.10149. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Giralt M, Moos T, Thomsen PS, Hernandez J, Hidalgo J. Impaired inflammatory response to glial cell death in genetically metallothionein-I- and -II-deficient mice. Exp Neurol. 1999b;156:149–64. doi: 10.1006/exnr.1998.7009. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Hidalgo J. Metallothionein I+II expression and their role in experimental autoimmune encephalomyelitis. Glia. 2000a;32:247–63. doi: 10.1002/1098-1136(200012)32:3<247::AID-GLIA50>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Hidalgo J. Metallothionein I+II expression and their role in experimental autoimune encephalomyelitis. Glia. 2000b;32:247–63. doi: 10.1002/1098-1136(200012)32:3<247::AID-GLIA50>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Hidalgo J. Metallothionein treatment reduces proinflammatory cytokines IL-6 and TNF-alpha and apoptotic cell death during experimental autoimmune encephalomyelitis (EAE) Exp Neurol. 2001;170:1–14. doi: 10.1006/exnr.2001.7675. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Hidalgo J. Treatment with metallothionein prevents demyelination and axonal damage and increases oligodendrocyte precursors and tissue repair during experimental autoimmune encephalomyelitis. J Neurosci Res. 2003;72:574–86. doi: 10.1002/jnr.10615. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Moos T, Carrasco J, Hadberg H, Molinero A, Bluethmann H, et al. Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia. 1999c;25:343–57. [PubMed] [Google Scholar]
- Philbert MA, Beiswanger CM, Waters DK, Reuhl KR, Lowndes HE. Cellular and regional distribution of reduced glutathione in the nervous system of the rat: histochemical localization by mercury orange and o-phthaldialdehyde-induced histofluorescence. Toxicol Appl Pharmacol. 1991;107:215–27. doi: 10.1016/0041-008x(91)90204-r. [DOI] [PubMed] [Google Scholar]
- Portala K, Levander S, Westermark K, Ekselius L, von Knorring L. Pattern of neuropsychological deficits in patients with treated Wilson’s disease. Eur Arch Psychiatry Clin Neurosci. 2001;251:262–8. doi: 10.1007/pl00007543. [DOI] [PubMed] [Google Scholar]
- Poulsen CB, Penkowa M, Borup R, Nielsen FC, Caceres M, Quintana A, et al. Brain response to traumatic brain injury in wild-type and interleukin-6 knockout mice: a microarray analysis. J Neurochem. 2005;92:417–32. doi: 10.1111/j.1471-4159.2004.02877.x. [DOI] [PubMed] [Google Scholar]
- Puttaparthi K, Gitomer W, Krishnan U, CSon M, Rajendran B, Elliot J. Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J Neurosci. 2002a;22:8790–6. doi: 10.1523/JNEUROSCI.22-20-08790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttaparthi K, Gitomer WL, Krishnan U, Son M, Rajendran B, Elliott JL. Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J Neurosci. 2002b;22:8790–6. doi: 10.1523/JNEUROSCI.22-20-08790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, et al. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–9. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Quintana A, Molinero A, Florit S, Manso Y, Comes G, Carrasco J, et al. Diverging mechanisms for TNF-alpha receptors in normal mouse brains and in functional recovery after injury: From gene to behavior. J Neurosci Res. 2007;85:2668–85. doi: 10.1002/jnr.21126. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Fairhurst MT. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. XI The binding of methylmercury by sulfhydryl-containing amino acids and by glutathione. J Am Chem Soc. 1975;97:2086–92. doi: 10.1021/ja00841a015. [DOI] [PubMed] [Google Scholar]
- Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, et al. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. Embo J. 1993;12:1355–62. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsvik T, Norseth T. Methyl mercuric compounds in rat bile. Acta Pharmacol Toxicol (Copenh) 1975;36:67–78. doi: 10.1111/j.1600-0773.1975.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Rising L, Vitarella D, Kimelberg HK, Aschner M. Cadmium chloride (CdCl2)-induced metallothionein (MT) expression in neonatal rat primary astrocyte cultures. Brain Res. 1995;678:91–8. doi: 10.1016/0006-8993(95)00170-u. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, et al. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25:9735–45. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Kawazoe Y, Uchida Y, Hozumi I, Inuzuka T, Watabe K. Growth inhibitory factor prevents degeneration of injured adult rat motoneurons. Neuroreport. 2003;14:2147–2151. doi: 10.1097/00001756-200312020-00003. [DOI] [PubMed] [Google Scholar]
- Samson SL, Gedamu L. Molecular analyses of metallothionein gene regulation. Prog Nucleic Acid Res Mol Biol. 1998;59:257–88. doi: 10.1016/s0079-6603(08)61034-x. [DOI] [PubMed] [Google Scholar]
- Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–37. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- Searle PF, Davison BL, Stuart GW, Wilkie TM, Norstedt G, Palmiter RD. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984;4:1221–30. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell AK, Jensen LT, Erickson JC, Palmiter RD, Winge DR. Bioactivity of metallothionein-3 correlates with its novel beta domain sequence rather than metal binding properties. Biochemistry. 1995;34:4740–7. doi: 10.1021/bi00014a031. [DOI] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001a;914:159–65. doi: 10.1016/s0006-8993(01)02791-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001b;902:156–63. doi: 10.1016/s0006-8993(01)02342-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Mutkus LA, Walker SJ, Aschner M. Methylmercury enhances arachidonic acid release and cytosolic phospholipase A2 expression in primary cultures of neonatal astrocytes. Brain Res Mol Brain Res. 2002;106:1–11. doi: 10.1016/s0169-328x(02)00403-5. [DOI] [PubMed] [Google Scholar]
- Shi YWW, Mo J, Du L, Yao S, Tang W, Shi Y, Wang W, Mo J, Du L, Yao S, Tang W. Interactions of growth inhibitory factor with hydroxyl and superoxide radicals. Biometals. 2003;16:383–389. doi: 10.1023/a:1022582209196. [DOI] [PubMed] [Google Scholar]
- Sillevis Smitt PA, Blaauwgeers HG, Troost D, de Jong JM. Metallothionein immunoreactivity is increased in the spinal cord of patients with amyotrophic lateral sclerosis. Neurosci Lett. 1992;144:107–10. doi: 10.1016/0304-3940(92)90727-o. [DOI] [PubMed] [Google Scholar]
- Sillevis Smitt PA, Mulder TP, Verspaget HW, Blaauwgeers HG, Troost D, de Jong JM. Metallothionein in amyotrophic lateral sclerosis. Biol Signals. 1994;3:193–7. doi: 10.1159/000109545. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakajima K, Otaki N, Kimura M, Kawaharada U, Uehara K, et al. Localization of metallothionein in aged human brain. Pathol Int. 1994;44:20–6. doi: 10.1111/j.1440-1827.1994.tb02581.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lu A, Aronow BJ, Wagner KR, Sharp FR. Genomic responses of the brain to ischemic stroke, intracerebral haemorrhage, kainate seizures, hypoglycemia, and hypoxia. Eur J Neurosci. 2002;15:1937–52. doi: 10.1046/j.1460-9568.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- Thomas DJSC. Effects of coadministered low molecular weight thiol compounds on short term distribution of methylmercury in the rat. Toxicol Appl Pharmacol. 1979;62:104–110. doi: 10.1016/0041-008x(82)90106-5. [DOI] [PubMed] [Google Scholar]
- Trendelenburg G, Prass K, Priller J, Kapinya K, Polley A, Muselmann C, et al. Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J Neurosci. 2002;22:5879–88. doi: 10.1523/JNEUROSCI.22-14-05879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S, Kobayashi H, Uchida Y, Ihara Y, Miyatake T. Molecular cloning of human growth inhibitory factor cDNA and its down-regulation in Alzheimer’s disease. Embo J. 1992;11:4843–50. doi: 10.1002/j.1460-2075.1992.tb05590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Atkin JD, Farg MA, Zang DW, Rembach A, Lopes EC, et al. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25:108–17. doi: 10.1523/JNEUROSCI.4253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y. Growth-inhibitory factor, metallothionein-like protein, and neurodegenerative diseases. Biol Signals. 1994a;3:211–5. doi: 10.1159/000109547. [DOI] [PubMed] [Google Scholar]
- Uchida Y. Growth-inhibitory factor, metallothionein-like protein, and neurodegenerative diseases. Biol Signals. 1994b;3:211–5. doi: 10.1159/000109547. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Gomi F, Masumizu T, Miura Y. Growth inhibitory factor prevents neurite extension and the death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging. J Biol Chem. 2002;277:32353–9. doi: 10.1074/jbc.M111263200. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron. 1991a;7:337–47. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron. 1991b;7:337–47. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- Vahter M, Mottet NK, Friberg L, Lind B, Shen DD, Burbacher T. Speciation of mercury in the primate blood and brain following long-term exposure to methyl mercury. Toxicol Appl Pharmacol. 1994;124:221–9. doi: 10.1006/taap.1994.1026. [DOI] [PubMed] [Google Scholar]
- Vasak M, Hasler DW. Metallothioneins: new functional and structural insights. Curr Opin Chem Biol. 2000;4:177–83. doi: 10.1016/s1367-5931(00)00082-x. [DOI] [PubMed] [Google Scholar]
- Vašák MKJ. Metallothionein. In: King RB, editor. Encyclopedia of Inorganic Chemistry. New York: J. Wiley & Sons Ltd; 1994. pp. 229–2241. [Google Scholar]
- Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74:1434–42. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- West AK, Chuah MI, Vickers JC, Chung RS. Protective role of metallothioneins in the injured mammalian brain. Rev Neurosci. 2004;15:157–66. doi: 10.1515/revneuro.2004.15.3.157. [DOI] [PubMed] [Google Scholar]
- West AK, Stallings R, Hildebrand CE, Chiu R, Karin M, Richards RI. Human metallothionein genes: structure of the functional locus at 16q13. Genomics. 1990;8:513–8. doi: 10.1016/0888-7543(90)90038-v. [DOI] [PubMed] [Google Scholar]
- Westin G, Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. Embo J. 1988;7:3763–70. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N, Abouhamed M, Verroust P, Thevenod F. Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured retinal proximal tubule cells. J Pharmacol Exp Therap. 2006;318:782–91. doi: 10.1124/jpet.106.102574. [DOI] [PubMed] [Google Scholar]
- Xie T, Tong L, McCann UD, Yuan J, Becker KG, Mechan AO, et al. Identification and characterization of metallothionein-1 and -2 gene expression in the context of (+/-)3,4-methylenedioxymethamphetamine-induced toxicity to brain dopaminergic neurons. J Neurosci. 2004;24:7043–50. doi: 10.1523/JNEUROSCI.1626-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagle MK, Palmiter RD. Coordinate regulation of mouse metallothionein I and II genes by heavy metals and glucocorticoids. Mol Cell Biol. 1985;5:291–4. doi: 10.1128/mcb.5.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CP, Allen JW, Conklin DR, Aschner M. Transfection and overexpression of metallothionein-I in neonatal rat primary astrocyte cultures and in astrocytoma cells increases their resistance to methylmercury-induced cytotoxicity. Brain Res. 1999;818:414–20. doi: 10.1016/s0006-8993(98)01229-3. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Nakano A, Hirayama K. Induction by mercury compounds of brain metallothionein in rats: Hg0 exposure induces long-lived brain metallothionein. Arch Toxicol. 1998;72:187–91. doi: 10.1007/s002040050486. [DOI] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, et al. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Watanabe C, Horie K, Satoh M, Sawada M, Shimada A. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicol Lett. 2005;155:361–8. doi: 10.1016/j.toxlet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Youn J, Hwang D, Ryoo ZY, Lynes MA, Paik DJ, Chung HS, et al. Metallothionein suppresses collagen-induced arthritis via induction of TGF- and down-regulation of proinflammatory mediators. Clin Exp Immunol. 2002;129:232–239. doi: 10.1046/j.1365-2249.2002.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Lukiw WJ, Bergeron C, Niznik HB, Fraser PE. Metallothionein III is reduced in Alzheimer’s disease. Brain Res. 2001;894:37–45. doi: 10.1016/s0006-8993(00)03196-6. [DOI] [PubMed] [Google Scholar]
- Yuguchi T, Kohmura E, Yamada K, Sakaki T, Yamashita T, Otsuki H, et al. Expression of growth inhibitory factor mRNA following cortical injury in rat. J Neurotrauma. 1995a;12:299–306. doi: 10.1089/neu.1995.12.299. [DOI] [PubMed] [Google Scholar]
- Yuguchi T, Kohmura E, Yamada K, Sakaki T, Yamashita T, Otsuki H, et al. Changes in growth inhibitory factor mRNA expression compared with those in c-jun mRNA expression following facial nerve transection. Brain Res Mol Brain Res. 1995b;28:181–5. doi: 10.1016/0169-328x(94)00205-s. [DOI] [PubMed] [Google Scholar]
- Zambenedetti P, Giordano R, Zatta P. Metallothioneins are highly expressed in astrocytes and microcapillaries in Alzheimer’s disease. J Chem Neuroanat. 1998;15:21–6. doi: 10.1016/s0891-0618(98)00024-6. [DOI] [PubMed] [Google Scholar]