Abstract
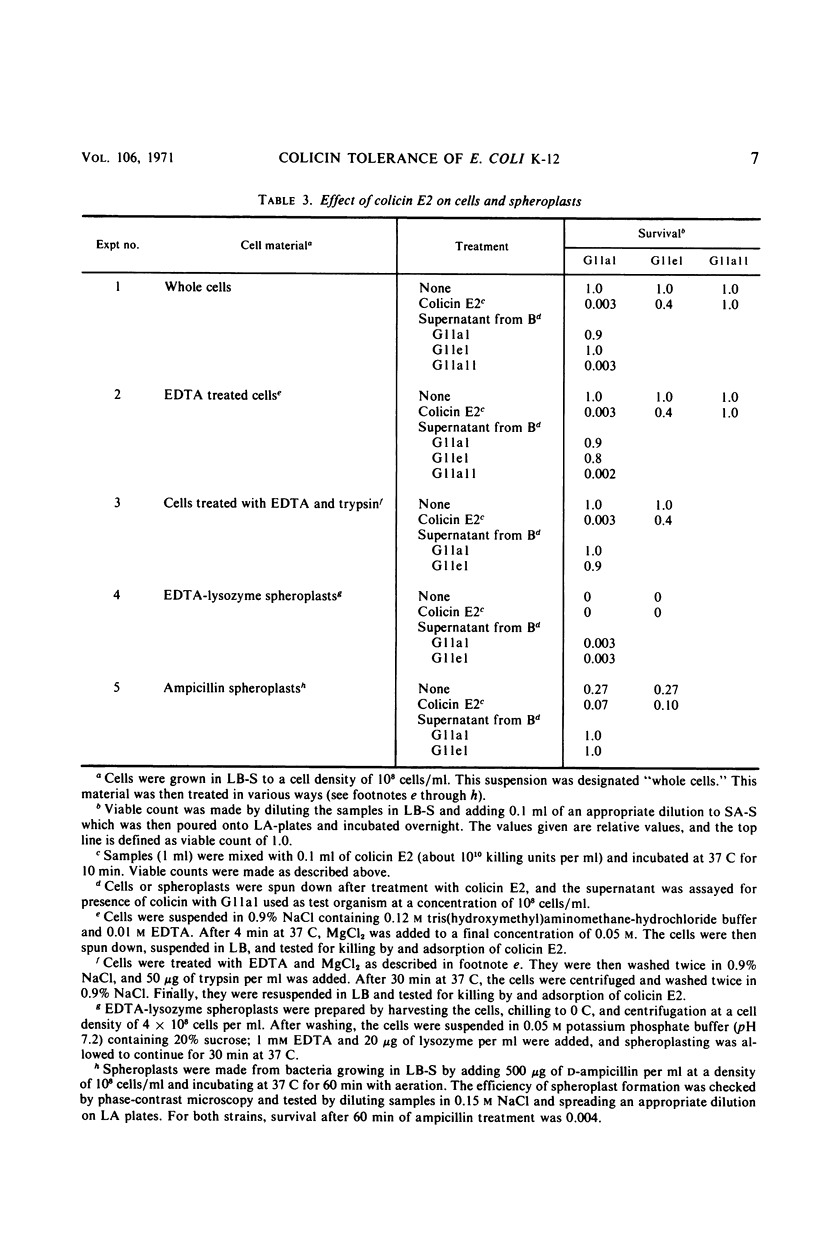
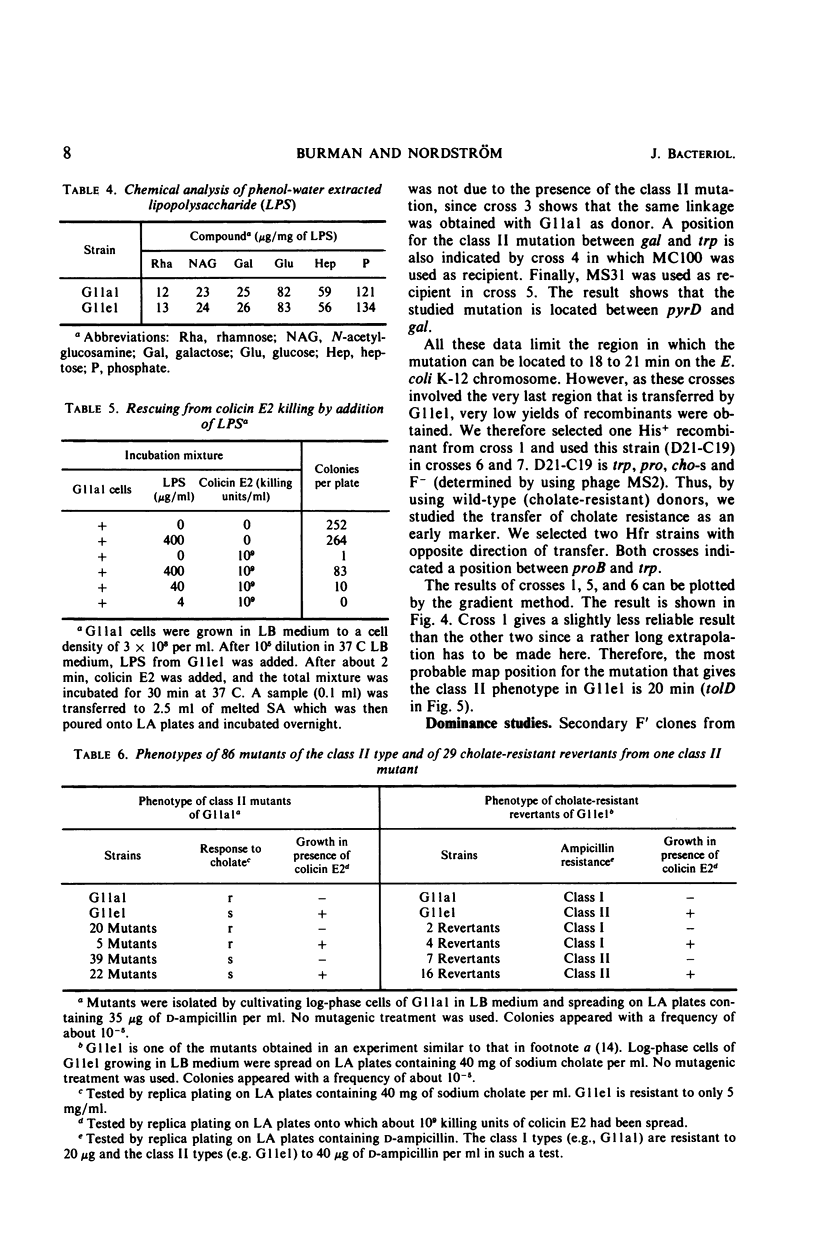
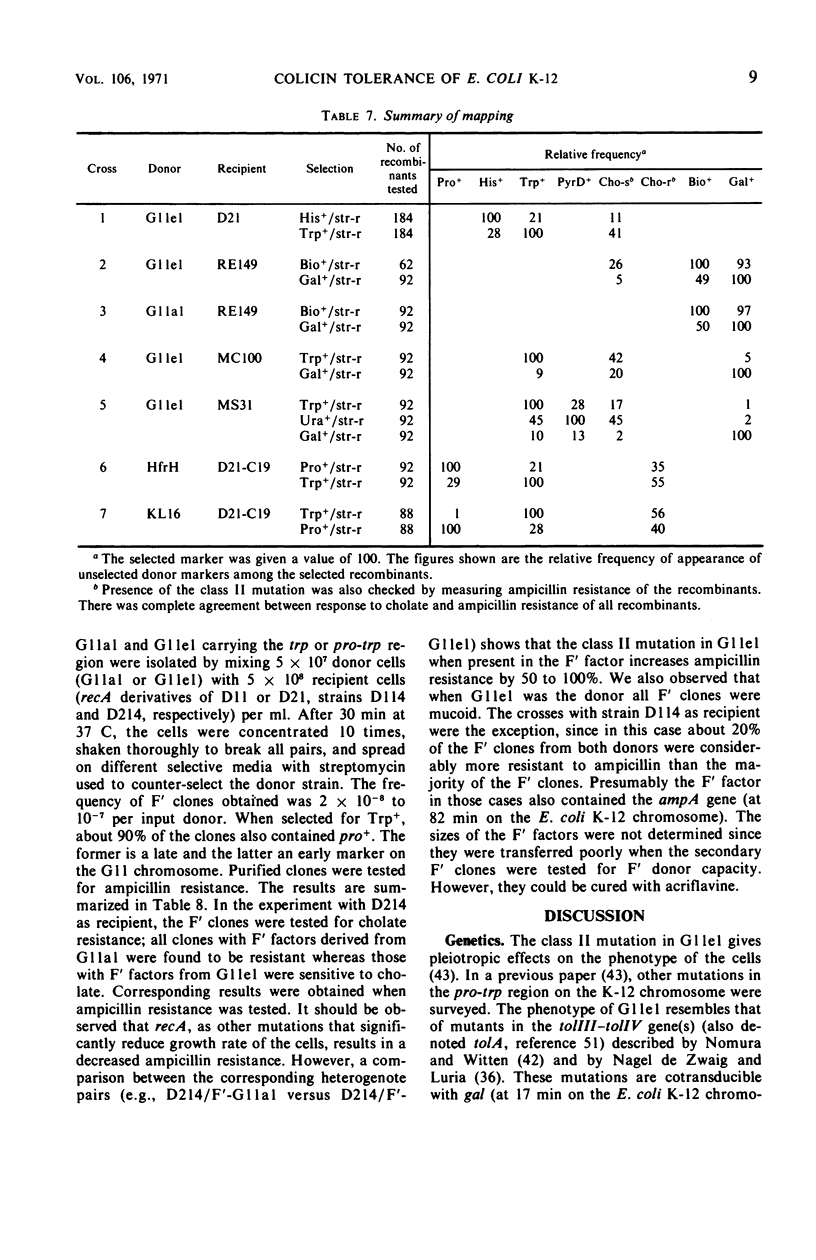
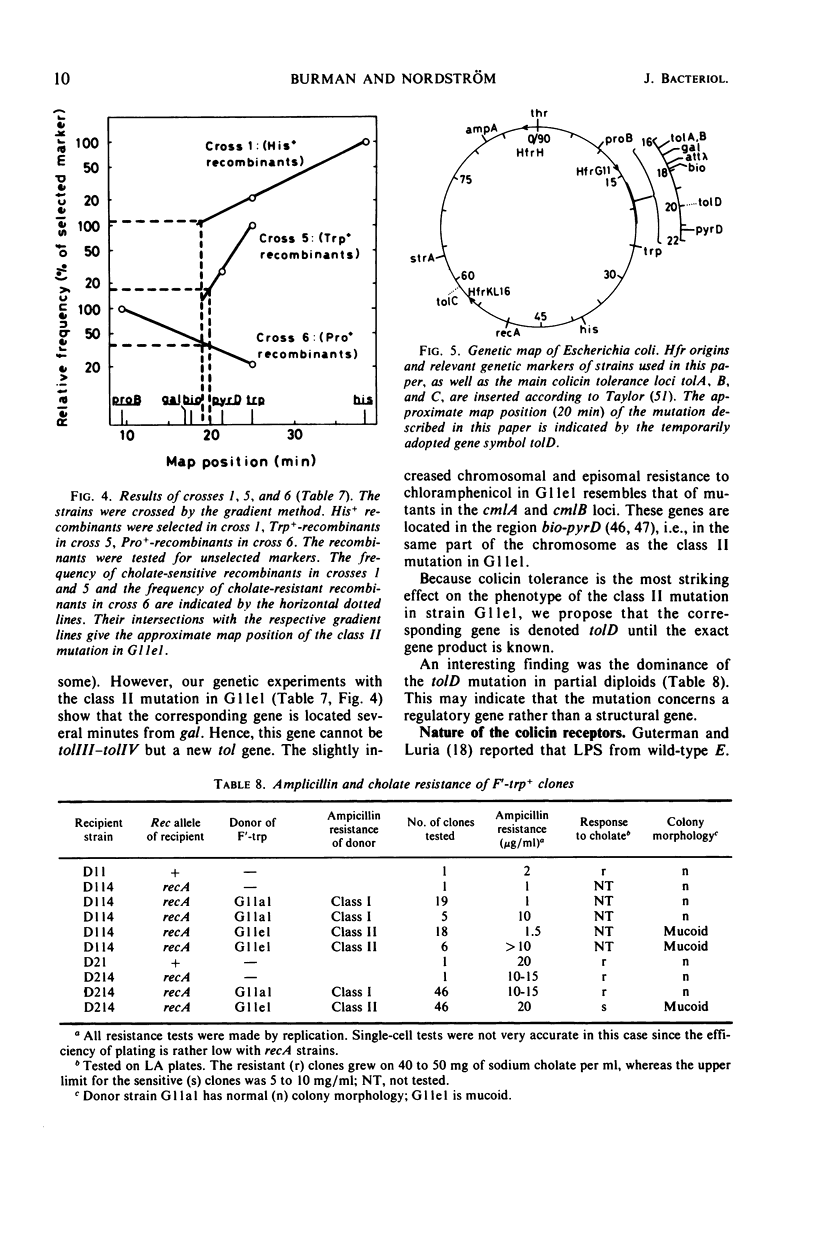
A mutant (G11el) of Escherichia coli selected as being resistant to ampicillin and showing signs of an envelope defect was also found to be tolerant to colicins E2 and E3. The colicin tolerance of G11el could be partially repressed by Mg2+ ions. Transition from tolerance to sensitivity and vice versa by shifting the concentration of Mg2+ in the growth medium required several generations. This indicated that synthesis of new envelope material was needed for transition. Previous physiological results have indicated a change in the envelope lipopolysaccharide (LPS) of G11el. However, chemical analyses revealed no differences in carbohydrate composition between LPS from G11el and its parent strain G11al. Genetic experiments showed that the mutation in G11el is located at about 20 min on the E. coli K-12 chromosome. The mutation was dominant over wild type in partial diploids with the mutation located on the episome. Because colicin tolerance was the most striking phenotypic effect as a result of mutation in the actual locus, this gene will be named tolD until the exact gene product is known. Spheroplasts formed from G11al and G11el by ethylenediaminetetraacetate-lysozyme treatment did not adsorb colicin E2; however, penicillin spheroplasts of G11al and G11el were tolerant to colicin E2. Thus, colicin tolerance can be induced biochemically. It is suggested that colicin tolerance often is a secondary consequence of a change in the cell envelope.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Carson K. J., Eagon R. G. Lysozyme sensitivity of the cell wall of Pseudomonas aeruginosa. Further evidence for the role of the non-peptidoglycan components in cell wall rigidity. Can J Microbiol. 1966 Feb;12(1):105–108. doi: 10.1139/m66-015. [DOI] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins and colicinogenic factors. Symp Soc Exp Biol. 1958;12:104–122. [PubMed] [Google Scholar]
- Gordon R. C., MacLeod R. A. MG++ phospholipids in cell envelopes of a marine and a terrestrial pseudomonad. Biochem Biophys Res Commun. 1966 Sep 8;24(5):684–690. doi: 10.1016/0006-291x(66)90378-0. [DOI] [PubMed] [Google Scholar]
- Guterman S. K., Luria S. E. Escherichia coli: strains that excrete an inhibitor of colicin B. Science. 1969 Jun 20;164(3886):1414–1414. doi: 10.1126/science.164.3886.1414. [DOI] [PubMed] [Google Scholar]
- Hill C., Holland I. B. Genetic basis of colicin E susceptibility in Escherichia coli. I. Isolation and properties of refractory mutants and the preliminary mapping of their mutations. J Bacteriol. 1967 Sep;94(3):677–686. doi: 10.1128/jb.94.3.677-686.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I. B. Properties of Escherichia coli K12 mutants which show conditional refractivity to colicin E2. J Mol Biol. 1968 Jan 28;31(2):267–275. doi: 10.1016/0022-2836(68)90443-9. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Threlfall E. J. Identification of closely linked loci controlling ultraviolet sensitivity and refractivity to colicin E2 in Escherichia coli. J Bacteriol. 1969 Jan;97(1):91–96. doi: 10.1128/jb.97.1.91-96.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. Resistance to colicin E as a genetic marker in E. coli K12. Nature. 1955 Apr 30;175(4461):779–779. doi: 10.1038/175779a0. [DOI] [PubMed] [Google Scholar]
- Konisky J., Nomura M. Interaction of colicins with bacterial cells. II. Specific alteration of Escherichia coli ribosomes induced by colicin E3 in vivo. J Mol Biol. 1967 Jun 14;26(2):181–195. doi: 10.1016/0022-2836(67)90290-2. [DOI] [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Nomura M. Interaction of colicins with bacterial cells. I. Studies with radioactive colicins. J Bacteriol. 1966 Feb;91(2):685–694. doi: 10.1128/jb.91.2.685-694.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Baker B. Suppression of radiation sensitivity and capsular polysaccharide synthesis in Escherichia coli K-12 by ochre suppressors. J Bacteriol. 1967 Aug;94(2):388–395. doi: 10.1128/jb.94.2.388-395.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- NOMURA M. MECHANISM OF ACTION OF COLICINES. Proc Natl Acad Sci U S A. 1964 Dec;52:1514–1521. doi: 10.1073/pnas.52.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M., NAKAMURA M. Reversibility of inhibition of nucleic acids and protein synthesis by colicin K. Biochem Biophys Res Commun. 1962 May 4;7:306–309. doi: 10.1016/0006-291x(62)90196-1. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R. New class of conditional colicin-tolerant mutants. J Bacteriol. 1969 Jul;99(1):78–84. doi: 10.1128/jb.99.1.78-84.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Doherty P. Linkage relationships of two genes causing partial resistance to chloramphenicol in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1450–1451. doi: 10.1128/jb.96.4.1450-1451.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C. Genetic analysis of some mutations causing resistance to tetracycline in Escherichia coli K12. Genet Res. 1968 Jun;11(3):303–309. doi: 10.1017/s0016672300011484. [DOI] [PubMed] [Google Scholar]
- Reeves P. Mutants resistant to colicin CA42-E2: cross resistance and genetic mapping of a special class of mutants. Aust J Exp Biol Med Sci. 1966 Jun;44(3):301–315. doi: 10.1038/icb.1966.29. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wilson J. H., Luftig R. B., Wood W. B. Interaction of bacteriophage T4 tail fiber components with a lipopolysaccharide fraction from Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):423–434. doi: 10.1016/0022-2836(70)90152-x. [DOI] [PubMed] [Google Scholar]


