Abstract
Objective
The 6 month assessment of the response to antiretroviral therapy (ART) is a critical step. In sub-Saharan Africa, few people have access to plasma viral-load measurement. We assessed the gain or loss in body mass index (BMI), alone or in combination with the gain or loss in CD4+ T-cell count (CD4), as a tool for predicting the response to ART.
Methods
In a cohort of 622 adults in Abidjan, Côte d’Ivoire, we calculated the sensitivity, specificity and predictive values of BMI and CD4 for treatment success defined as viral-load undetectability (< 300 copies/ml) as gold standard.
Findings
After 6 months of ART, the median change in BMI was an increase of 1.0 kg/m² (interquartile range, IQR: 0.0–2.1), the median change in CD4 an increase of 148/μl (IQR: 54–230) and 84% of patients reached viral-load undetectability. The distribution of change in BMI was similar among patients who reached undetectability and those who did not (increases of 1.06 kg/m² versus 0.99 kg/m², P = 0.51). With larger changes in BMI, the specificity for treatment success increased but its sensitivity decreased and its positive predictive value was stable around 85%. All results remained similar when combining changes in BMI with those in CD4 and when stratifying by groups of baseline BMI or CD4.
Conclusion
In settings where viral-load measurement is not available, a high BMI gain does not reflect virological success, even when combined with a high CD4 gain. In our population, most patients with detectable viral-load had probably adhered to the drug regimen sufficiently to reach significant gains in body mass and CD4 count but had adhered insufficiently to reach viral suppression.
Résumé
Objectif
L’évaluation sur 6 mois de la réponse au traitement antirétroviral (ART) est une étape critique. En Afrique sub-saharienne, peu de personnes ont accès à la mesure de la charge virale plasmatique. Nous avons évalué le gain ou la perte d’indice de masse corporelle (IMC), isolément ou en association avec le gain ou la perte de cellules T CD4+ (CD4), en tant qu’outils pour prédire la réponse au traitement ART.
Méthodes
Parmi une cohorte de 622 adultes d’Abidjan, en Côte d’Ivoire, nous avons calculé la sensibilité, la spécificité et les valeurs prédictives de l’IMC et des CD4 pour le succès du traitement, défini comme l’impossibilité de détecter une charge virale (< 300 copies/ml), à titre normatif.
Résultats
Après 6 mois de traitement ART, on obtenait comme valeur médiane de la variation de l’IMC une augmentation de 1,0 kg/m² (intervalle interquartile, IIQ : 0,0-2,1) et comme médiane de la variation des CD4 une augmentation de 148/μl (IIQ : 54-230), ainsi qu’une indétectabilité de la charge virale chez 84 % des patients. La distribution de la variation de l’IMC était similaire chez les patients ayant atteint l’indétectabilité et chez ceux ne l’ayant pas atteint (augmentation de 1,06 kg/m² au lieu de 0,99 kg/m², p < 0,51). Lorsque la variation de l’IMC augmente, sa spécificité à l’égard du succès thérapeutique diminue et sa valeur prédictive positive reste stable autour de 85 %. Les résultats ne varient guère lorsqu’on combine les variations de l’IMC, dans leur ensemble, et celles des CD4 et qu’on opère une stratification par groupes d’IMC ou de CD4 de départ.
Conclusion
Dans les situations où l’on ne dispose pas de mesure de la charge virale, un fort gain d’IMC n’implique pas un succès virologique, même s’il s’accompagne d’une importante élévation des CD4. Dans la population étudiée, la plupart des patients présentant une charge virale détectable avaient probablement respecté assez longtemps le schéma thérapeutique pour bénéficier d’augmentations notables de la masse corporelle et de la numération des CD4, mais pas suffisamment pour atteindre la suppression virale.
Resumen
Objetivo
La evaluación a los 6 meses de la respuesta a la terapia antirretroviral (TAR) es una medida esencial, pero en el África subsahariana son muy pocas las personas con acceso a análisis de la carga viral en plasma. Evaluamos el aumento o disminución del índice de masa corporal (IMC), aisladamente o en combinación con el aumento o disminución del recuento de linfocitos T CD4+ (CD4), como estrategia para predecir la respuesta a la TAR.
Métodos
En una cohorte de 622 adultos de Abidján, Côte d’Ivoire, calculamos la sensibilidad, la especificidad y los valores predictivos del IMC y los niveles de CD4 como indicadores de la eficacia terapéutica, definida como una carga viral indetectable (< 300 copias/ml).
Resultados
A los 6 meses de iniciada la TAR, el IMC mediano había aumentado en 1,0 kg/m² (intervalo intercuartílico, IIC: 0,0-2,1) y el nivel mediano de CD4, en 148/μl (IIC: 54-230), y la carga viral había pasado a ser indetectable en un 84% de los pacientes. La distribución de la variación del IMC fue similar en los pacientes que alcanzaron niveles indetectables y en los demás (aumento de 1,06 kg/m² frente a 0,99 kg/m², p = 0,51). Conforme aumentaba la variación del IMC, la especificidad respecto de la eficacia terapéutica aumentaba, pero su sensibilidad disminuía, y su valor predictivo positivo se mantenía estable, en torno al 85%. Se obtenían siempre resultados similares al combinar distintos cambios del IMC y del recuento de CD4, así como al estratificar los grupos en función de los valores basales de IMC o CD4.
Conclusión
En los entornos donde no es posible medir la carga viral, un gran aumento del IMC no refleja la eficacia virológica, aun cuando se combine con un incremento importante de los niveles de CD4. En la población aquí estudiada, la mayoría de los pacientes con carga viral detectable probablemente habían seguido el tratamiento en la medida suficiente para conseguir aumentos importantes de la masa corporal y del recuento de CD4, pero no en la medida necesaria para lograr la supresión viral.
ملخص
الغرض
يعد تقيـيم الاستجابة للمعالجة المضادة للفيروسات القەقرية، الذي يمتد ستة أشەر، خطوة حاسمة. ففي البلدان الواقعة جنوبي الصحراء الأفريقية، يتاح لقلة من الناس قياس الحمل الفيروسي في البلاسما. وقد قام الباحثون في ەذە الدراسة بتقييم الزيادة والنقصان في منسب كتلة الجسم، سواء أُجري ذلك منفصلا أم مع تعداد الخلايا التائية (CD4)، كأداة لتوقع مدى الاستجابة للمعالجة المضادة للفيروسات القەقرية.
الطريقة
في مجموعة متساوية العمر قوامەا 622 بالغاً في مدينة أبيدجان في كوت ديفوار، قام الباحثون بحساب قيم الحساسية والنوعية والقيم التنبؤية لمنسب كتلة الجسم والخلايا التائية (CD4) لقياس مدى نجاح المعالجة، والذي يعرَّف بعدم إمكانية كشف الحمل الفيروسي (أقل من 300 نسخة/ ميلي لتر) بوصفە المعيار الذەبي.
الموجودات
بعد مرور ستة أشەر على المعالجة بمضادات الفيروسات القەقرية، ازداد متوسط التغير في منسب كتلة الجسم بمقدار 1.0 كغ/م² (بمدى ضمن الشريحة الربعية يتراوح بين 0.0 – 2.1)، فيما ازداد متوسط التغير في خلايا CD4 بمقدار 148 خلية/مم³ (بمدى ضمن الشريحة الربعية يتراوح بين 54 و230)، كما وصل 84% من المرضى إلى مرحلة عدم إمكانية كشف الحمل الفيروسي. وقد كان توزُّع التغيُّر في منسب كتلة الجسم متشابەاً بين المرضى الذين وصلوا إلى مرحلة عدم إمكانية كشف الحمل الفيروسي وبين من لم يصلوا إلى ەذە المرحلة (بزيادة 1.06 كغ/م² مقابل 0.99 كغ/م²، وكانت نسبة الاحتمال 0.51). وبازدياد شدة التغيرات في منسب كتلة الجسم ازدادت النوعية في نجاح المعالجة، ولكن تناقصت حساسيتەا، بينما استقرت القيمة التنبؤية الإيجابية حول 85%. كما بقيت معظم النتائج متشابەة عندما ضُمَّت التغيرات في منسب كتلة الجسم إلى التغيرات في الخلايا CD4 وعند التوزيع على مجموعات طبقية وفقاً للقيم الأساسية لمنسب كتلة الجسم وتعدد الخلايا CD4.
الاستنتاج
عندما لا يتاح قياس الحمل الفيروسي فإن الزيادة الكبيرة في منسب كتلة الجسم لا تعطي صورة واضحة عن النجاح من وجەة نظر علم الفيروسات، حتى لو اقترن ذلك بزيادة في تعداد الخلايا CD4. وفي المجموعة التي تمت دراستەا، كان الراجح التزام معظم المرضى، الذين أمكن كشف الحمل الفيروسي لديەم، بالنظام العلاجي الدوائي، بما يكفل تحقيق زيادة ملحوظة في كتلة الجسم وفي تعداد الخلايا CD4، إلا أن امتثالەم لم يكف لإيصالەم إلى مرحلة الكبت الفيروسي.
Introduction
International guidelines recommend that plasma viral load undetectability should be reached within the first 6 months of antiretroviral therapy (ART).1 In patients with plasma viral load still detectable at 6 months, the most common cause of virological failure is poor adherence to the treatment regimen. Reinforcing the importance of adherence is key to improving long-term outcomes.
In sub-Saharan Africa, few people have access to viral-load measurement. For the millions of African patients who will start ART within the next few years, treatment will have to be monitored with other tools, including clinical examination and CD4+ T-cell (CD4) count.2 Given the importance attached to viral suppression shortly after initiation of therapy, whether clinical and immunological indicators can predict viral-load undetectability at 6 months is an interesting question; or are these variables independently associated with initiation of antiretroviral therapy and unable to predict virological suppression?
Previous studies have found that the CD4 change at 6 months poorly predicts viral-load undetectability.3,4 To our knowledge, no study has assessed the predictive value for virological success of a gain in body mass index (BMI) in sub-Saharan adults starting ART. BMI is widely measurable, contrary to viral load, CD4 count, and WHO clinical staging, which is largely based on etiological diagnosis often requiring laboratory investigations. BMI has been repeatedly associated with the prognosis of patients with HIV on and off ART.5–10 Before the therapy became available, BMI was shown to increase in African patients taking co-trimoxazole prophylaxis, even though co-trimoxazole had no effect on change in CD4 count.11 Hence, BMI could be of use not only as a predictor for prognosis but also as a marker for HIV-treatment efficacy.
We assessed the change in BMI alone or in combination with change in CD4 between initiation of ART and the 6-month visit as a tool for predicting virological success or virological failure at 6 months in HIV-infected adults followed in a prospective cohort study in Abidjan, Côte d’Ivoire.
Methods
Patients
In December 2002, a multicentre randomized trial (Trivacan ANRS 1269 trial) was launched in five outpatient clinics of Abidjan.12 The main objective of this trial was to assess various structured treatment interruption strategies of ART. The trial was designed in two phases. Patients were included in the first phase (prerandomization phase) if they met the following criteria: age ≥ 18 years, naive to curative ART, CD4 count between 150/μl and 350/μl or CD4 percentage between 12.5% and 20%, absence of pregnancy, absence of severe renal or hepatic failure and written informed consent. In this prerandomization phase, all patients received continuous ART. After at least 6 months in this phase, patients with undetectable plasma HIV-1 RNA and CD4 count > 350/μl were randomly assigned into the antiretroviral-therapy interruptions strategies phase.
Here we present data on BMI, CD4 count and viral-load evolution during the first 6 months of continuous ART within the prerandomization phase of the Trivacan trial. All patients infected with HIV-1 included in the prerandomization phase of the Trivacan trial were eligible for the present study if they had BMI < 25 kg/m², CD4 count < 500/μl, and plasma viral load was detectable before initiation of ART and if they were still alive and followed up at 6 months. Patients were excluded from the analysis if they had at least one missing value for BMI, CD4 or viral load at baseline or at 6 months.
The protocol of the Trivacan trial was approved by the ethics committee of the Ivorian Ministry of Health and the Institutional Review Board of the French National Agency for AIDS Research (ANRS).
Follow-up
The procedures of the prerandomization phase have been previously described.13,14 In summary, at enrolment, patients started zidovudine–lamivudine in combination with: (i) preferably efavirenz for patients infected with HIV-1 or people infected with both HIV-1 and HIV-2 with effective contraception and no history of nevirapine prophylactic treatment; (ii) indinavir and ritonavir (800/100 mg twice daily) for HIV-2 infected patients and women not desiring contraception or with a history of nevirapine prophylaxis. Co-trimoxazole prophylaxis was systematically given to all patients, in accordance with WHO 2006 guidelines.15 After inclusion, participants were asked to return monthly to their study clinic. At enrolment and at each monthly visit, a standardized questionnaire was used to record clinical characteristics, including height, weight and self-reported adherence to treatment during the previous 4 days. Weight was measured at each visit using the same scales. Between these scheduled visits, patients had free access to the study clinics.
The CD4 count (True Count® technique on FACScan®, Becton Dickinson) and plasma HIV-1 RNA load [real-time polymerase chain reaction (PCR) on Taq Man technology Abi Prism 7000, Applied Biosystems, quantification limit 300 copies/ml] were measured every 3 months.16 In case of viral load > 300 copies/ml, resistance genotype was tested with automated population full-sequence analysis (ABI system) with the ANRS consensus technique. French resistance algorithm 2006 was used for interpretation (available at: http://www.hivfrenchresistance.org). All care was free-of-charge.
Statistical analyses
Baseline was the date of enrolment in the prerandomization phase. The end of study date was the 6 month visit. In the main set of analysis, virological success at 6 months was defined as a plasma viral load below the threshold of detectability (300 copies/ml). In a second set of analysis, virological success was defined as a plasma viral load below 3 log10 copies/ml.
First, at each scheduled visit, we described the distribution of CD4 count, BMI and the difference between the CD4 and BMI values and their baseline values. The mean changes in CD4 and BMI at each point were compared between groups of baseline values with Kruskal–Wallis tests.
Second, we estimated the sensitivity, specificity, and positive and negative predictive value for virological success or failure of the changes in CD4, BMI or both with different thresholds of change and using successively the 3-month and 6-month values of changes in BMI and CD4.
Third, the distributions of changes in BMI and CD4 at 3 months and 6 months were compared between patients who reached virological success at 6 months and those who did not with Kruskal–Wallis tests.
Finally, the association between adherence and virological success at 6 months was analysed with a multivariate logistic regression model, adjusted on baseline CD4 count, WHO clinical stage, plasma HIV-1 viral load and care centre. Non-adherence was defined as self-reporting at least one ARV drug dose missed. We successively analysed the role of non-adherence during the overall follow-up (i.e. reporting at least one ARV drug dose missed at any of the six visits) and the role of early non-adherence (i.e. reporting at least one ARV drug dose missed at the first month visit). All analyses were made using SAS 8.2 software (SAS Institute, Cary NC, United States of America).
Results
Patients
Of the 840 patients included in the prerandomization phase of the Trivacan trial, 622 were included in the present study; 32 were eligible but were excluded from the analyses because they had at least one missing value for baseline or 6 month CD4, BMI or viral load; and 186 were non-eligible for at least one of the following reasons: they had a baseline BMI > 25/m² (n = 138, 16%), they were infected with HIV-2 only (n = 16, 2%), they had a baseline CD4 count > 500/μl (n = 20, 2%), they had an undetectable viral load at baseline (n = 25, 3%), they died before 6 month follow-up (n = 10, 1%) or they were lost to follow-up before 6 months (n = 9, 1%). As shown in Table 1, the 622 patients included in the study were mostly female. Their median CD4 count, BMI and plasma HIV-1 viral load were 250/μl, 20.8 kg/m² and 5.0 log10 copies/ml, respectively.
Table 1. Patients’ baseline characteristics (N = 622).
| Characteristic | n | n/N (%) | Median | IQR |
|---|---|---|---|---|
| Women | 471 | 76 | ||
| Age (years) | 34 | 29–39 | ||
| Schooling | ||||
| Illiterate | 126 | 20 | ||
| Primary school level | 180 | 29 | ||
| ≥ secondary school level | 312 | 51 | ||
| Monthly family income | ||||
| None | 238 | 38 | ||
| < US$ 90 | 262 | 42 | ||
| ≥ US$ 90 | 122 | 20 | ||
| Body mass index (kg/m²) | 20.8 | 19.1–22.6 | ||
| WHO clinical stage | ||||
| 1 | 111 | 18 | ||
| 2 | 256 | 41 | ||
| 3 | 208 | 33 | ||
| 4 | 47 | 8 | ||
| CD4 (cells/μl) | 250 | 185–315 | ||
| Plasma HIV-1 RNA log10/ml | 5.0 | 4.5–5.5 | ||
| Haemoglobin level (g/l) | ||||
| Men | 12.6 | 11.4–13.6 | ||
| Women | 10.9 | 10–11.8 | ||
| Positive plasma HBs antigen | 86 | 14 |
CD4, CD4+ T-cell count; IQR, interquartile range.
Virological success at 6 months
At 6 months, 523 patients (84%) had undetectable plasma viral load. Of the remaining 99 patients with detectable viral load, 20 had a viral load < 3 log10 copies/ml, 36 had a viral load between 3 log10 copies/ml and 4 log10 copies/ml, and 43 had a viral load ≥ 4 log10 copies/ml. Of these 99 patients, 88 had available results for genotype resistance tests, showing no resistance to any antiretroviral drugs in 62 (70%) and at least one resistance mutation in 26 (30%). In the latter, the mutations were K103N alone (n = 14), M184V alone (n = 6), K103N and M184V (n = 5) and M41L (n = 1).
Change in BMI and CD4 count
At 6 months, the median change in BMI was an increase of 1.0 kg/m² (interquartile range, IQR: 0.0–2.1) and the median change in CD4 was an increase of 148/μl (IQR: 54–230).
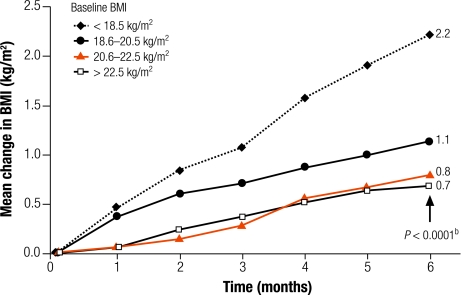
Fig. 1 shows the mean change in BMI at each monthly visit, by groups of baseline BMI. There was a significant difference between groups in terms of mean change at 6 months, which ranged from an increase of 0.7 kg/m² in patients with baseline BMI of 22.5–25 kg/m² to an increase of 2.2 kg/m² in patients with baseline BMI < 18.5 kg/m² (P < 0.001).
Fig. 1.
Mean change in BMI from baselinea
BMI, body mass index.
a No. of patients in each sub-group: < 18.5 kg/m² (n = 115), 18.6–20.5 kg/m² (n = 165), 20.6–22.5 kg/m² (n = 181), > 22.5 kg/m² (n = 161).
b P-value, Kruskal–Wallis test.
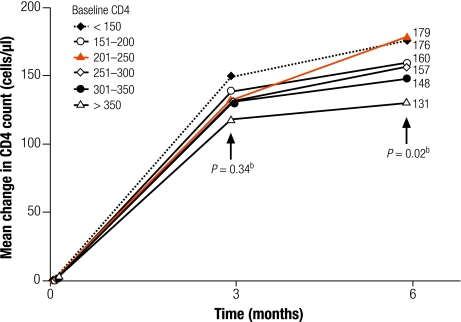
Fig. 2 shows the mean change in CD4 count at 3 months and 6 months, by groups of baseline CD4. Change in CD4 at 6 months was significantly different between groups, ranging from an increase in 131/μl in patients with baseline CD4 at 350–500/μl to an increase of 176/μl in patients with baseline CD4 < 150/μl (P = 0.02).
Fig. 2.
Mean change in CD4 count from baselinea
CD4, CD4+ T-cell count.
a No. of patients in each sub-group: < 150/μl (n = 75), 151–200/μl (n = 114), 201–250/μl (n = 124), 251–300/μl (n = 120), 301–350/μl (n = 91), > 350/μl (n = 98).
b P-values, Kruskal–Wallis test.
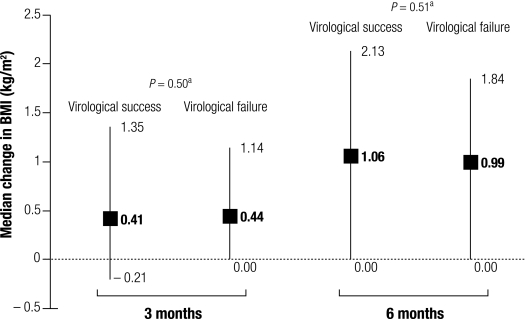
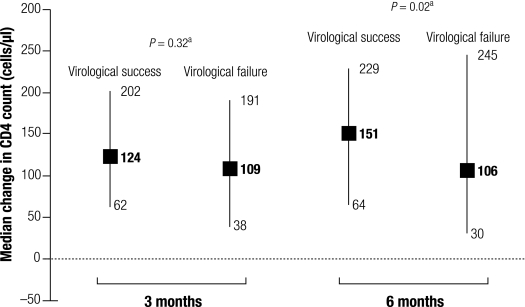
Fig. 3 and Fig. 4 show the distribution of changes in BMI and in CD4, respectively, at 3 months and at 6 months in patients who reached virological success at the latter follow-up and in those who did not. Patients reaching viral-load undetectability had distributions of change in BMI at 6 months comparable to those who did not. By contrast, patients reaching viral load undetectability had slightly but significantly higher CD4 count at 6 months than those who did not.
Fig. 3.
Median (IQR) change in BMI from baseline at months 3 and 6, according to virological success at 6 months
BMI, body-mass index. IQR, interquartile range.
a P-values, Kruskal–Wallis test.
Fig. 4.
Median (IQR) change in CD4 count at months 3 and 6, according to virological success at 6 months
CD4, CD4+ T-cell count; IQR, interquartile range.
a P-values, Kruskal–Wallis test.
Morbidity from baseline to 6 months
During the first 6 months, 26 (4.2%) of the 622 participants had at least one new WHO stage-3 or stage-4 morbidity episode not present at baseline (total number of new episodes: 29, median time between last episode and 6 month follow-up: 82 days, IQR: 50–133). This included 22 (4.2%) of the 523 patients with undetectable plasma viral load at 6 months (total number of episodes: 24) and four (4.0%) of the 99 patients with detectable viral load at 6 months (total number of episodes: 5). Patients with at least one severe morbidity episode had a significantly lower change in BMI from baseline to 6 months than those who did not experience severe morbidity [median increase: 0.3 (IQR: –1.5 to 1.7) versus median increase: 1.1 (IQR: 0.0 to 2.1), respectively]. The 29 episodes of severe morbidity were episodes of tuberculosis (n = 14), invasive bacterial diseases (n = 11, including four pneumonias, two isolated bacteraemias, one sinusitis, one deep abscess, one meningitis and one pyelonephritis), isosporiasis (n = 1), cryptosporidiosis (n = 1), chronic genital herpes simplex virus infection (n = 1) and unexplained diarrhoea > 30 days (n = 1).
Predictors of viral load at 6 months
Table 2 and Table 3 show the sensitivity, specificity and predictive values for virological success (Table 2) or virological failure (Table 3) of changes in BMI or CD4 at 6 months. The specificity of a gain in BMI for virological success rapidly increased with increasing gain, but it almost never rose above 90%. Meanwhile, its sensitivity rapidly fell with increasing gain, and its positive predictive value remained stable around 85% (i.e. close to the percentage of patients reaching virological success in the overall population). This remained true even for the highest gains and even when combining gain in BMI with a gain in CD4 cells.
Table 2. Sensitivity, specificity and predictive values of a gain in BMI, a gain in CD4 cells or both, for predicting virological success at 6 months.
| Gain in BMI and/or CD4 cells | No. of patients (N)a | No. with virological success (n)b | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Gain in BMI | ||||||
| ≥ 0 kg/m² | 482 | 406 | 78 | 23 | 84 | 16 |
| ≥ +1 kg/m² | 318 | 269 | 51 | 50 | 85 | 16 |
| ≥ +2 kg/m² | 160 | 139 | 27 | 79 | 87 | 17 |
| ≥ +3 kg/m² | 78 | 67 | 13 | 89 | 86 | 16 |
| ≥ +4 kg/m² | 38 | 32 | 6 | 94 | 84 | 16 |
| Gain in CD4 | ||||||
| ≥ 0/μl | 555 | 474 | 91 | 18 | 85 | 27 |
| ≥ +50/μl | 474 | 407 | 78 | 32 | 86 | 22 |
| ≥ +100/μl | 393 | 341 | 65 | 46 | 87 | 20 |
| ≥ +150/μl | 302 | 263 | 51 | 61 | 87 | 19 |
| ≥ +200/μl | 205 | 172 | 33 | 67 | 84 | 16 |
| ≥ +250/μl | 127 | 105 | 20 | 78 | 83 | 16 |
| ≥ +300/μl | 81 | 70 | 14 | 89 | 87 | 16 |
| Combined gains | ||||||
| ≥ 0 kg/m² and ≥ 0/μl | 440 | 373 | 71 | 32 | 85 | 18 |
| ≥ +1 kg/m² and ≥ +50/μl | 256 | 219 | 42 | 63 | 86 | 17 |
| ≥ +1 kg/m² and ≥ +100/μl | 209 | 181 | 35 | 72 | 87 | 17 |
| ≥ +1 kg/m² and ≥ +200/μl | 114 | 94 | 18 | 80 | 82 | 16 |
| ≥ +2 kg/m² and ≥ +50/μl | 135 | 116 | 22 | 81 | 86 | 16 |
| ≥ +2 kg/m² and ≥ +100/μl | 113 | 98 | 19 | 85 | 87 | 16 |
| ≥ +2 kg/m² and ≥ +200/μl | 65 | 55 | 10 | 90 | 85 | 16 |
BMI, body mass index; CD4, CD4+ T-cell count; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity. a No. of patients in whom the gain in BMI and/or the gain in CD4 is higher than the corresponding threshold at 6 months. b No. of patients with undetectable viral load at 6 months.
Table 3. Sensitivity, specificity and predictive values of a loss in BMI, a loss in CD4 cells or both, for predicting virological failure at 6 months.
| Loss in BMI and/or CD4 | No. of patients (N)a | No. with virological failure (n)b | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Loss in BMI | ||||||
| < –4 kg/m² | 2 | 0 | 0 | 99 | 0 | 84 |
| < –3 kg/m² | 6 | 1 | 1 | 99 | 17 | 84 |
| < –2 kg/m² | 23 | 4 | 4 | 96 | 17 | 84 |
| < –1 kg/m² | 61 | 8 | 8 | 90 | 13 | 84 |
| < 0 kg/m² | 140 | 23 | 23 | 78 | 16 | 84 |
| Loss in CD4 | ||||||
| < –150/μl | 2 | 0 | 0 | 99 | 0 | 84 |
| < –100/μl | 6 | 1 | 1 | 99 | 17 | 84 |
| < –50/μl | 29 | 6 | 6 | 96 | 21 | 84 |
| < 0/μl | 67 | 18 | 18 | 90 | 27 | 85 |
| Combined losses | ||||||
| < –1 kg/m² and < –50/μl | 8 | 1 | 1 | 99 | 13 | 84 |
| < 0 kg/m² and < 0/μl | 25 | 9 | 9 | 97 | 36 | 85 |
BMI, body mass index; CD4, CD4+ T-cell count; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity. a No. of patients in whom the gain in BMI and/or the gain in CD4 is higher than the corresponding threshold at 6 months. b No. of patients with undetectable viral load at 6 months.
These results remained similar when virological success was defined as a viral load < 3 log10 copies/ml, when stratifying analyses by groups of baseline BMI values or by groups of baseline CD4 values, and when using changes in BMI and CD4 at 3 months to predict virological success at 6 months (data not shown).
Adherence
The proportion of patients self-reporting at least one missed dose for at least one antiretroviral drug during the preceding 4 days was 11%, 12%, 11%, 9%, 10% and 8% at months 1, 2, 3, 4, 5 and 6, respectively. The percentage of patients self-reporting at least one missed dose at any of the six visits was 39%. There was no association between missing at least one dose at any of the six visits and virological failure at 6 months (univariate analysis, P = 0.14). However, self-reporting at least one missed dose at 1 month was significantly associated with virological failure at 6 months both in the univariate (P = 0.03) and multivariate analysis (P = 0.04). Adjusted on baseline CD4 count, WHO clinical staging, plasma viral load and care centre, the odds ratio of virological failure at 6 months was 1.87 in patients declaring at least one missed dose during the preceding 4 days at 1 month compared with patients declaring no missed dose (95% confidence interval: 1.03–3.41).
Discussion
We reported BMI, CD4 count and viral-load change between initiation of ART and 6 months in 622 adults who started therapy with a BMI < 25 kg/m² in Côte d’Ivoire. At 6 months, we found that the overall rate of patients reaching undetectable viral load was 84%. Patients reaching undetectable viral load had distributions of change in BMI at 3 months and 6 months comparable to those who had detectable viral load. The percentage of patients who succeeded in suppressing their plasma viral load remained comparable among patients reaching anthropometric or immunological markers of success (i.e. high BMI or CD4 gain) and among those reaching markers of failure (high BMI or CD4 loss). The gain or loss in BMI and CD4, alone or in combination, was not useful in predicting virological success or failure at 6 months, even when considering the highest gains or losses.
In countries where plasma viral load can be routinely measured, a detectable viral load at 6 months leads to subsequent investigation of the reason for insufficient suppression. Among these reasons, the most common is incomplete adherence.17 At this stage, improving adherence in patients is crucial to avoid the emergence of resistance mutations and therefore to ensure long-term success of ART.18 In our study, most patients with detectable viral load were probably insufficiently adherent to reach complete viral-load suppression but sufficiently adherent to reach significant gains in BMI and CD4. Conversely, most patients who presented a loss in BMI or CD4 cells probably were adherent to treatment, as 87% of patients who lost at least 1 kg/m² and 79% of patients who lost at least 50 CD4/μl between baseline and 6 months had undetectable viral load at 6 months. These findings have two consequences: first, they plead for making plasma viral-load quantification routinely available in low-resource settings; second, in settings where viral-load measurement is not available, clinicians should be aware that a high gain in CD4 – as previously shown3,4 – but also a high BMI gain, alone or in combination with a high CD4 gain – as shown in our study – should not be seen as reflecting optimum adherence to treatment. Similarly, a BMI loss, alone or in combination with a CD4 loss, should not lead to the conclusion that a patient is not adherent. Thus, in these settings, direct markers for adherence should be even more closely monitored than in high-resource settings.
In three large cohorts from low-resource settings, the median gain in weight after 6 months on ART was estimated at 3 kg (IQR: 1–6), 5.0 kg (IQR: 1.5–9.6) and 4.0 kg (IQR: 0.9–7.7) in patients with a median CD4 count at 48/μl, 43/μl and 131/μl, respectively, before ART therapy.19–21 To our knowledge, the gain in BMI on ART has never been reported in sub-Saharan Africa. Our study focused only on the 6-month response to ART. It cannot be inferred from our data that BMI change over longer follow-up (e.g. a break in the BMI change curve in a patient who previously reached criteria for treatment success) could not be useful for predicting later treatment failure. This should be assessed in studies with BMI being systematically recorded over longer periods of follow-up. As BMI change could be independently associated with non-ART treatments (e.g. co-trimoxazole prophylaxis or antituberculous treatments),11 further studies should include homogeneous populations with regards to non-ART drugs received by the patients, or have sufficient power to adjust for co-treatments.
Our study had some limitations. First, our population was not representative of the overall population of adults receiving ART in sub-Saharan Africa. Participants started ART with less advanced immunosuppression, compared with most adults starting ART in sub-Saharan Africa.5,7,8 They were followed under cohort conditions, with low rates of loss-to-follow-up and high rates of virological success. Because patients received care, including ART, free of charge they were under optimum conditions for adherence to treatment. In patients at a more advanced stage of immunosuppression and lower BMI at baseline, or in patients followed in programme conditions with lower rates of success at 6 months, the association between BMI gain and virological success might differ from our population. However, as our results remained similar when stratifying analyses by groups of baseline BMI and CD4 values, repeating our analyses in populations starting ART at a more advanced stage would be likely to give similar results.
Second, we did not test for resistance at baseline and were therefore unable to distinguish patients with ART failure because of primary drug resistance from those with failure for other reasons, including poor adherence. However, primary resistance to ART drugs is still rare in Côte d’Ivoire (5.6%).22,23 Furthermore, at 6 months, only 30% of patients with detectable viral load had resistance to at least one drug. Therefore, the assumption that most patients with detectable viral load could have been imperfectly adherent may be reasonable.
Third, we measured plasma HIV-1 RNA viral load by means of the automated TaqMan real-time reverse transcription PCR assay, with a detection threshold of 300 copies/ml. Although it is impossible to rule out that using an assay with lower detection threshold might have had consequences on our findings, BMI and CD4 gain would be unlikely to be more strongly predictive of virological success than in our study. Of note, our results did not vary in the sensitivity analysis with 1000 copies/ml as the threshold for defining success.
In conclusion, the 6 month assessment of the response to ART is a crucial step. At this stage, markers for unsatisfactory response to ART, even though not useful for decisions regarding therapeutic switch, could help elucidate responsible factors for early therapeutic failure. In low-resource settings, plasma viral-load quantification has now become much more affordable due to the generalization of real-time PCR.14 Making viral-load measurement widely available at 6 months would have two benefits. On the one hand, it would allow the identification of patients with virological failure among those who show markers of clinical and immunological success. These patients with discordant responses have impaired prognosis compared with those with concordant responses17,24 and may benefit from support for adherence. On the other hand, viral-load measurement would also be useful for patients with loss of CD4 or BMI. In these patients, finding that viral load is undetectable would help to actively search for concurrent conditions and not focus on adherence issues only. When viral load is not available, clinical and immunological markers cannot predict virological success. In settings where viral load cannot be measured, direct markers for adherence should be closely monitored. ■
Acknowledgements
We thank Joanna Orne-Gliemann and Besigin Towne-Gold (INSERM U593) for grammatical and editing contributions.
Footnotes
Funding: This study was supported by the French Agence Nationale de Recherches sur le SIDA (ANRS) and the Ivorian Ministry of Public Health within the collaborative Programme PAC-CI.
Competing interests: None declared.
References
- 1.Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach Geneva: WHO; 2006. Available from: http://www.who.int/hiv/pub/guidelines/WHO%20Adult%20ART%20Guidelines.pdf [accessed on 23 April 2008].
- 2.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 3.Moore DM, Mermin J, Awor A, Yip B, Hogg RS, Montaner JS. Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006;43:436–9. doi: 10.1097/01.qai.0000243105.80393.42. [DOI] [PubMed] [Google Scholar]
- 4.Bisson GP, Gross R, Strom JB, Rollins C, Bellamy S, Weinstein R, et al. Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy. AIDS. 2006;20:1613–9. doi: 10.1097/01.aids.0000238407.00874.dc. [DOI] [PubMed] [Google Scholar]
- 5.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 6.Castetbon K, Anglaret X, Touré S, Chêne G, Ouassa T, Attia A, et al. Prognostic value of cross-sectional anthropometric indices on short-term risk of mortality in HIV-infected adults in Abidjan, Côte d’Ivoire. Am J Epidemiol. 2001;154:75–84. doi: 10.1093/aje/154.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–60. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 8.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 9.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–9. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 10.van der Sande MA.Schim van der Loeff MF, Aveika AA, Sabally S, Togun T, Sarge-Njie R, et alBody mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr 2004371288–94. 10.1097/01.qai.0000122708.59121.03 [DOI] [PubMed] [Google Scholar]
- 11.Castetbon K, Anglaret X, Attia A, Toure S, Dakoury-Dogbo N, Messou E, et al. Effect of early chemoprophylaxis with co-trimoxazole on nutritional status evolution in HIV-1 infected adults in Abidjan, Côte d’Ivoire. AIDS. 2001;15:869–76. doi: 10.1097/00002030-200105040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–9. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 13.Danel C, Moh R, Anzian A, Abo Y, Chenal H, Guehi C, et al. Tolerance and acceptability of an efavirenz-based regimen in 740 adults (Predominantly women) in West Africa. J Acquir Immune Defic Syndr. 2006;42:29–35. doi: 10.1097/01.qai.0000219777.04927.50. [DOI] [PubMed] [Google Scholar]
- 14.Danel C, Moh R, Peytavin G, Anzian A, Minga A, Ba Gomis O, et al. Lack of indinavir-associated nephrological complications in HIV-infected adults (predominantly women) with high indinavir plasma concentration in Abidjan, Côte d’Ivoire. AIDS Res Hum Retroviruses. 2007;23:62–6. doi: 10.1089/aid.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO expert consultation on co-trimoxazole prophylaxis in HIV infection WHO technical report series. Geneva: WHO; 2006. Available from: http://www.who.int/hiv/pub/meetingreports/ctx/en [accessed on 23 April 2008].
- 16.Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, et al. Transfer and Evaluation of an Automated, Low-Cost Real-Time Reverse Transcription-PCR Test for Diagnosis and Monitoring of Human Immunodeficiency Virus Type 1 Infection in a West African Resource-Limited Setting. J Clin Microbiol. 2005;43:2709–17. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, et al. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40:288–93. doi: 10.1097/01.qai.0000182847.38098.d1. [DOI] [PubMed] [Google Scholar]
- 18.Kantor R, Shafer RW, Follansbee S, Taylor J, Shilane D, Hurley L, et al. Evolution of resistance to drugs in HIV-1-infected patients failing antiretroviral therapy. AIDS. 2004;18:1503–11. doi: 10.1097/01.aids.0000131358.29586.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassie JM, Szumilin E, Calmy A, Goemaere E. Highly active antiretroviral therapy in resource-poor settings: the experience of Medecins Sans Frontieres. AIDS. 2003;17:1995–7. doi: 10.1097/00002030-200309050-00023. [DOI] [PubMed] [Google Scholar]
- 20.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 21.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 22.Toni T, Masquelier B, Bonard D, Faure M, Huet C, Caumont A, et al. Primary HIV-1 drug resistance in Abidjan (Cote d’Ivoire): a genotypic and phenotypic study. AIDS. 2002;16:488–91. doi: 10.1097/00002030-200202150-00024. [DOI] [PubMed] [Google Scholar]
- 23.Toni TD, Recordon-Pinson P, Minga A, Ekouevi D, Bonard D, Bequet L, et al. Presence of key drug resistance mutations in isolates from untreated patients of Abidjan, Cote d’Ivoire: ANRS 1257 study. AIDS Res Hum Retroviruses. 2003;19:713–7. doi: 10.1089/088922203322280946. [DOI] [PubMed] [Google Scholar]
- 24.Schechter M, Tuboi SH. Discordant immunological and virological responses to antiretroviral therapy. J Antimicrob Chemother. 2006;58:506–10. doi: 10.1093/jac/dkl263. [DOI] [PubMed] [Google Scholar]